NeuroSkin®: AI-Driven Wearable Functional Electrical Stimulation for Post-Stroke Gait Recovery—A Multicenter Feasibility Study
Abstract
Highlights
- The NeuroSkin® system was safely implemented in seven rehabilitation centers and achieved excellent usability (mean SUS score: 84.6), with no adverse events reported.
- Patients showed statistically significant improvements in gait speed, endurance, balance, and ambulation level following NeuroSkin®-assisted therapy.
- AI-driven wearable FES can be integrated into routine stroke rehabilitation with minimal training, enabling real-time, multi-muscle stimulation adapted to each patient.
- These findings support the feasibility of deploying personalized, sensor-based FES in clinical practice and lay the groundwork for future controlled trials assessing its efficacy.
Abstract
1. Introduction
2. Materials and Methods
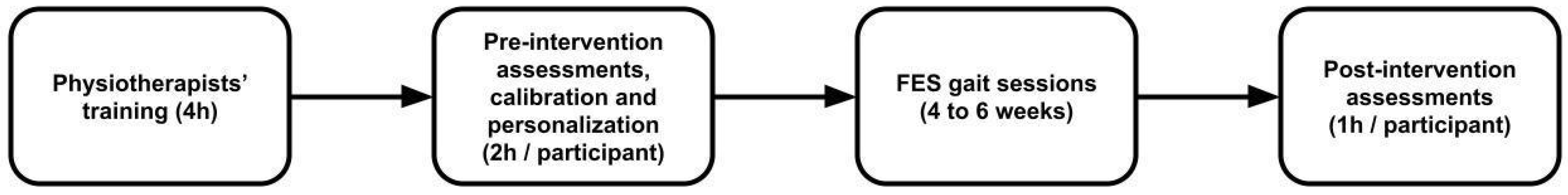
2.1. Study Design
- First-ever supratentorial ischemic or hemorrhagic stroke;
- Subacute stage (≤6 months post-stroke);
- Hemiparetic gait requiring assistance with sufficient motor skills to walk with help from a single person or technical aids (0 < NFAC < 5—New Functional Ambulation Category [21]);
- Medically stable and able to participate in therapy;
- Responsive to FES.
- Orthopedic or cardiorespiratory contraindications to walking;
- Implanted electrical devices;
- Cognitive or communication impairments precluding evaluation;
- Not responsive to FES.
2.2. Intervention
- -
- Personalization: Before the first session, a brief personalization phase was conducted, during which the patient walked approximately 20 steps without stimulation. Gait data from this recording were used to refine the pre-trained gait model and improve the accuracy of gait phase detection for stimulation control.
- -
- Calibration of stimulation intensities: For each of the six targeted muscle groups (gluteus maximus, quadriceps, hamstrings, tibialis anterior, fibularis, gastrocnemius), the maximum tolerable stimulation intensity was determined individually at baseline. These limits could later be adjusted as needed during sessions to account for habituation, fatigue, or recovery progress.
- -
- Gait training activities: Most sessions involved repeated overground walking at a comfortable pace along a flat indoor corridor, using technical aids (e.g., canes, walkers) if required. Therapists focused on correcting gait deficits common after stroke, such as drop foot, knee hyperextension, or equinovarus deformity, by tuning stimulation timing and intensity in real time.
- -
- Session structure and progression: Sessions typically alternated between short walking bouts and brief rest periods depending on patient fatigue and tolerance. Progression across sessions was individualized and could involve increased walking distance, higher stimulation intensities, or focusing on additional gait phases and muscle groups as recovery advanced.
- -
- Real-time adjustments: During each session, therapists used the NeuroSkin® tablet interface and the remote control to modify stimulation parameters on the fly, allowing them to adapt to patient-specific gait deficits and comfort levels dynamically.
2.3. Outcome Measures and Statistical Analysis
- 10-Meter Walk Test (10MWT): evaluates gait speed by timing how long it takes to walk 10 m at a comfortable pace [23];
- 6-Minute Walk Test (6MWT): measures walking endurance by recording the total distance walked in six minutes [24];
- Timed Up and Go (TUG): assesses functional mobility and dynamic balance by timing how long it takes to stand up from a chair, walk 3 m, turn, return, and sit down [25];
- New Functional Ambulation Classification (NFAC): categorizes the level of walking autonomy on a nine-point scale, ranging from non-functional ambulation to independent walking [21].
2.4. Usability and Satisfaction
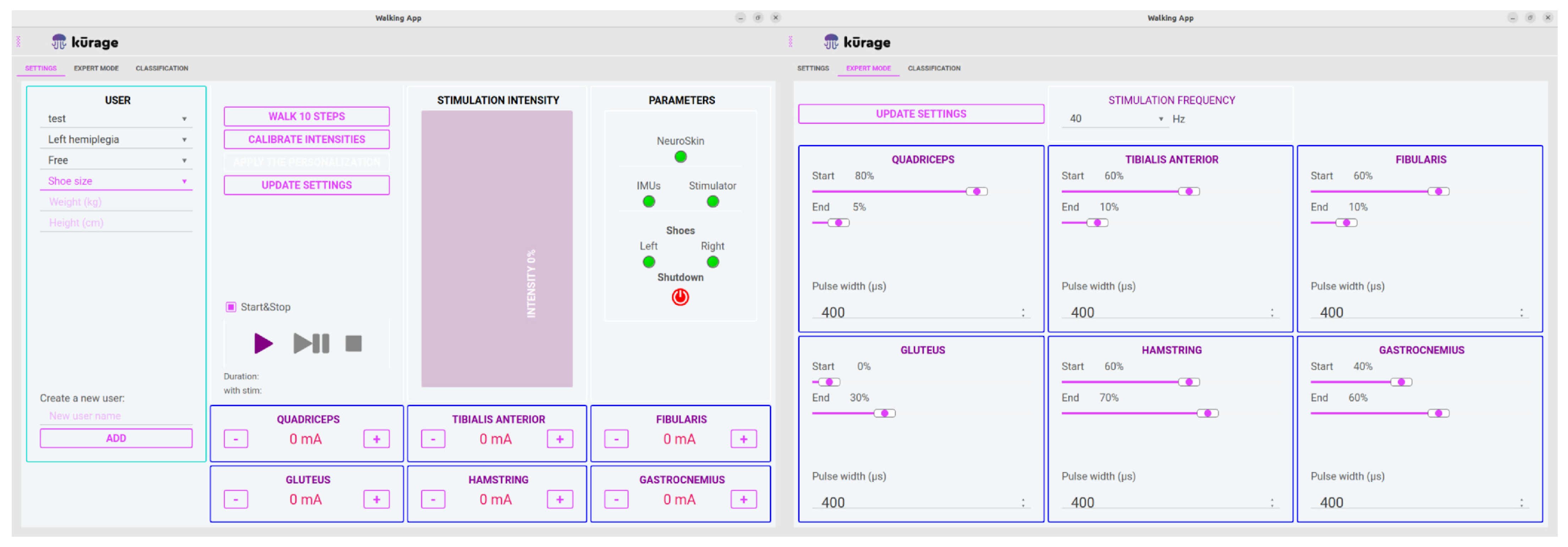
2.5. The Neuroskin System
- A lower-extremity garment with embedded FES dry electrodes targeting the six following muscle groups: Gluteus Maximus, Quadriceps, Hamstrings, Tibialis Anterior, Fibularis, and Gastrocnemius;
- A set of sensors: seven Inertial Measurement Units (IMU) placed on the pelvis, upper and lower leg segments, and feet; eight Ground Reaction Force (GRF) sensors integrated into the insoles of the shoes;
- An AI-driven real-time gait phase detector incorporated into a microcomputer positioned on the back of a vest worn by the patient;
- A MotiMove (3F-Fit Fabricando Faber, Belgrade, Serbia) electrical stimulator [26];
- A remote controller used to regulate the overall intensity of stimulation during the sessions;
- An application allowing therapists to manage individual patient profiles, including stimulation parameters (see Figure 3).
2.6. AI Model and Personalization Procedure
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| AIM | Acceptability of Intervention Measure |
| CE | Conformité Européenne (European Conformity) |
| CNIL | Commission Nationale de l’Informatique et des Libertés (National Commission on Informatics and Liberty) |
| CNN | Convolutional Neural Network |
| d | Cohen’s d (effect size measure) |
| FES | Functional Electrical Stimulation |
| FIM | Feasibility of Intervention Measure |
| GRF | Ground Reaction Force |
| IMU | Inertial Measurement Unit |
| MCID | Minimal Clinically Important Difference |
| MDC | Minimal Detectable Change |
| MSE | Mean Squared Error |
| NFAC | New Functional Ambulation Classification |
| SRD | Smallest Real Change |
| QUEST | Quebec User Evaluation of Satisfaction with Assistive Technology |
| SUS | System Usability Scale |
| TENS | Transcutaneous Electrical Nerve Stimulation |
| TUG | Timed Up and Go test |
| 6MWT | 6-Minute Walk Test |
| 10MWT | 10-Meter Walk Test |
References
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Dobkin, B.H. Rehabilitation after Stroke. N. Engl. J. Med. 2005, 352, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Popović, D.B.; Popović-Maneski, L. Neuroprosthesis and Functional Electrical Stimulation (Peripheral). In Handbook of Neuroengineering; Springer: Singapore, 2022; pp. 1–40. [Google Scholar] [CrossRef]
- Bogataj, U.; Gros, N.; Maležič, M.; Kelih, B.; Kljajić, M.; Ačimović, R. Restoration of Gait During Two to Three Weeks of Therapy with Multichannel Electrical Stimulation. Phys. Ther. 1989, 69, 319–327. [Google Scholar] [CrossRef]
- Kralj, A.; Grobelnik, S. Functional electrical stimulation of paraplegic patients-a feasibility study. Bul. Pros. Res. 1973, 20, 75–102. [Google Scholar]
- Sheffler, L.R.; Chae, J. Neuromuscular Electrical Stimulation in Neurorehabilitation. Muscle Nerve 2007, 35, 562–590. [Google Scholar] [CrossRef]
- De Kroon, J.R.; van der Lee, J.H.; Ijzerman, M.J.; Lankhorst, G.J. Therapeutic Electrical Stimulation to Improve Motor Control and Functional Abilities of the Upper Extremity after Stroke: A Systematic Review. Clin. Rehabil. 2002, 16, 350–360. [Google Scholar] [CrossRef]
- Pomeroy, V.M.; King, L.M.; Pollock, A.; Baily-Hallam, A.; Langhorne, P. Electrostimulation for Promoting Recovery of Movement or Functional Ability after Stroke. Cochrane Database Syst. Rev. 2006, 2006, CD003241. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gao, J.; Zhou, Y.; Zheng, B.; Liu, Y.; Cao, M.; Huang, H.; Su, X.; Chen, J. Implications of neuromuscular electrical stimulation on gait ability, balance and kinematic parameters after stroke: A systematic review and meta-analysis. J. Neuroeng. Rehabil. 2024, 21, 164. [Google Scholar] [CrossRef]
- He, R.; Dong, Y.; Li, Y.; Zheng, M.; Peng, S.; Tong, R.K.-Y.; Song, R. Therapeutic and orthotic effects of an adaptive functional electrical stimulation system on gait biomechanics in participants with stroke. J. Neuroeng. Rehabil. 2025, 22, 62. [Google Scholar] [CrossRef]
- Howlett, O.A.; Lannin, N.A.; Ada, L.; McKinstry, C. Functional electrical stimulation improves activity after stroke: A systematic review with meta-analysis. Arch. Phys. Med. Rehabil. 2015, 96, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Stanič, U.; Trnkoczy, A.; Kljajić, M.; Bajd, T. Optimal stimulating sequences to normalize hemiplegic’s gait. In Proceedings of the 3rd Conference on Bioengineering, Budapest, Hungary, 24–30 June 1974. [Google Scholar]
- Stanič, U.; Acimović-Janezic, R.; Gros, N.; Trnkoczy, A.; Bajd, T.; Kljajić, M. Multichannel electrical stimulation for correction of hemiplegic gait. Methodology and preliminary results. Scand. J. Rehabil. Med. 1978, 10, 75–92. [Google Scholar] [PubMed]
- Chaplin, E. Functional neuromuscular stimulation for mobility in people with spinal cord injuries. The Parastep I System. J Spinal Cord Med. 1996, 19, 99–105. [Google Scholar] [PubMed]
- Prenton, S.; Hollands, K.; Kenney, L. Functional electrical stimulation versus ankle foot orthoses for foot-drop: A meta-analysis of orthotic effects. J. Rehabil. Med. 2016, 48, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Kottink, A.; Tenniglo, M.; Vries, W.; Hermens, H.; Buurke, J. Effects of an implantable two-channel peroneal nerve stimulator versus conventional walking device on spatiotemporal parameters and kinematics of hemiparetic gait. J. Rehabil. Med. 2012, 44, 51–57. [Google Scholar] [CrossRef] [PubMed]
- The Case for the Odstock Dropped Foot Stimulator (ODFS®). Salisbury NHS Foundation Trust, Salisbury, UK, 2021. Available online: https://odstockmedical.com/wp-content/uploads/the_case_for_the_odstock_dropped_foot_stimulator_28th_june_2021.pdf (accessed on 5 September 2025).
- NICE—ODFS Pace and Pace XL Functional Electrical Stimulation Devices for Treating Drop Foot. Medtech Innovation Briefing [MIB56], 2016. Available online: https://www.nice.org.uk/advice/mib56 (accessed on 5 September 2025).
- Feppon, N.; Metani, A.; Mohammed, S. Assessing Personalized FES Patterns for Gait Rehabilitation in a Clinical Setting. IEEE Int. Conf. Rehabil. Robot 2025, 2025, 1724–1731. [Google Scholar] [CrossRef] [PubMed]
- Brooke, J. SUS—A Quick and Dirty Usability Scale. In Usability Evaluation in Industry; Jordan, P.W., Thomas, B., Weerdmeester, B.A., McClelland, I.L., Eds.; Taylor & Francis: London, UK, 1996; pp. 189–194. [Google Scholar]
- Brun, V.; Mousbeh, Z.; Jouet-Pastre, B.; Benaim, C.; Kunnert, J.; Dhoms, G.; d’ANgeli-Chevassut, M.; Torres, B.; Pélissier, J. Évaluation clinique de la marche de l’hémiplégique vasculaire: Proposition d’une modification de la functional ambulation classification. Ann. Réadaptation Médecine Phys. 2000, 43, 14–20. [Google Scholar] [CrossRef]
- Dromerick, A.W.; Geed, S.; Barth, J.; Brady, K.; Giannetti, M.L.; Mitchell, A.; Edwardson, M.A.; Tan, M.T.; Zhou, Y.; Newport, E.L.; et al. Critical Period After Stroke Study (CPASS): A phase II clinical trial testing an optimal time for motor recovery after stroke in humans. Proc. Natl. Acad. Sci. USA 2021, 118, e2026676118. [Google Scholar] [CrossRef]
- Watson, M.J. Refining the Ten-metre Walking Test for Use with Neurologically Impaired People. Physiotherapy 2002, 88, 386–397. [Google Scholar] [CrossRef]
- ATS Statement. Guidelines for the Six-Minute Walk Test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Popović-Maneski, L.; Mateo, S. MotiMove: Multi-purpose Transcutaneous Functional Electrical Stimulator. Artif. Organs 2022, 46, 1970–1979. [Google Scholar] [CrossRef]
- Chantraine, F.; Filipetti, P.; Schreiber, C.; Remacle, A.; Kolanowski, E.; Moissenet, F.; Trumbower, R.D. Proposition of a Classification of Adult Patients with Hemiparesis in Chronic Phase. PLoS ONE 2016, 11, e0156726. [Google Scholar] [CrossRef]
- Kang, I.; Molinaro, D.D.; Duggal, S.; Chen, Y.; Kunapuli, P.; Young, A.J. Real-Time Gait Phase Estimation for Robotic Hip Exoskeleton Control During Multimodal Locomotion. IEEE Robot. Autom. Lett. 2021, 6, 3491–3497. [Google Scholar] [CrossRef]
- Perera, S.; Mody, S.H.; Woodman, R.C.; Studenski, S.A. Meaningful change and responsiveness in common physical performance measures in older adults. J. Am. Geriatr. Soc. 2006, 54, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Flansbjer, U.B.; Holmbäck, A.M.; Downham, D.; Patten, C.; Lexell, J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J. Rehabil. Med. 2005, 37, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Jaqueline da Cunha, M.; Rech, K.D.; Salazar, A.P.; Pagnussat, A.S. Functional electrical stimulation of the peroneal nerve improves post-stroke gait speed when combined with physiotherapy. A systematic review and meta-analysis. Ann. Phys. Rehabil. Med. 2021, 64, 101388. [Google Scholar] [CrossRef]
- Gil-Castillo, J.; Alnajjar, F.; Koutsou, A.; Torricelli, D.; Moreno, J.C. Advances in neuroprosthetic management of foot drop: A review. J. Neuroeng. Rehabil. 2020, 17, 46. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, S.; Chia Bejarano, N.; Ambrosini, E.; Nardone, A.; Turcato, A.M.; Monticone, M.; Ferrigno, G.; Pedrocchi, A. A Personalized Multi-Channel FES Controller Based on Muscle Synergies to Support Gait Rehabilitation after Stroke. Front. Neurosci. 2016, 10, 425. [Google Scholar] [CrossRef] [PubMed]
- Berkelmans, S.; Dominici, N.; Afschrift, M.; Bruijn, S.; Janssen, T.W.J. Feasibility and safety of automated multi-channel FES-assisted gait training in incomplete spinal cord injury. J. Rehabil. Med. 2025, 57, jrm42638. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Fares, H.; Ghayvat, H.; Brunner, I.C.; Puthusserypady, S.; Razavi, B.; Lansberg, M.; Poon, A.; Meador, K.J. A systematic review on functional electrical stimulation based rehabilitation systems for upper limb post-stroke recovery. Front. Neurol. 2023, 14, 1272992. [Google Scholar] [CrossRef]
- Imatz-Ojanguren, E.; Sánchez-Márquez, G.; Asiain-Aristu, J.R.; Cueto-Mendo, J.; Jaunarena-Goicoechea, E.; Zabaleta, H.; Keller, T. A foot drop compensation device based on surface multi-field functional electrical stimulation-Usability study in a clinical environment. J. Rehabil. Assist. Technol. Eng. 2019, 6, 2055668319862141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Demers, L.; Weiss-Lambrou, R.; Ska, B. The Quebec User Evaluation of Satisfaction with Assistive Technology (QUEST 2.0): An overview and recent progress. Technol. Disabil. 2002, 14, 101–105. [Google Scholar] [CrossRef]
- Weiner, B.J.; Lewis, C.C.; Stanick, C.; Powell, B.J.; Dorsey, C.N.; Clary, A.S.; Boynton, M.H.; Halko, H. Psychometric assessment of three newly developed implementation outcome measures. Implement. Sci. 2017, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Ballester, B.R.; Maier, M.; Duff, A.; Cameirão, M.; Bermúdez, S.; Duarte, E.; Cuxart, A.; Rodríguez, S.; San Segundo Mozo, R.M.; Verschure, P.F.M.J. A critical time window for recovery extends beyond one-year post-stroke. J. Neurophysiol. 2019, 122, 350–357. [Google Scholar] [CrossRef] [PubMed]

| Patient | Age | Sex | Type of Stroke | Days Since Stroke | Paretic Side | Center | Number of Sessions |
|---|---|---|---|---|---|---|---|
| 1 | 62 | M | Ischemic | 78 | Right | 1 | 20 |
| 2 | 71 | F | Ischemic | 89 | Right | 1 | 19 |
| 3 | 35 | F | Hemorrhagic | 60 | Left | 2 | 20 |
| 4 | 66 | M | Ischemic | 71 | Left | 3 | 11 |
| 5 | 49 | F | Ischemic | 65 | Left | 3 | 11 |
| 6 | 59 | M | Ischemic | 35 | Right | 4 | 15 |
| 7 | 72 | M | Ischemic | 29 | Left | 4 | 20 |
| 8 | 47 | M | Ischemic | 45 | Right | 5 | 20 |
| 9 | 72 | M | Ischemic | 85 | Right | 5 | 20 |
| 10 | 57 | M | Hemorrhagic | 29 | Right | 5 | 20 |
| 11 | 46 | F | Ischemic | 112 | Left | 5 | 20 |
| 12 | 72 | M | Ischemic | 42 | Right | 6 | 12 |
| 13 | 51 | M | Hemorrhagic | 86 | Left | 6 | 10 |
| 14 | 76 | M | Ischemic | 89 | Left | 7 | 12 |
| 15 | 74 | F | Ischemic | 10 | Left | 7 | 12 |
| Patient | NFAC | 10MWT | 6MWT | TUG | ||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| 1 | 3 | 7 | 0.56 | 0.83 | 197 | 303 | - | - |
| 2 | 2 | 6 | 0.2 | 0.45 | 63 | 270 | - | - |
| 3 | 2 | 5 | 0.21 | 0.43 | 71 | 143 | 54 | 32 |
| 4 | 3 | 6 | 0.57 | 1.05 | 155 | 375 | 30 | 13.45 |
| 5 | 2 | 5 | 0.16 | 0.24 | 43 | 67 | 45.53 | 39.07 |
| 6 | 3 | 7 | 1.01 | 1.21 | 322 | 367 | 8.99 | 7.26 |
| 7 | 2 | 5 | 0.45 | 0.72 | 23 | 230 | 47 | 16.58 |
| 8 | 2 | 5 | - | - | 28 | 200 | 170 | 15.4 |
| 9 | 2 | 6 | 0.44 | 1.02 | 135 | 250 | 27.4 | 13.63 |
| 10 | 4 | 7 | 0.64 | 0.66 | 210 | 210 | 21.26 | 18.06 |
| 11 | 5 | 6 | 1.03 | 1.8 | 356 | 394 | 11.28 | 9.69 |
| 12 | 6 | 7 | 0.7 | 1.04 | 245 | 495 | 11.26 | 8.56 |
| 13 | 1 | 2 | 0.1 | 0.17 | 45 | 50 | 191.28 | 48.58 |
| 14 | 5 | 6 | 0.29 | 0.56 | 85 | 125 | 24.89 | 23.41 |
| 15 | 1 | 8 | 0 | 0.97 | 0 | 351 | unable | 10.3 |
| Normality | No | Yes | Yes | No | ||||
| (p-value) | (p = 0.0169) | (p = 0.0677) | (p = 0.1666) | (p = 0.0001301) | ||||
| Significance | Yes | Yes | Yes | Yes | ||||
| (p-value) | (p = 0.000632) | (p = 0.0004046) | (p = 0.0004718) | (p = 0.0004883) | ||||
| Effect size (Cohen’s d/r-value) | Large | Large | Large | Large | ||||
| (r = 0.88) | (d = 1.26) | (d = 1.17) | (r = 1.01) | |||||
| Mean change | +3 | +70.3% | +176% | −39.1% | ||||
| Operator | 1 | 2 | 3 | 4 | 5 | 6 | 7 | AVG | STD |
| SUS | 87.5 | 90 | 90 | 82.5 | 90 | 62.5 | 90 | 84.6 | 10.1 |
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | AVG | STD |
| SUS | 4 | 3 | - | 10 | 9 | 8 | 6 | 8 | - | 10 | 9 | 5 | - | 8 | 8 | 7.33 | 2.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metani, A.; Popović-Maneski, L.; Seguin, P.; Di Marco, J. NeuroSkin®: AI-Driven Wearable Functional Electrical Stimulation for Post-Stroke Gait Recovery—A Multicenter Feasibility Study. Sensors 2025, 25, 5614. https://doi.org/10.3390/s25185614
Metani A, Popović-Maneski L, Seguin P, Di Marco J. NeuroSkin®: AI-Driven Wearable Functional Electrical Stimulation for Post-Stroke Gait Recovery—A Multicenter Feasibility Study. Sensors. 2025; 25(18):5614. https://doi.org/10.3390/s25185614
Chicago/Turabian StyleMetani, Amine, Lana Popović-Maneski, Perrine Seguin, and Julie Di Marco. 2025. "NeuroSkin®: AI-Driven Wearable Functional Electrical Stimulation for Post-Stroke Gait Recovery—A Multicenter Feasibility Study" Sensors 25, no. 18: 5614. https://doi.org/10.3390/s25185614
APA StyleMetani, A., Popović-Maneski, L., Seguin, P., & Di Marco, J. (2025). NeuroSkin®: AI-Driven Wearable Functional Electrical Stimulation for Post-Stroke Gait Recovery—A Multicenter Feasibility Study. Sensors, 25(18), 5614. https://doi.org/10.3390/s25185614






