Colorimetric Detection of Nitrosamines in Human Serum Albumin Using Cysteine-Capped Gold Nanoparticles
Abstract
1. Introduction
2. Material and Methods
3. Results and Discussion
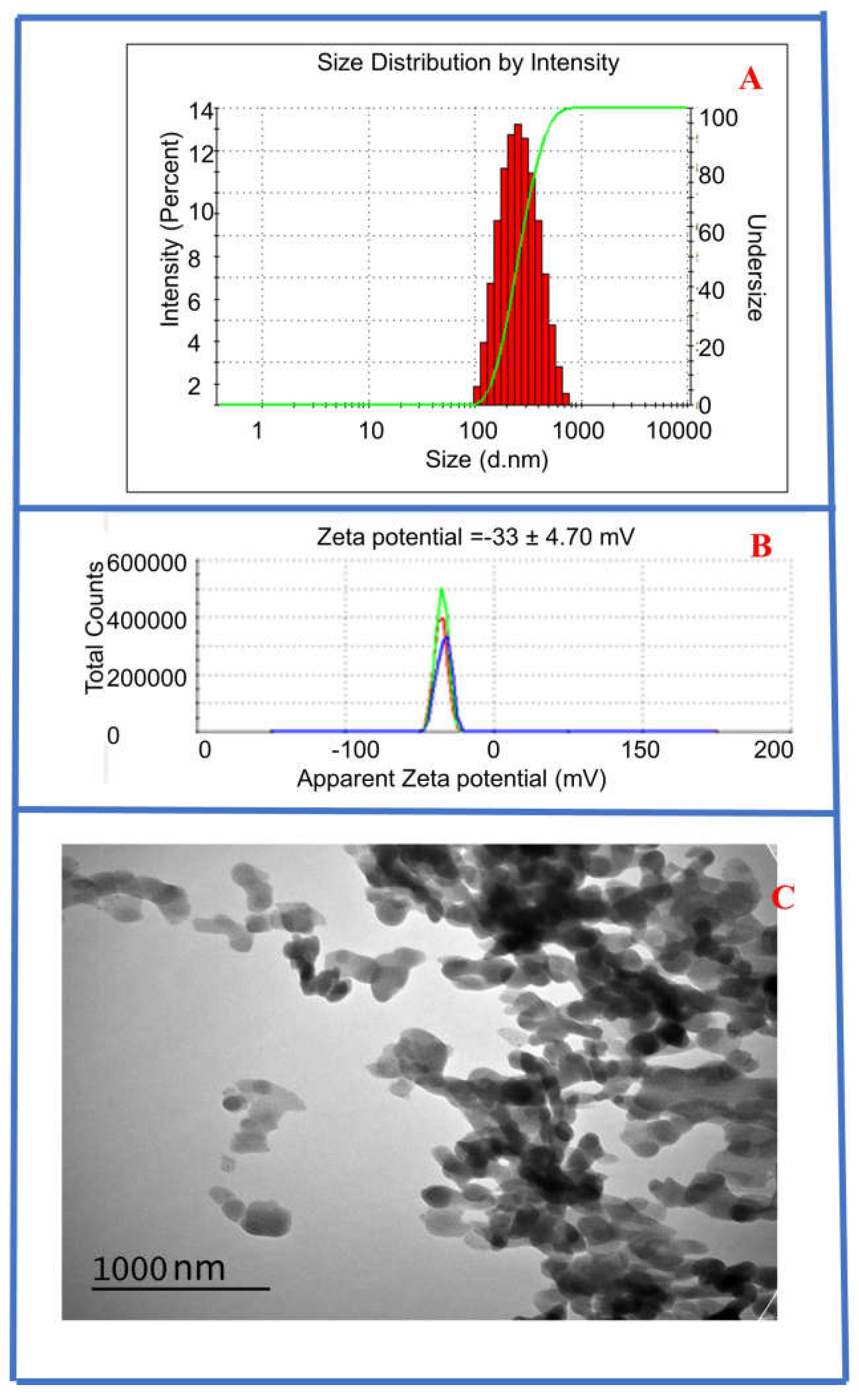
3.1. Characterization of Synthesized CysAuNPs
3.2. Colorimetric Detection of NDEA in HSA Samples
3.3. Job Plot
3.4. Proposed NDEA–CysAuNPs–HSA Interaction Mechanism
3.5. MD and MDS Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- US Food and Drugs Administration; Current Good Manufacturing in Manufacture, Processing, Packing, or Holding. 1963. Available online: https://tinyurl.com/tu5mrl8 (accessed on 17 December 2023).
- Department of Health and Human Services, U.S. Food and Drug Administration. Pharmaceutical cGMPs for the 21st Century—A Risk-Based Approach; Spring: Silver, MD, USA, 2004; pp. 1–32. [Google Scholar]
- Abdin, A.Y.; Yeboah, P.; Jacob, C. Chemical Impurities: An Epistemological Riddle with Serious Side Effects. Int. J. Environ. Res. Public Health 2020, 17, 1030. [Google Scholar] [CrossRef]
- ICH Expert Working Group. The Value and Benefits of ICH to Drug Regulatory Authorities: Advancing Harmonization for Better Health; International Conference on Harmonization: Geneva, Switzerland, 2010; pp. 1–36. [Google Scholar]
- Humfrey, C.D.N. Recent Developments in the Risk Assessment of Potentially Genotoxic Impurities in Pharmaceutical Drug Substances. Toxicol. Sci. 2007, 100, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Szekely, G.; Amores de Sousa, M.C.; Gil, M.; Castelo Ferreira, F.; Heggie, W. Genotoxic Impurities in Pharmaceutical Manufacturing: Sources, Regulations, and Mitigation. Chem. Rev. 2015, 115, 8182–8229. [Google Scholar] [CrossRef]
- U. S. Food and Drugs Administration. FDA Updates Recalled Valsartan-Containing Product Information and Reminds API Manufacturers to Evaluate Processes for Unsafe Impurities. 2018. Available online: https://tinyurl.com/y57kyulh (accessed on 16 December 2023).
- European Medicines Agency EMA. Update on Review of Valsartan Medicines Following Detection of Impurity in Active Substance Assessing Potential Impact on Patients is Priority; European Medi-cines Agency: London, UK, 2018. [Google Scholar]
- US Food and Drugs Administration. Updates and Press Announcements on NDMA in Zantac (Ranitidine); Spring: Silver, MD, USA, 2019. Available online: https://tinyurl.com/qnfdflt (accessed on 16 December 2023).
- European Medicines Agency. EMA to Review Ranitidine Medicines Following Detection of NDMA. 2023. Available online: https://tinyurl.com/y2k879my (accessed on 14 December 2023).
- Shaik, K.M.; Sarmah, B.; Wadekar, G.S.; Kumar, P. Regulatory Updates and Analytical Methodolo-gies for Nitrosamine Impurities Detection in Sartans, Ranitidine, Nizatidine, and Metformin along with Sample Preparation Techniques. Crit. Rev. Anal. Chem. 2022, 52, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, I.W.; Blanazs, A.; Byrne, J.J.; Dirat, O.; Fennell, J.W.; Nadine Kuhl, N.; Stuart, L.; Wells, S.L.; Whiting, M.P. Approaches and Considerations for the Investigation and Synthesis of N-Nitrosamine Drug Substance-Related Impurities (NDSRIs). Org. Process Res. Dev. 2023, 27, 1784–1791. [Google Scholar] [CrossRef]
- FDA Updates and Press Announcements on Angiotensin II Receptor Blocker (ARB) Recalls (Valsartan, Losartan, and Irbesartan), United States Food and Drug Administration (USFDA). 2019. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-angiotensin-ii-receptor-blocker-arb-recalls-valsartan-losartan (accessed on 17 December 2023).
- Snodin, D.J.; Elder, D.P. Short commentary on NDMA (N-nitrosodimethylamine) contamination of valsartan products. Regul. Toxicol. Pharmacol. 2019, 103, 325–329. [Google Scholar] [CrossRef]
- FDA. Control of Nitrosamine Impurities in Human Drugs. US Food and Drug Administration. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents (accessed on 15 December 2023).
- World Health Organization (WHO). Information Note Nitrosamine Impurities. Available online: https://cdn.who.int/media/docs/default-source/essential-medicines/medical-alert-2019/informationnotenitrosamine-impurities-nov2019en.pdf (accessed on 15 December 2023).
- Cioc, R.C.; Joyce, C.; Mayr, M.; Bream, R.N. Formation of N-Nitrosamine Drug Substance Related Impurities in Medicines: A Regulatory Perspective on Risk Factors and Mitigation Strategies. Org. Process Res. Dev. 2023, 27, 1736–1750. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Angiotensin-II-Receptor Antagonists (Sartans) Containing a te-Trazole Group-Referral. 2021. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/angiotensin-ii-receptor-antagonists-sartans-containing-tetrazole-group (accessed on 17 December 2023).
- European Medicines Agency (EMA). Ranitidine-Containing Medicinal Products-Referral. 2020. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/ranitidine-containing-medicinal-products (accessed on 17 December 2023).
- Eisenbrand, G.; Spiegelhalder, B.; Kann, J.; Klein, R.; Preussmann, R. Carcinogenic N-Nitrosodimethylamine as a Contamination in Drugs Containing 4-Dimethylamino-2,3-Dimethyl-1-Phenyl-3-Pyrazolin-5-One (Amidopyrine, Aminophena-zone). Arzneim.-Forsch. 1979, 29, 867–869. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, J.; Schmidt, F.; Blumrich, M.; Amberg, A.; Czich, A. Predicting DNA-Reactivity of N-Nitrosamines: A Quantum Chemical Approach. Chem. Res. Toxicol. 2022, 35, 2068–2084. [Google Scholar] [CrossRef]
- Li, X.; He, X.; Le, Y.; Guo, X.; Bryant, M.S.; Atrakchi, A.H.; McGovern, T.J.; Davis-Bruno, K.L.; Keire, D.A.; Heflich, R.H.; et al. Genotoxicity evaluation of nitrosamine impurities using human TK6 cells transduced with cytochrome P450s. Arch. Toxicol. 2022, 96, 3077–3089. [Google Scholar] [CrossRef]
- Farrag, M.A.; Ezz, M.K.; Ibrahim, N.K.; Ahmed, E.K. Chemopreventive Potential of Myrtenal against Nitrosamine-Initiated, Radiation-Promoted Rat Bladder Carcinogenesis. Nutr Cancer. 2022, 74, 288–298. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, C.; Zhou, J.; Zhang, H.; Zhang, Y.; Wang, S.; Pu, Y.; Yin, L. Reactive oxygen spe-cies-mediated activation of NLRP3 inflammasome associated with pyroptosis in Het-1A cells induced by the co-exposure of nitrosamines. J. Appl. Toxicol. 2022, 42, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Thresher, A.; Foster, R.; Ponting, D.J.; Stalford, S.A.; Tennant, R.E.; Thomas, R. Are all nitrosa-mines concerning? A review of mutagenicity and carcinogenicity data. Regul. Toxicol. Pharmacol. 2020, 116, 104749. [Google Scholar] [CrossRef]
- Voluntary Recall of Several Medicines Containing Valsartan Following Impurity Detection. Available online: https://www.pharmacytimes.com/view/voluntary-recall-of-several-medicines-containing-valsartan-following-impurity-detection- (accessed on 16 December 2023).
- Bharate, S.S. Critical Analysis of Drug Product Recalls Due to Nitrosamine Impurities. J. Med. Chem. 2021, 64, 2923–2936. [Google Scholar] [CrossRef]
- Wichitnithad, W.; Nantaphol, S.; Noppakhunsomboon, K.; Rojsitthisak, P. An update on the current status and prospects of nitrosation pathways and possible root causes of nitrosamine formation in vari-ous pharmaceuticals. Saudi Pharm. J. 2023, 31, 295–311. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ai, J.; Hassink, M.; Taylor, K.M.; Kuklenyik, P.; Valentin-Blasini, L.; Watson, C. Tobacco-Specific Nitrosamines in Current Commercial Large Cigars, Cigarillos, and Little Cigars. Chem. Res. Toxicol. 2024, 37, 220–226. [Google Scholar] [CrossRef]
- Stanfill, S.B.; Hecht, S.S.; Joerger, A.C.; Gonzalez, P.J.; Maia, L.B.; Rivas, M.G.; Moura, J.J.G.; Gupta, A.K.; Le Brun, N.E.; Crack, J.C.; et al. From culti-vation to cancer: Formation of N-nitrosamines and other carcinogens in smokeless tobacco and their mutagenic implications. Crit. Rev. Toxicol. 2023, 53, 658–701. [Google Scholar] [CrossRef]
- Hashemi, S.; Park, J.; Yang, M.; Kim, J.; Oh, Y.; Pyo, H.; Yang, J. Long-term monitoring and risk as-sessment of N-nitrosamines in the finished water of drinking water treatment plants in South Korea. Environ. Sci. Pollut. Res. 2022, 29, 3930–3943. [Google Scholar] [CrossRef]
- Vizioli, B.D.C.; Hantao, L.W.; Montagner, C.C. Drinking water nitrosamines in a large metropolitan region in Brazil. Environ. Sci. Pollut. Res. 2021, 28, 32823–32830. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhu, B.; Huang, H.; Chen, W.; Li, H.; Chen, Y.; Liang, Y.; Zeng, H. Analysing N-nitrosamine occurrence and sources in karst reservoirs, Southwest China. Environ. Geochem. Health 2024, 46, 112. [Google Scholar] [CrossRef]
- Xia, J.; Chen, Y.; Huang, H.; Haixiang Li, H.; Huang, D.; Liang, Y.; Zeng, H.; Chen, W. Occurrence and mass loads of N-nitrosamines discharged from different anthropogenic activities in Desheng River, South China. Environ. Sci. Pollut. Res. 2023, 30, 57975–57988. [Google Scholar] [CrossRef]
- Lee, H. S Literature compilation of volatile N-nitrosamines in processed meat and poultry products—An update. Food Addit. Contam. Part A 2019, 36, 1491–1500. [Google Scholar] [CrossRef]
- Özbay, S.; Şireli, U.T. Volatile N-nitrosamines in processed meat products and salami from Turkey. Food Addit. Contam. Part B 2021, 14, 110–114. [Google Scholar] [CrossRef]
- Tayell, D.I.; Farrag, N.K.; Aborhyem, S.M. Dietary intake and risk assessment of nitrosamine in pro-cessed meat products among medical staff during their night shift. Sci. Rep. 2025, 15, 1898. [Google Scholar] [CrossRef]
- Chang, S.; Chang, C.; Wang, L.; Chen, W.; Fan, S.; Zang, C.; Hsu, Y.; Lin, M.; Tseng, S.; Wang, D. A multi-analyte LC-MS/MS method for screening and quantification of nitrosamines in sartans. J. Food Drug Anal. 2020, 28, 98–107. [Google Scholar] [CrossRef]
- Choudhary, S.; Sharma, A.; Chandrasekar, M.P.; Kotikalapudi, S.; SCIEX, Gurgaon, India. LC-MS/MS Quantitative Analysis of NDMA in Ranitidine Active Pharmaceutical Ingredient (API) and Drug Product Using the SCIEX 4500 QTRAP. Phenomenex India. Available online: https://www.phenomenex.com/documents/2022/05/20/19/23/lcmsms-quantitative-analysis-of-ndma-in-ranitidine-active-pharmaceutical-ingredient-api-and-drug-pro?_gl=1*v2doci*_up*MQ..*_gs*MQ..&gclid=EAIaIQobChMI1OWX6KG-jwMVR6dmAh0VOR6KEAAYASAAEgJ4lvD_BwE&gbraid=0AAAAADp0uGYFcbpAAQG-2SSBV4Ya14j3U (accessed on 15 December 2023).
- Wichitnithad, W.; Sudtanon, O.; Srisunak, P.; Cheewatanakornkool, K.; Nantaphol, S.; Rojsitthisak, P. Development of a Sensitive Headspace Gas Chromatography–Mass Spectrometry Method for the Simultaneous Determination of Nitrosamines in Losartan Active Pharmaceutical Ingredients. ACS Omega 2021, 6, 11048–11058. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, A.B.; Giebułtowicz, J.; Dąbrowska, M.; Stolarczyk, E.U. Development of a Sensitive Screening Method for Simultaneous Determination of Nine Genotoxic Nitrosamines in Active Phar-maceutical Ingredients by GC-MS. Int. J. Mol. Sci. 2022, 23, 12125. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xie, B.; Mai, B.; Cai, Q.; He, R.; Guo, D.; Zhang, Z.; Fan, J.; Zhang, W. Development of a Sensitive and Stable GC-MS/MS Method for Simultaneous Determination of Four N-Nitrosamine Genotoxic Impurities in Sartan Substances. J. Anal. Sci. Technol. 2021, 12, 3. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Combined Headspace N-Nitrosodimethylamine (NDMA), N-Nitrosodiethylamine (NDEA), N-Nitrosoethylisopropylamine (NEIPA), and N-Nitrosodiisopropylamine (NDIPA) Impurity Assay by GC-MS/MS; US Food and Drug Administration; Spring: Silver, MD, USA, 2019; pp. 1–7.
- Tsutsumi, T.; Akiyama, H.; Demizu, Y.; Uchiyama, N.; Masada, S.; Tsuji, G.; Arai, R.; Abe, Y.; Haka-matsuka, T.; Izutsu, K.; et al. Analysis of an Impurity, N-Nitrosodimethylamine, in Valsartan Drug Substances and Associated Products Using GC-MS. Biol. Pharm. Bull. 2019, 42, 547–551. [Google Scholar] [CrossRef]
- Bahrani, S.H.; Raofie, F. Supercritical fluid extraction assisted by magnetic solid-phase extraction method using MWCNTs/Fe3O4@coPANI-PTH as preconcentration and gas chromatography mass spectrometry analysis for determining nitrosamines in Valsartan and Losartan tablets. J. Chromatogr. 2025, 1756, 466050. [Google Scholar] [CrossRef]
- Lee, D.H.; Hwang, S.H.; Park, S.; Lee, J.; Oh, H.B.; Han, S.B.; Liu, K.-H.; Lee, Y.-M.; Pyo, H.S.; Hong, J. A Solvent-Free Headspace GC/MS Method for Sensitive Screening of N-Nitrosodimethylamine in Drug Products. Anal. Methods 2021, 13, 3402–3409. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Thakur, D.; Chowdhury, D. DNA Carbon-Nanodots based Electrochemical Biosensor for Detection of Mutagenic Nitrosamines. ACS Appl. Biomater. 2020, 3, 1796–1803. [Google Scholar] [CrossRef]
- Beard, J.C.; Wang, C.; Sridharan, A.; Croy, R.G.; Essigmann, J.M.; TSwager, T.M. Colorimetric Detection of Aqueous N-Nitrosodimethylamine via Photonitrosation of a Naphtholsulfonate Indicator. ACS Sens. 2024, 9, 4655–4661. [Google Scholar] [CrossRef]
- Pu, C.; Zeng, T. Comparative Evaluation of Chemical and Photolytic Denitrosation Methods for Chemiluminescence Detection of Total N-Nitrosamines in Wastewater Samples. Environ. Sci. Technol. 2023, 57, 7526–7536. [Google Scholar] [CrossRef]
- Montaño-Priede, J.L.; Sanromán-Iglesias, M.; Zabala, N.; Grzelczak, M.; Aizpurua, J. Robust Rules for Optimal Colorimetric Sensing Based on Gold Nanoparticle Aggregation. ACS Sens. 2023, 8, 1827–1834. [Google Scholar] [CrossRef]
- Shahbazi, N.; Zare-Dorabei, R. A Facile Colorimetric and Spectrophotometric Method for Sensitive Determination of Metformin in Human Serum Based on Citrate-Capped Gold Nanoparticles: Central Composite Design Optimization. ACS Omega 2019, 4, 17519–17526. [Google Scholar] [CrossRef] [PubMed]
- Budlayan, M.L.M.; Oracion, J.P.L.; De La Rosa, L.B.; Rodriguez, M.J.D.; Patricio, J.N.; Perez, S.J.L.P.; Arco, S.D.; Manigo, J.P.; Austria, E.S.; Alguno, A.C.; et al. Y Preparation of Spin-Coated Poly(vinyl alcohol)/ chitosan/ Gold Nanoparticles Composite and Its Po-tential for Colorimetric Detection of Cyanide in Water. Pol. J. Environ. Stud. 2022, 31, 1569–1576. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, T.; Zheng, Y.; Guo, Q.; Wang, B.; Zhu, S. Sensitive Colorimetric Determination of Cyromazine Using a Gold Nanoparticle (Au NP) Based Sensor with Smartphone Detec-tion. Anal. Lett. 2022, 56, 1896–1910. [Google Scholar] [CrossRef]
- Unabia, R.B.; Reazo, R.L.D.; Rivera, R.B.P.; Lapening, M.A.; Omping, J.L.; Lumod, R.M.; Ruda, A.G.; Sayson, N.L.B.; Dumancas, G.; Malaluan, R.M.; et al. Dopamine-Functionalized Gold Nanoparticles for Colorimetric Detection of Histamine. ACS Omega 2024, 9, 17238–17246. [Google Scholar] [CrossRef]
- Omping, J.; Unabia, R.; Reazo, R.L.; Lapening, M.; Lumod, R.; Ruda, A.; Rivera, R.B.; Sayson, N.L.; Latayada, F.; Capangpangan, R.; et al. Facile Synthesis of PEGylated Gold Nanoparticles for Enhanced Colorimetric Detection of Histamine. ACS Omega 2024, 9, 14269–14278. [Google Scholar] [CrossRef] [PubMed]
- He, X.M.; Carter, D.C. Atomic structure and chemistry of human serum albumin. Nature 1992, 358, 209–215. [Google Scholar] [CrossRef]
- Yue, Y.; Chen, X.; Qin, J.; Yao, X. A study of the binding of C.I. Direct Yellow 9 to human serum albumin using optical spectroscopy and molecular modeling. Dye. Pigment. 2008, 79, 176–182. [Google Scholar] [CrossRef]
- Bai, J.; Sun, X.; Ma, X. Interaction of tebuconazole with bovine serum albumin: Determination of the binding mechanism and binding site by spectroscopic methods. J. Environ. Sci. Health—Part B Pestic. Food Contam. Agric. Wastes 2020, 55, 509–516. [Google Scholar] [CrossRef]
- Noh, E.; Moon, J.M.; Chun, B.J.; Cho, Y.S.; Ryu, S.J.; Kim, D. The clinical role of serum albumin in Organophospate poisoning. Basic Clin. Pharmacol. Toxicol. 2021, 128, 605–614. [Google Scholar] [CrossRef]
- Golianová, K.; Havadej, S.; Verebová, V.; Uličný, J.; Holečková, B.; Staničová, J. Interaction of conazole pesticides epoxiconazole and prothioconazole with human and bovine serum albumin studied using spectroscopic methods and molecular modeling. Int. J. Mol. Sci. 2021, 22, 1925. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Xing, Y.; Hou, C.; Zhou, Q.; Sun, Y.; Sun, Y.; Xu, H.; Gao, J. Mechanism of the interaction between benthiavalicarb-isopropyl and human serum albumin. Spectrosc. Lett. 2020, 53, 360–371. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Z.; Xie, Y.; Xue, X.; Tang, S.F.; Hou, X. Molecular mechanism investigation on the interaction of Clothianidin with human serum albumin. Spectrosc. Lett. 2019, 52, 246–252. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Z.; He, S.; Zhao, J.; Lai, X.; Xu, J. L-Cysteine Modified Gold Nanoparticles for Tube-Based Fluorometric Determination of Mercury (II) Ions. Microchim. Acta 2019, 186, 632. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Guex, N.; Peitsch, M.C. SWISS-MODEL and the Swiss-Pdb Viewer: An Environment for Compar-ative Protein Modeling. Electrophoresis 1997, 18, 2714–2723. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Gan, J.; Chen, S.; Xiao, Z.-X.; Cao, Y. CB-Dock2: Improved Protein–Ligand Blind Docking by Integrating Cavity Detection, Docking and Homologous Template Fitting. Nucleic Acids Res. 2022, 50, W159–W164. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Huang, J.; MacKerell, A.D. CHARMM36 All-Atom Additive Protein Force Field: Validation Based on Comparison to NMR Data. J. Comput. Chem. 2013, 34, 2135–2145. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM General Force Field: A Force Field for Drug-like Molecules Compatible with the CHARMM All-atom Additive Biological Force Fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef]
- Valdés-Tresanco, M.S.; Valdés-Tresanco, M.E.; Valiente, P.A.; Moreno, E. Gmx_MMPBSA: A New Tool to Perform End-State Free Energy Calculations with GROMACS. J. Chem. Theory Comput. 2021, 17, 6281–6291. [Google Scholar] [CrossRef]
- Devi, S.; Singh, B.; Paul, A.K.; Tyagi, S. Highly Sensitive and Selective Detection of Trinitrotolu-ene Using Cysteine-Capped Gold Nanoparticles. Anal. Methods 2016, 8, 4398–4405. [Google Scholar] [CrossRef]
- Ditta, S.A.; Yaqub, A.; Tanvir, F.; Rashid, M.; Ullah, R.; Zubair, M.; Ali, S.; Anjum, K.M. Gold Nanoparticles Capped with L-Glycine, L-Cystine, and L-Tyrosine: Toxicity Profiling and Antioxi-dant Potential. J. Mater. Sci. 2023, 58, 2814–2837. [Google Scholar] [CrossRef] [PubMed]
- Knecht, M.R.; Sethi, M. Bio-Inspired Colorimetric Detection of Hg2+ and Pb2+ Heavy Metal Ions Using Au Nanoparticles. Anal. Bioanal. Chem. 2009, 394, 33–46. [Google Scholar] [CrossRef]
- Huang, C.-C.; Chang, H.-T. Parameters for Selective Colorimetric Sensing of Mercury(II) in Aqueous Solutions Using Mercaptopropionic Acid-Modified Gold Nanoparticles. Chem. Commun. 2007, 12, 1215–1217. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Yuan, X.; Yu, Y.; Zhang, Q.; Leong, D.T.; Lee, J.Y.; Xie, J. From Aggregation-Induced Emission of Au(I)–Thiolate Complexes to Ultrabright Au(0)@Au(I)–Thiolate Core–Shell Nanoclus-ters. J. Am. Chem. Soc. 2012, 134, 16662–16670. [Google Scholar] [CrossRef]
- Acres, R.G.; Feyer, V.; Tsud, N.; Carlino, E.; Prince, K.C. Mechanisms of Aggregation of Cysteine Functionalized Gold Nanoparticles. J. Phys. Chem. C 2014, 118, 10481–10487. [Google Scholar] [CrossRef]
- Pace, C.N.; Vajdos, F.; Fee, L.; Grimsley, G.; Gray, T. How to measure and predict the molar absorp-tion coefficient of a protein. Protein Sci. 1995, 4, 2411–2423. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Chen, C.; Yin, M.; Li, H.; Li, W.; Zhang, Z.; Wang, Q.; Du, Z.; Xu, X.; Wang, Y. Interactions between gold nanoparticles with different morphologies and human serum albumin. Front. Chem. 2023, 11, 1273388. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Huang Re Yu, Y.; Su, R.; Qi, W.; He, Z. Gold Nanoparticle-Aptamer-Based LSPR Sensing of Ochratoxin A at a Widened Detection Range by Double Calibration Curve Method. Front. Chem. 2018, 6, 1–9. [Google Scholar] [CrossRef]
- Lim, S.I.; Zhong, C.-J. Molecularly Mediated Processing and Assembly of Nanoparticles: Exploring the Interparticle Interactions and Structures. Acc. Chem. Res. 2009, 42, 798–808. [Google Scholar] [CrossRef]
- Mocanu, A.; Cernica, I.; Tomoaia, G.; Bobos, L.D.; Horovitz, O.; Tomoaia-Cotisel, M. Self-Assembly Characteristics of Gold Nanoparticles in the Presence of Cysteine. Colloids Surf. A Physicochem. Eng. Asp. 2009, 338, 93–101. [Google Scholar] [CrossRef]
- Sudeep, P.K.; Joseph, S.S.; Thomas, K.G. Selective Detection of Cysteine and Glutathione Using Gold Nanorods. J. Am. Chem. Soc. 2005, 127, 6516–6517. [Google Scholar] [CrossRef]
- Sen, T.; Mandal, S.; Haldar, S.; Chattopadhyay, K.; Patra, A. Interaction of Gold Nanoparticle with Human Serum Albumin (HSA) Protein Using Surface Energy Transfer. J. Phys. Chem. C 2011, 115, 24037–24044. [Google Scholar] [CrossRef]





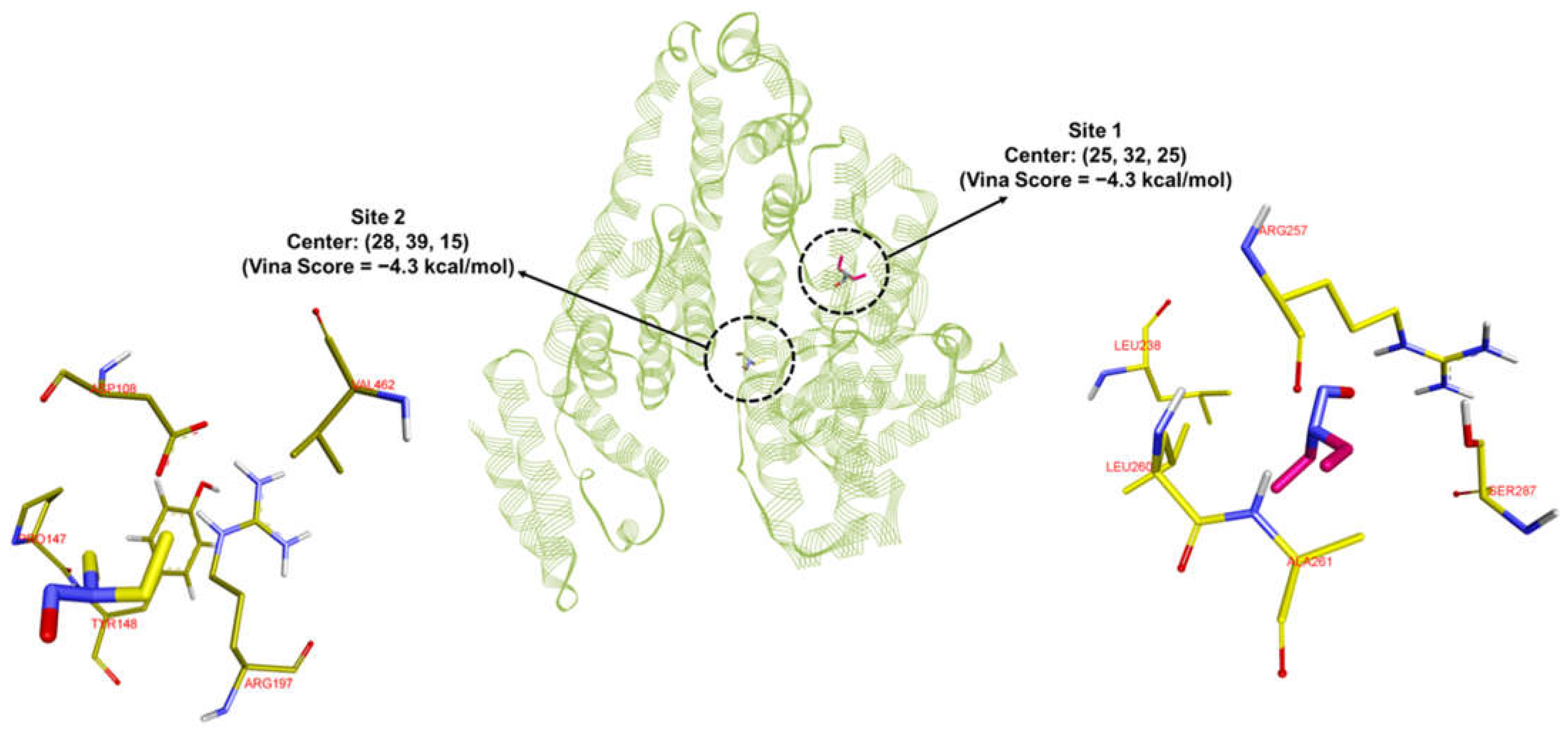

| Binding Sites | van der Waals (kcal/mol) | Electrostatics (kcal/mol) | Polar Solvation Energy (kcal/mol) | Non-Polar Solvation Energy (kcal/mol) | TOTAL ΔG (kcal/mol) |
|---|---|---|---|---|---|
| Site 1 | −17.3 | −0.5 | 6.1 | −2.7 | −14.4 |
| Site 2 | −15.6 | −0.3 | 6.2 | −2.5 | −12.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fakayode, S.O.; Bwambok, D.K.; Banerjee, S.; Rai, P.; Okoth, R.; Kuiters, C.; Benjamin, U. Colorimetric Detection of Nitrosamines in Human Serum Albumin Using Cysteine-Capped Gold Nanoparticles. Sensors 2025, 25, 5505. https://doi.org/10.3390/s25175505
Fakayode SO, Bwambok DK, Banerjee S, Rai P, Okoth R, Kuiters C, Benjamin U. Colorimetric Detection of Nitrosamines in Human Serum Albumin Using Cysteine-Capped Gold Nanoparticles. Sensors. 2025; 25(17):5505. https://doi.org/10.3390/s25175505
Chicago/Turabian StyleFakayode, Sayo O., David K. Bwambok, Souvik Banerjee, Prateek Rai, Ronald Okoth, Corinne Kuiters, and Ufuoma Benjamin. 2025. "Colorimetric Detection of Nitrosamines in Human Serum Albumin Using Cysteine-Capped Gold Nanoparticles" Sensors 25, no. 17: 5505. https://doi.org/10.3390/s25175505
APA StyleFakayode, S. O., Bwambok, D. K., Banerjee, S., Rai, P., Okoth, R., Kuiters, C., & Benjamin, U. (2025). Colorimetric Detection of Nitrosamines in Human Serum Albumin Using Cysteine-Capped Gold Nanoparticles. Sensors, 25(17), 5505. https://doi.org/10.3390/s25175505







