Assessment of Corrosion in Naval Steels Submerged in Artificial Seawater Utilizing a Magnetic Non-Destructive Sensor
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Experimental Procedure
2.2.1. Electrochemical Corrosion Tests
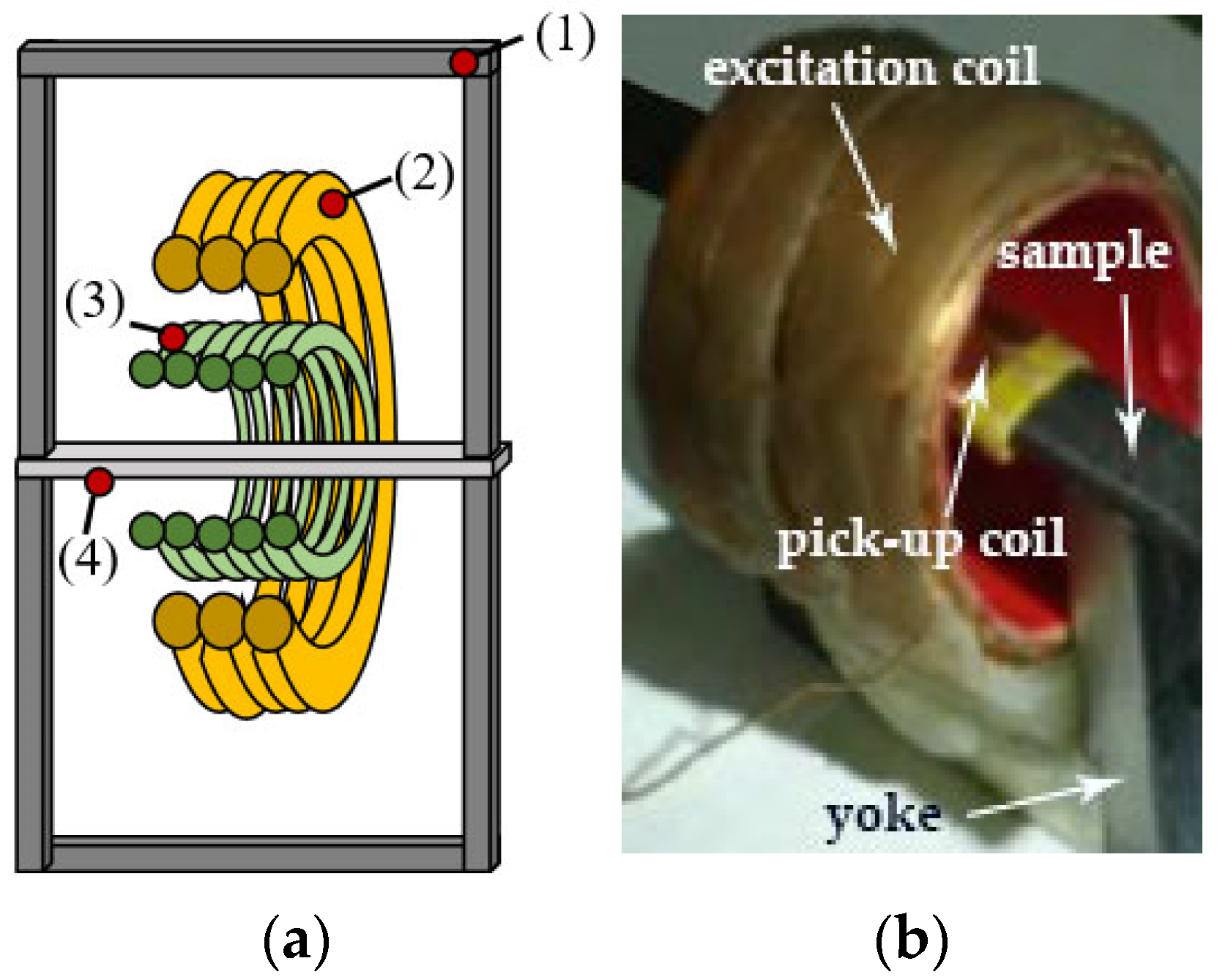
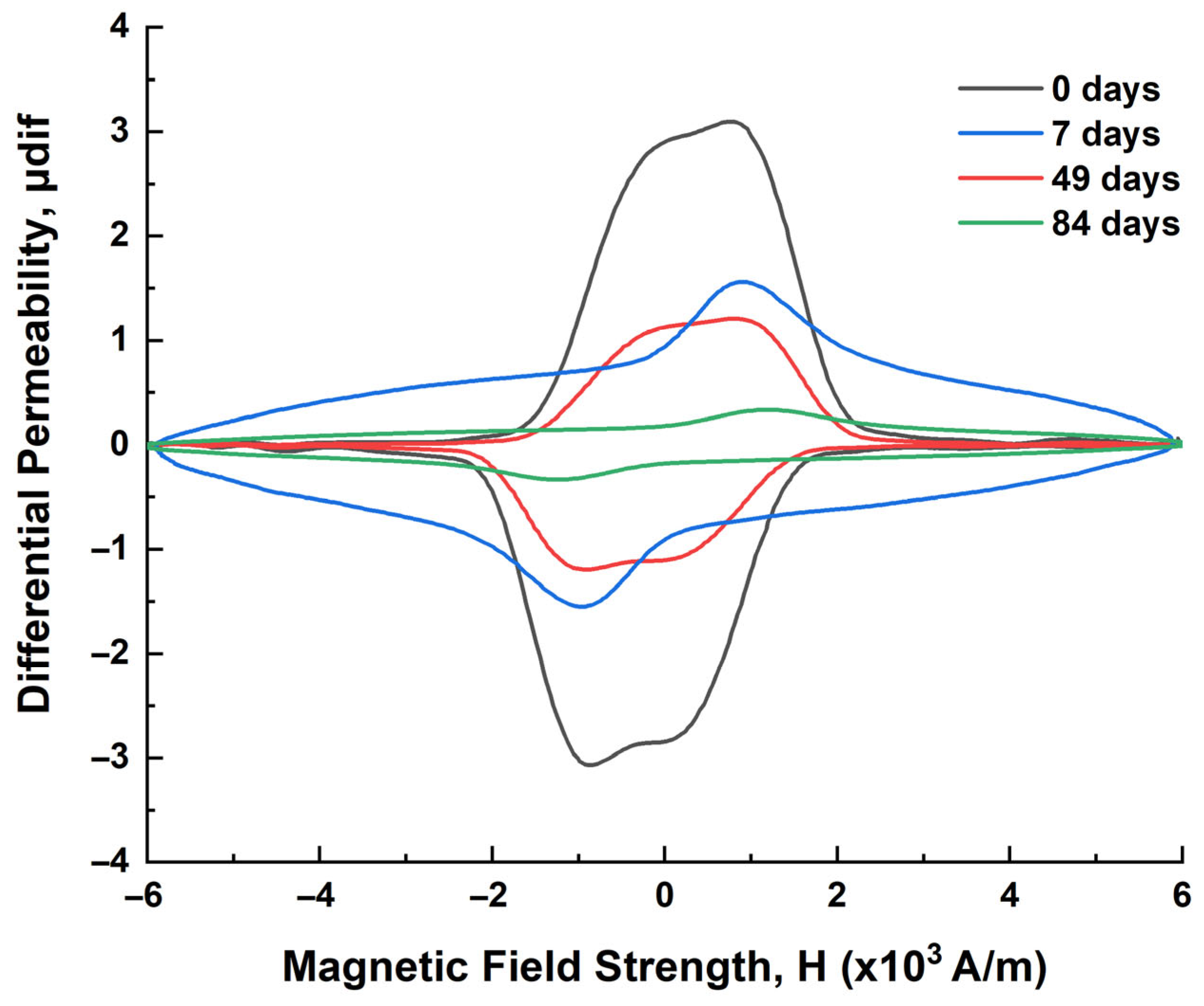
2.2.2. Magnetic Measurements
2.2.3. In Situ Static Immersion Tests
3. Results
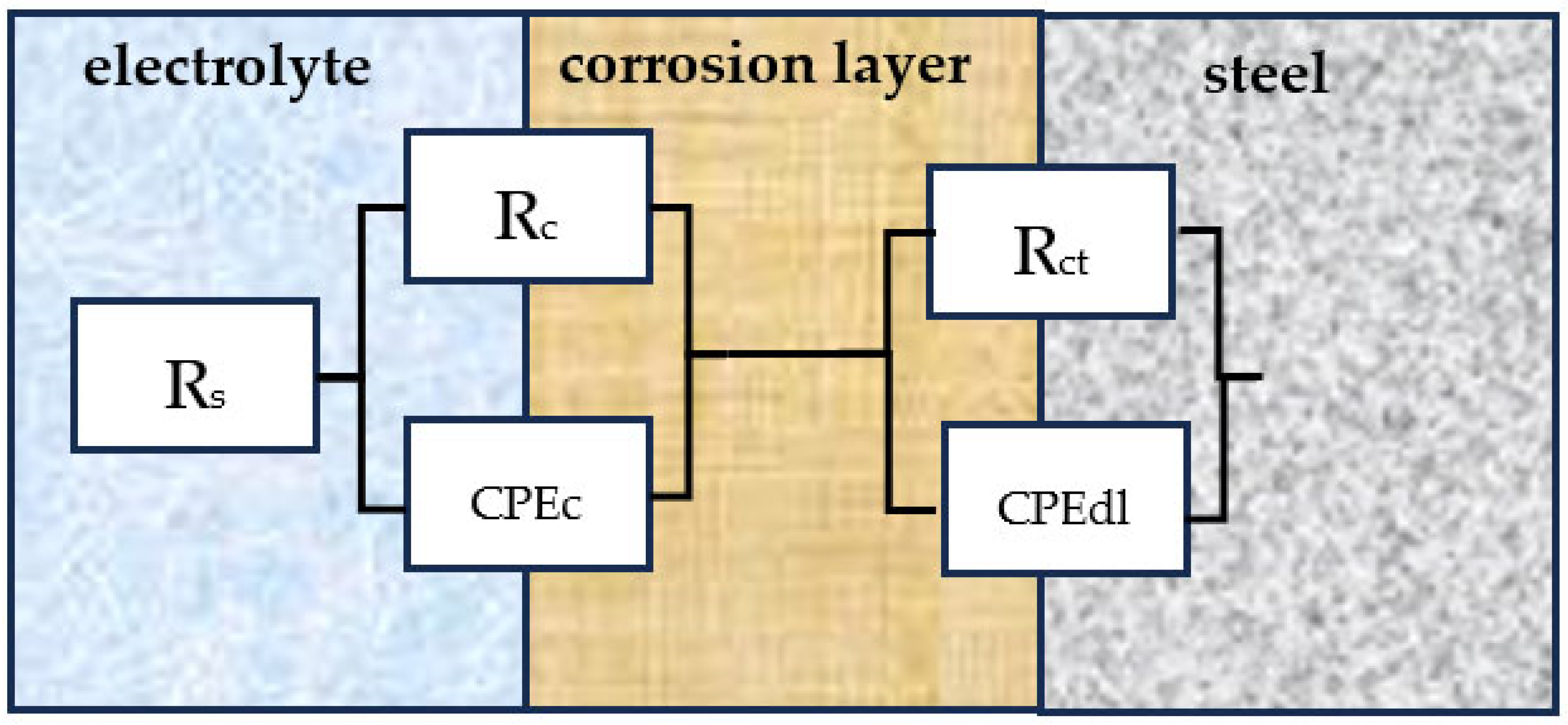
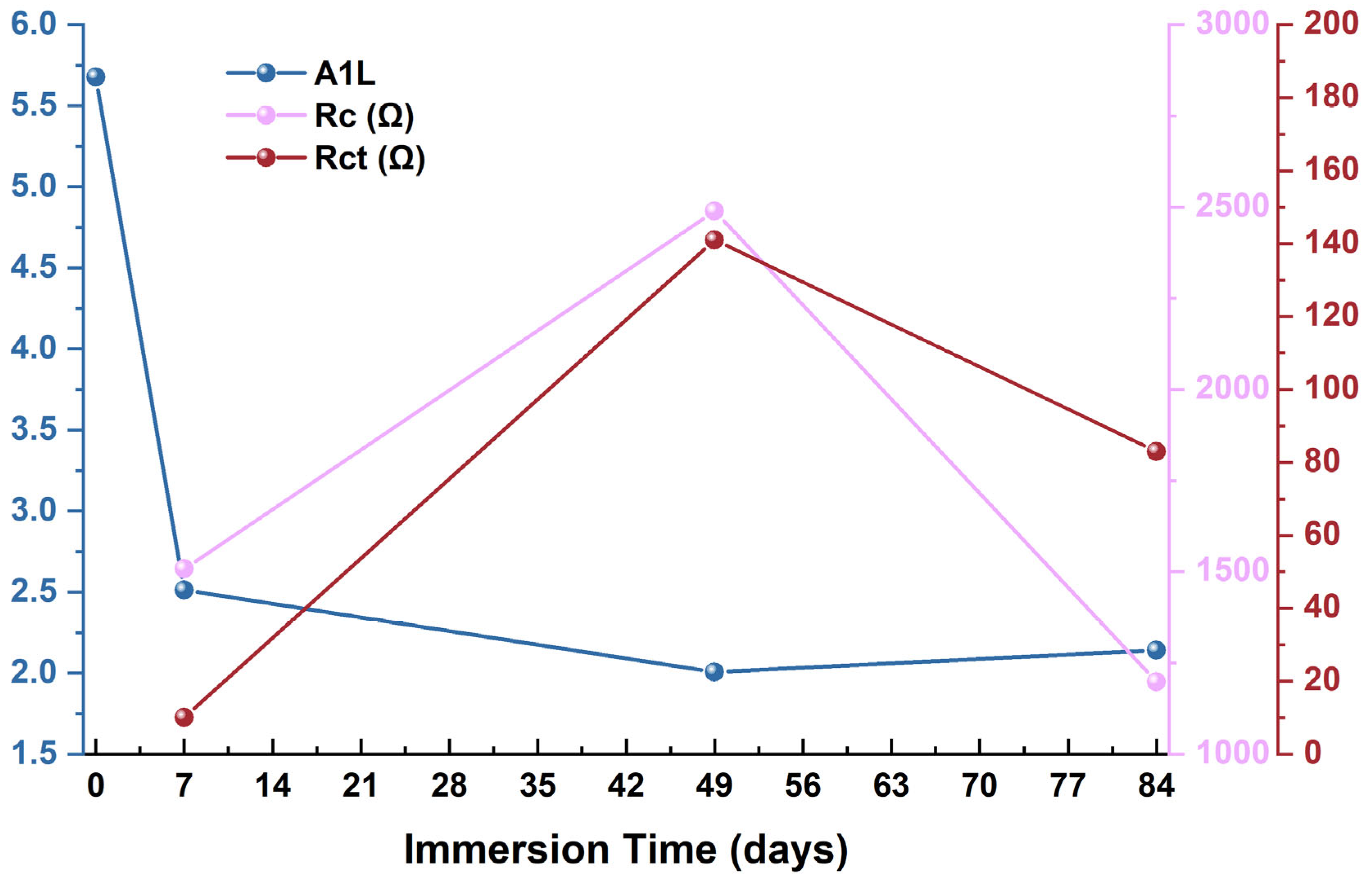
3.1. Electrochemical Impedance Spectroscopy (EIS) Tests
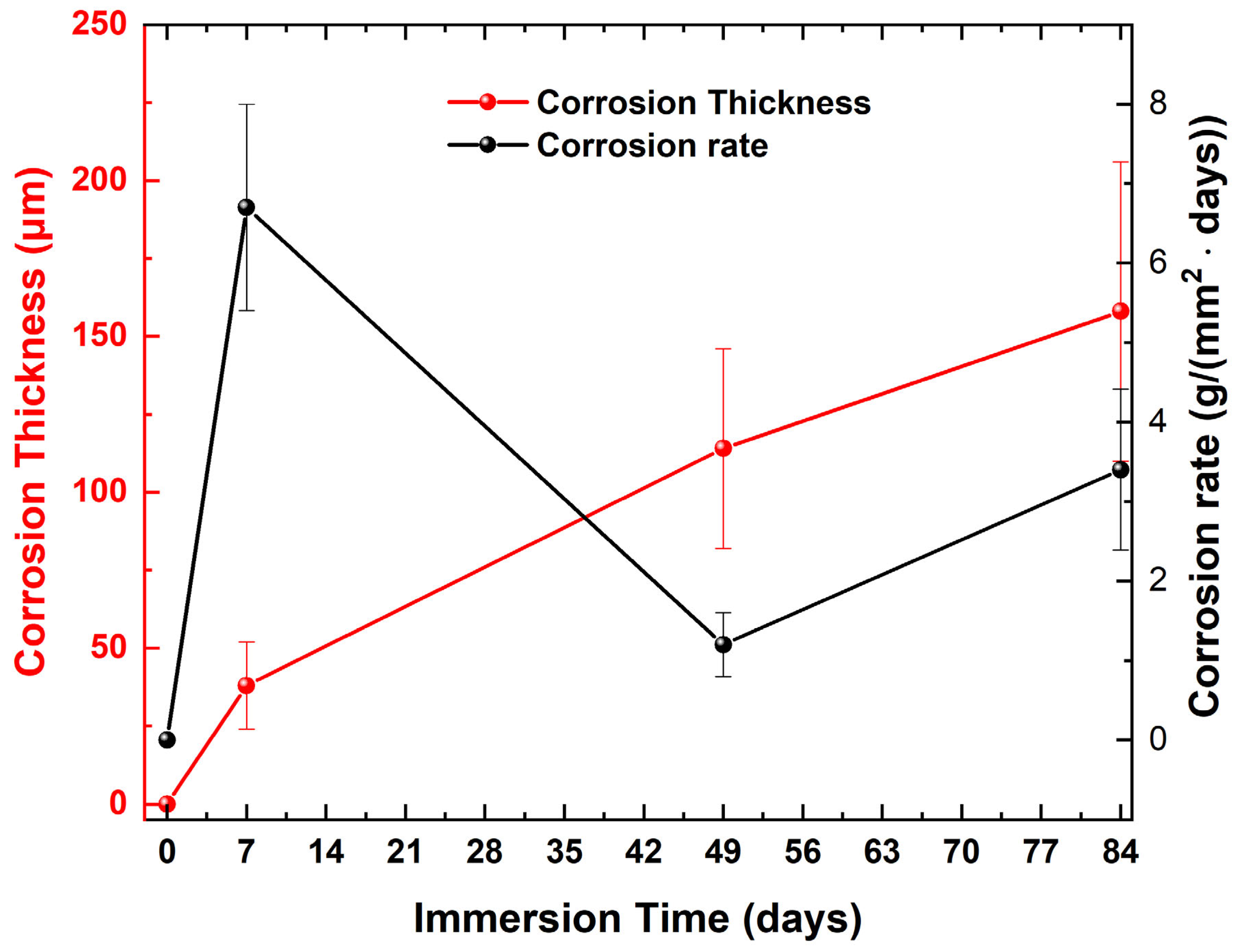
3.2. In Situ Corrosion Tests
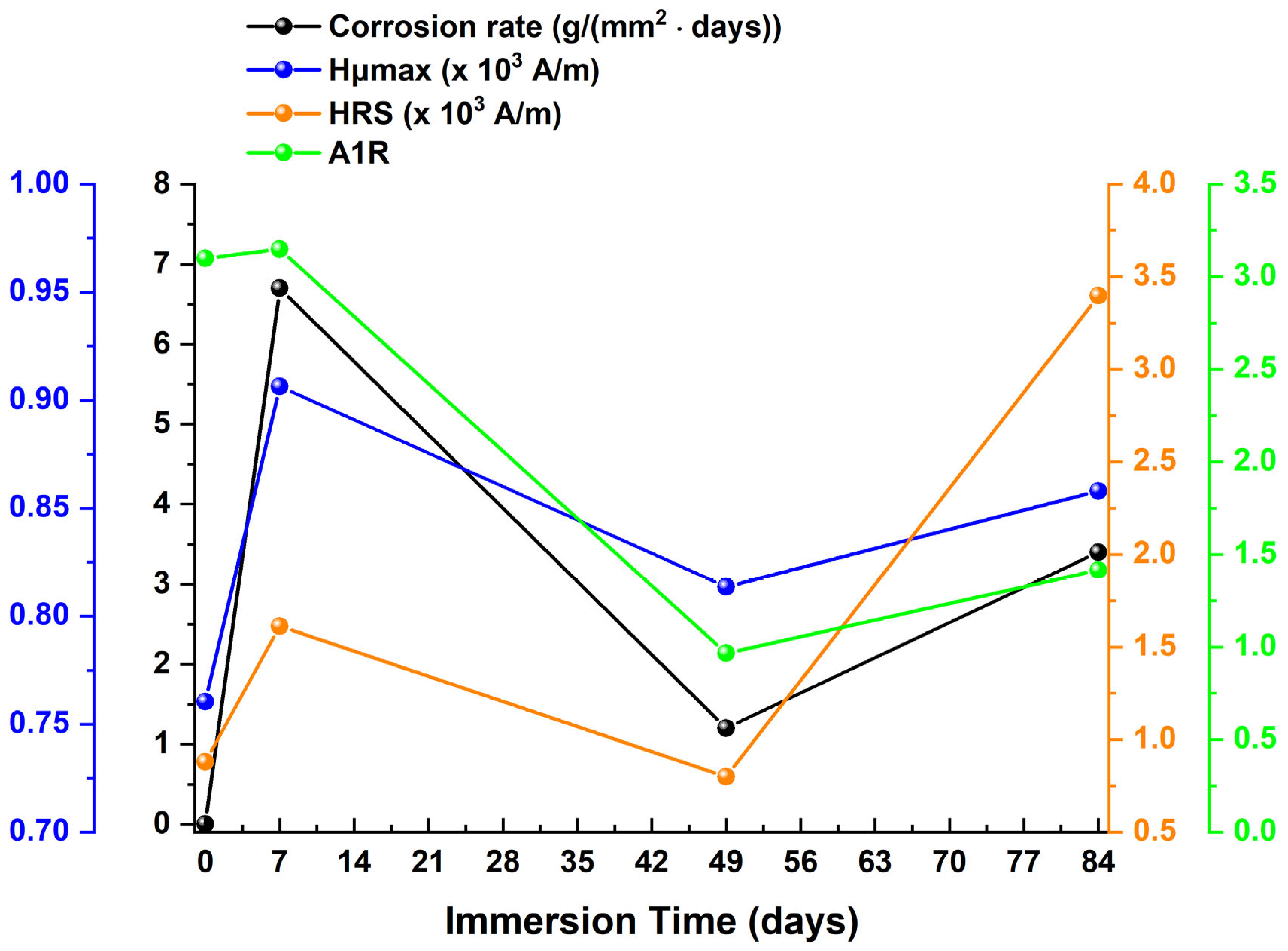
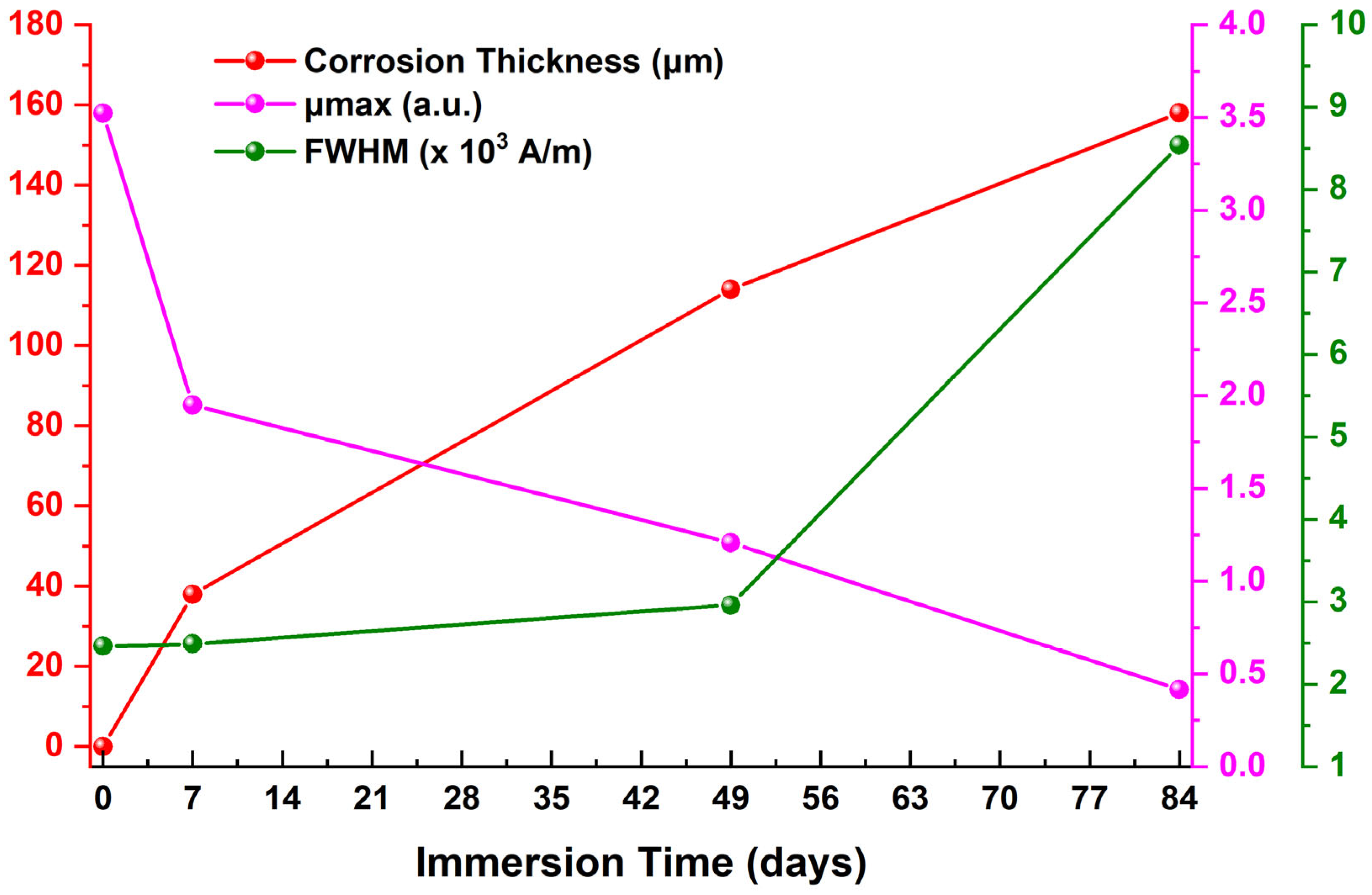
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASW | artificial seawater |
| EIS | Electrochemical Impedance Spectroscopy |
| RE | Reference Electrode |
| CE | Counter Electrode |
| WE | Working Electrode |
| OCP | Open Circuit Potential |
| WL | weight loss ratio |
| wi | weight prior to corrosion |
| wf | weight subsequent to corrosion |
| CR | Corrosion Rate |
| A | surface area of the specimen experiencing corrosion |
| t | time of the corrosion process |
| SEM | Scanning Electron Microscopy |
| μdiff-H | permeability profile |
| Rs | electrolyte resistance |
| Rct | charge transfer resistance |
| Rc | corrosion layer resistance |
| CPEdl | constant-phase element of double layer |
| CPEc | corrosion layer capacitance |
| BH | Magnetic hysteresis loop |
| μmax | Peak height |
| Hμmax | Peak Position |
| FWHM | Full Width at half peak height |
| HLS | Position of right shoulder |
| HRS | Position of left shoulder |
| A1L | Area under the BH curve to the left of the intersection |
| A1Ρ | Area under the BH curve to the right of the intersection |
References
- Imanian Ghazanlou, S.; Mobasher Amini, A.; Carrier, F.-A.; Sarkar, D.K.; Rehman, K.; Javidani, M. Study of the Microstructure and Mechanical Properties of Steel Grades for Ship Hull Construction. Materials 2024, 17, 5687. [Google Scholar] [CrossRef] [PubMed]
- Vourna, P.; Papadopoulos, N.D.; Falara, P.P.; Hristoforou, E. Barkhausen Noise Emission of Naval Steel: The Impact of Seawater Corrosion Coverage and Depth. NDT E Int. 2025, 151, 103319. [Google Scholar] [CrossRef]
- Falara, P.P.; Papadopoulos, N.D.; Vourna, P. Microstructure and Performance of Antibiofouling Coatings on High-Strength Steel Substrates Immersed in the Marine Environment. Micro 2022, 2, 277–294. [Google Scholar] [CrossRef]
- Dalmora, G.P.V.; Borges Filho, E.P.; Maraschin Conterato, A.A.; Roso, W.S.; Pereira, C.E.; Dettmer, A. Methods of Corrosion Prevention for Steel in Marine Environments: A Review. Results Surf. Interfaces 2025, 18, 100430. [Google Scholar] [CrossRef]
- Bogatu, N.; Buruiana, D.L.; Muresan, A.C.; Ghisman, V.; Lupu, A.; Mardare, L.; Herbei, E.E.; Basliu, V.; Ceoromila, A.; Florescu, S. Assessment of the Effectiveness of Protective Coatings in Preventing Steel Corrosion in the Marine Environment. Polymers 2025, 17, 378. [Google Scholar] [CrossRef]
- Knudsen, O.Ø.; Hagen, C.H.M.; Skilbred, A.W.B.; Bruaas, T.K.; Nærland, J. Root Causes for Corrosion on Painted Steel Structures in Marine Environments. Mater. Corros. 2025, 76, 802–811. [Google Scholar] [CrossRef]
- Abdel-Samad, A.; Soud, Y.; Zaki, M. Influence of Paint on Steel Corrosion for Marine Applications. JSEMAT 2014, 4, 189–195. [Google Scholar] [CrossRef]
- Martinelli-Orlando, F.; Mundra, S.; Angst, U.M. Cathodic Protection Mechanism of Iron and Steel in Porous Media. Commun. Mater. 2024, 5, 15. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, H.; Wang, H.; Tao, X.; Zhou, W. Study on the Corrosion Behavior of Hot-Dip Galvanized Steel in Simulated Industrial Atmospheric Environments. Int. J. Electrochem. Sci. 2024, 19, 100445. [Google Scholar] [CrossRef]
- Zhang, K.; Song, R.; Gao, Y. Corrosion Behavior of Hot-Dip Galvanized Advanced High Strength Steel Sheet in a Simulated Marine Atmospheric Environment. Int. J. Electrochem. Sci. 2019, 14, 1488–1499. [Google Scholar] [CrossRef]
- Pélissier, K.; Thierry, D. Powder and High-Solid Coatings as Anticorrosive Solutions for Marine and Offshore Applications? A Review. Coatings 2020, 10, 916. [Google Scholar] [CrossRef]
- Choe, H.-B.; Lee, H.-S.; Shin, J.-H. Experimental Study on the Electrochemical Anti-Corrosion Properties of Steel Structures Applying the Arc Thermal Metal Spraying Method. Materials 2014, 7, 7722–7736. [Google Scholar] [CrossRef]
- Qu, W.; Huang, Y.; Yu, X.; Zheng, M.; Lu, D. Effect of Petrolatum Tape Cover on the Hydrogen Permeation of AISI4135 Steel under Marine Splash Zone Conditions. Int. J. Electrochem. Sci. 2015, 10, 5892–5904. [Google Scholar] [CrossRef]
- Jiang, J.; Li, N.; Li, Q.; Jiang, Z.; Wang, B.; He, Y.; Liu, F.; Liu, C. Effect of Ca and Sb on the Corrosion Resistance of E690 Steel in Marine Atmosphere Environment. Metals 2023, 13, 826. [Google Scholar] [CrossRef]
- Vourna, P.; Falara, P.P.; Hristoforou, E.V.; Papadopoulos, N.D. Corrosion and Antifouling Behavior of a New Biocide-Free Antifouling Paint for Ship Hulls Under Both Artificially Simulated and Natural Marine Environment. Materials 2025, 18, 3095. [Google Scholar] [CrossRef] [PubMed]
- Khayatazad, M.; De Pue, L.; De Waele, W. Detection of Corrosion on Steel Structures Using Automated Image Processing. Dev. Built Environ. 2020, 3, 100022. [Google Scholar] [CrossRef]
- Chi, D.; Xu, Z.; Liu, H. Detection and Imaging of Corrosion Defects in Steel Structures Based on Ultrasonic Digital Image Processing. Metals 2024, 14, 390. [Google Scholar] [CrossRef]
- Vasagar, V.; Hassan, M.K.; Abdullah, A.M.; Karre, A.V.; Chen, B.; Kim, K.; Al-Qahtani, N.; Cai, T. Non-Destructive Techniques for Corrosion Detection: A Review. Corros. Eng. Sci. Technol. Int. J. Corros. Process. Corros. Control 2024, 59, 56–85. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, H.; Zhang, B.; Li, X.; Sang, X.; Si, Y.; Er, A.P.; Zheng, Y.; Yin, Q.; Jiang, J. A Corrosion Detection Method for Steel Strands Based on LC Electromagnetic Resonance. Adv. Mater. Sci. Eng. 2020, 2020, 8949821. [Google Scholar] [CrossRef]
- Bolton, T.; Bass, J.; Gaber, T. A Comparison of Deep Learning Techniques for Corrosion Detection. In Proceedings of the 8th International Conference on Advanced Intelligent Systems and Informatics 2022; Hassanien, A.E., Snášel, V., Tang, M., Sung, T.-W., Chang, K.-C., Eds.; Lecture Notes on Data Engineering and Communications Technologies; Springer International Publishing: Cham, Switzerland, 2023; Volume 152, pp. 189–198. ISBN 978-3-031-20600-9. [Google Scholar]
- Sanyal, S.; Park, S.; Chelliah, R.; Yeon, S.-J.; Barathikannan, K.; Vijayalakshmi, S.; Jeong, Y.-J.; Rubab, M.; Oh, D.H. Emerging Trends in Smart Self-Healing Coatings: A Focus on Micro/Nanocontainer Technologies for Enhanced Corrosion Protection. Coatings 2024, 14, 324. [Google Scholar] [CrossRef]
- Pallas Enguita, S.; Chen, C.-H.; Kovacic, S. A Review of Emerging Sensor Technologies for Tank Inspection: A Focus on LiDAR and Hyperspectral Imaging and Their Automation and Deployment. Electronics 2024, 13, 4850. [Google Scholar] [CrossRef]
- Committee D-19. D 1141-52 Standard Specifications for Substitute Ocean Water. In Manual on Industrial Water and Industrial Waste Water; ASTM: West Conshohocken, PA, USA; pp. 398–399. ISBN 978-0-8031-6884-8.
- ASTM G31-72; Standard Practice for Laboratory Immersion Corrosion Testing of Metals. ASTM International: West Conshohocken, PA, USA, 2004.
- Buruiană, D.L.; Mureşan, A.C.; Bogatu, N.; Ghisman, V.; Herbei, E.E.; Başliu, V. Corrosion Tendency of S235 Steel in 3.5% NaCl Solution and Drinking Water During Six Months of Exposure. Materials 2024, 17, 5979. [Google Scholar] [CrossRef]
- Duan, Z.; Huang, X.; Hou, J.; Chen, W.; Cai, L. Research on Intelligent Diagnosis of Corrosion in the Operation and Maintenance Stage of Steel Structure Engineering Based on U-Net Attention. Buildings 2024, 14, 3972. [Google Scholar] [CrossRef]
- Yang, Y.; Zhuang, Y.; Wang, H.; Chen, C. Corrosion Test and Corrosion Fatigue Numerical Simulation Research on Marine Structures. Ocean Eng. 2025, 316, 119931. [Google Scholar] [CrossRef]
- Ktena, A.; Hasicic, M.; Landgraf, F.J.G.; Moudilou, E.; Angelopoulos, S.; Hristoforou, E. On the Use of Differential Permeability and Magnetic Barkhausen Noise Measurements for Magnetic NDT Applications. J. Magn. Magn. Mater. 2022, 546, 168898. [Google Scholar] [CrossRef]
- Vourna, P.; Ktena, A.; Tsarabaris, P.; Hristoforou, E. Magnetic Residual Stress Monitoring Technique for Ferromagnetic Steels. Metals 2018, 8, 592. [Google Scholar] [CrossRef]
- Zhu, B.; Mei, Y.; Pei, J.; Wang, Z.; Zhang, Y. TRIP Effect Detection of Medium Manganese Steel Based on the MBN Signal. J. Magn. Magn. Mater. 2023, 580, 170766. [Google Scholar] [CrossRef]
- Fagan, P.; Zhang, S.; Sebald, G.; Uchimoto, T.; Ducharne, B. Barkhausen Noise Hysteresis Cycle: Theoretical and Experimental Understanding. J. Magn. Magn. Mater. 2023, 578, 170810. [Google Scholar] [CrossRef]
- Wang, L.; Xu, C.; Feng, L.; Wang, W. A Survey of the Magnetic Anisotropy Detection Technology of Ferromagnetic Materials Based on Magnetic Barkhausen Noise. Sensors 2024, 24, 7587. [Google Scholar] [CrossRef]
- Meyer, Y.A.; Menezes, I.; Bonatti, R.S.; Bortolozo, A.D.; Osório, W.R. EIS Investigation of the Corrosion Behavior of Steel Bars Embedded into Modified Concretes with Eggshell Contents. Metals 2022, 12, 417. [Google Scholar] [CrossRef]
- Betti, N.; Al-Amiery, A.A.; Al-Azzawi, W.K.; Isahak, W.N.R.W. Corrosion Inhibition Properties of Schiff Base Derivative against Mild Steel in HCl Environment Complemented with DFT Investigations. Sci. Rep. 2023, 13, 8979. [Google Scholar] [CrossRef]
- Feng, Y.; Feng, Y.; Zhou, X.; Wang, Q.; Cao, Y. Single and Double Alkyl Chain Quaternary Ammonium Salts as Environment-Friendly Corrosion Inhibitors for a Q235 Steel in 0.5 Mol/L H2SO4 Solution. Coatings 2023, 13, 1847. [Google Scholar] [CrossRef]
- Mazilu, A.; Benea, L.; Axente, E.R. Monitoring and Evaluation of the Corrosion Behavior in Seawater of the Low-Alloy Steels BVDH36 and LRAH36. IJMS 2024, 25, 6405. [Google Scholar] [CrossRef] [PubMed]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications, 1st ed.; Wiley: Hoboken, NJ, USA, 2018; ISBN 978-1-119-07408-3. [Google Scholar]
- Shu, S.; Baik, S.; Kazemzadeh-Atoufi, M.; Hu, X.; Liu, T.; Shang, A.; Davis, M.B.; Kumar, D.; Ziebarth, R.; Dhingra, S.; et al. On the High-Temperature Oxidation Behavior of a Niobium-Bearing High Nickel-Chromium Alloy: Microstructural Evolution and Implications on Oxidation Mechanisms. Corros. Sci. 2023, 220, 111261. [Google Scholar] [CrossRef]
- Zhang, R.; Sur, D.; Li, K.; Witt, J.; Black, R.; Whittingham, A.; Scully, J.R.; Hattrick-Simpers, J. Bayesian Assessment of Commonly Used Equivalent Circuit Models for Corrosion Analysis in Electrochemical Impedance Spectroscopy. npj Mater. Degrad. 2024, 8, 120. [Google Scholar] [CrossRef]
- Feliu, S. Electrochemical Impedance Spectroscopy for the Measurement of the Corrosion Rate of Magnesium Alloys: Brief Review and Challenges. Metals 2020, 10, 775. [Google Scholar] [CrossRef]
- Jović, V.D. Calculation of a Pure Double-Layer Capacitance from a Constant Phase Element in the Impedance Measurements. Zas. Mat. 2022, 63, 50–57. [Google Scholar] [CrossRef]
- Ramaraja, P.; Ramasamy, N.S. Electrochemical Impedance Spectroscopy for Microbial Fuel Cell Characterization. J. Microbial Biochem. Technol. 2013, 6, 1–14. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, J. Measurement and Interpretation of the Double Layer Capacitance of Pt(111)/Aqueous Solution Interfaces. Curr. Opin. Electrochem. 2023, 42, 101419. [Google Scholar] [CrossRef]
- Schalenbach, M.; Durmus, Y.E.; Tempel, H.; Kungl, H.; Eichel, R.-A. Double Layer Capacitances Analysed with Impedance Spectroscopy and Cyclic Voltammetry: Validity and Limits of the Constant Phase Element Parameterization. Phys. Chem. Chem. Phys. 2021, 23, 21097–21105. [Google Scholar] [CrossRef]
- Cheng, P.; Huang, X. Effect of Salinity on Corrosion Behavior of DH36 Steel in Seawater Immersion Zone. DTETR 2017. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Melchers, R.E. Probabilistic Models for Corrosion in Structural Reliability Assessment—Part 2: Models Based on Mechanics. J. Offshore Mech. Arct. Eng. 2003, 125, 272–280. [Google Scholar] [CrossRef]
- Vukelic, G.; Vizentin, G.; Ivosevic, S.; Bozic, Z. Analysis of Prolonged Marine Exposure on Properties of AH36 Steel. Eng. Fail. Anal. 2022, 135, 106132. [Google Scholar] [CrossRef]
- Vukelic, G.; Vizentin, G.; Brnic, J.; Brcic, M.; Sedmak, F. Long-Term Marine Environment Exposure Effect on Butt-Welded Shipbuilding Steel. JMSE 2021, 9, 491. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS Spectra of Fe2+ and Fe3+ Ions in Oxide Materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Ries, L.A.S.; Da Cunha Belo, M.; Ferreira, M.G.S.; Muller, I.L. Chemical Composition and Electronic Structure of Passive Films Formed on Alloy 600 in Acidic Solution. Corros. Sci. 2008, 50, 676–686. [Google Scholar] [CrossRef]
- Melchers, R.; Jeffrey, R. The Critical Involvement of Anaerobic Bacterial Activity in Modelling the Corrosion Behaviour of Mild Steel in Marine Environments. Electrochim. Acta 2008, 54, 80–85. [Google Scholar] [CrossRef]
- Liu, J.G.; Li, Y.T.; Hou, B.R. Corrosion Behaviour of DH36 Steel Used for Oil Platform in Splash Zones. Corros. Sci. Technol. 2015, 14, 190–194. [Google Scholar] [CrossRef]
- Shreir, L.L.; Jarman, R.A.; Burstein, G.T. Corrosion; Elsevier: Amsterdam, The Netherlands, 1994; ISBN 978-0-08-052351-4. [Google Scholar]
- Rémazeilles, C.; Refait, P. On the Formation of β-FeOOH (Akaganéite) in Chloride-Containing Environments. Corros. Sci. 2007, 49, 844–857. [Google Scholar] [CrossRef]
- Cook, W.G.; Olive, R.P. Pourbaix Diagrams for the Iron–Water System Extended to High-Subcritical and Low-Supercritical Conditions. Corros. Sci. 2012, 55, 326–331. [Google Scholar] [CrossRef]
- Cook, D.C. Spectroscopic Identification of Protective and Non-Protective Corrosion Coatings on Steel Structures in Marine Environments. Corros. Sci. 2005, 47, 2550–2570. [Google Scholar] [CrossRef]
- Wu, W.; Qin, L.; Cheng, X.; Xu, F.; Li, X. Microstructural Evolution and Its Effect on Corrosion Behavior and Mechanism of an Austenite-Based Low-Density Steel during Aging. Corros. Sci. 2023, 212, 110936. [Google Scholar] [CrossRef]
- Santana, J.J.; González-Guzmán, J.; Izquierdo, J.; González, S.; Souto, R.M. Sensing Electrochemical Activity in Polymer-Coated Metals during the Early Stages of Coating Degradation by Means of the Scanning Vibrating Electrode Technique. Corros. Sci. 2010, 52, 3924–3931. [Google Scholar] [CrossRef]
- Parapurath, S.; Ravikumar, A.; Vahdati, N.; Shiryayev, O. Effect of Magnetic Field on the Corrosion of API-5L-X65 Steel Using Electrochemical Methods in a Flow Loop. Appl. Sci. 2021, 11, 9329. [Google Scholar] [CrossRef]
- Pastorek, F.; Decký, M.; Neslušan, M.; Pitoňák, M. Usage of Barkhausen Noise for Assessment of Corrosion Damage on Different Low Alloyed Steels. Appl. Sci. 2021, 11, 10646. [Google Scholar] [CrossRef]
- Zgútová, K.; Pitoňák, M. Attenuation of Barkhausen Noise Emission Due to Variable Coating Thickness. Coatings 2021, 11, 263. [Google Scholar] [CrossRef]
- Neslušan, M.; Bahleda, F.; Minárik, P.; Zgútová, K.; Jambor, M. Non-Destructive Monitoring of Corrosion Extent in Steel Rope Wires via Barkhausen Noise Emission. J. Magn. Magn. Mater. 2019, 484, 179–187. [Google Scholar] [CrossRef]
- Deng, Y.; Li, Z.; Chen, J.; Qi, X. The Effects of the Structure Characteristics on Magnetic Barkhausen Noise in Commercial Steels. J. Magn. Magn. Mater. 2018, 451, 276–282. [Google Scholar] [CrossRef]
- Bénuffé, D.; Radouani, F.; Quemener, M.; Ozier, O.; Fauchon, M.; Toueix, Y.; Faӱ, F.; Magueresse, A.; Lescop, B.; Rioual, S.; et al. Multifactored Accelerated Marine Corrosion of Immersed Steels Influenced by Washed Ashore Sargassum Rafts. Mar. Environ. Res. 2025, 204, 106924. [Google Scholar] [CrossRef]
- Tuck, B.; Watkin, E.; Somers, A.; Machuca, L.L. A Critical Review of Marine Biofilms on Metallic Materials. npj Mater. Degrad. 2022, 6, 25. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, H.; Fan, Y.; Xu, D. Microbially Influenced Corrosion of Steel in Marine Environments: A Review from Mechanisms to Prevention. Microorganisms 2023, 11, 2299. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Li, K.; Liu, H.; Gu, T. Effects of Magnetic Fields on Microbiologically Influenced Corrosion of 304 Stainless Steel. Ind. Eng. Chem. Res. 2014, 53, 48–54. [Google Scholar] [CrossRef]
- Liu, N.; Qiu, L.; Qiu, L. Carbon Steel Corrosion Induced by Sulfate-Reducing Bacteria: A Review of Electrochemical Mechanisms and Pathways in Biofilms. Coatings 2024, 14, 1105. [Google Scholar] [CrossRef]
- Guo, N.; Zhao, Q.; Hui, X.; Guo, Z.; Dong, Y.; Yin, Y.; Zeng, Z.; Liu, T. Enhanced Corrosion Protection Action of Biofilms Based on Endogenous and Exogenous Bacterial Cellulose. Corros. Sci. 2022, 194, 109931. [Google Scholar] [CrossRef]
- Sheng, H.; Wang, P.; Yang, Y.; Tang, C. Stress and Microstructures Characterization Based on Magnetic Incremental Permeability and Magnetic Barkhausen Noise Techniques. Materials 2024, 17, 2657. [Google Scholar] [CrossRef]
- Elgamli, E.; Anayi, F. Advancements in Electrical Steels: A Comprehensive Review of Microstructure, Loss Analysis, Magnetic Properties, Alloying Elements, and the Influence of Coatings. Appl. Sci. 2023, 13, 10283. [Google Scholar] [CrossRef]
- Yang, B.; Liu, Z.; Gao, Y.; Wang, R.; Feng, Y.; Liu, X. Magnetization Changes Induced by Stress Noncoaxial with the Magnetic Field in a Low-Carbon Steel. Energies 2023, 16, 1103. [Google Scholar] [CrossRef]
- Nakai, T. Relationship of Magnetic Domain and Permeability for Clustered Soft Magnetic Narrow Strips with In-Plane Inclined Magnetization Easy Axis on Distributed Magnetic Field. Sensors 2024, 24, 706. [Google Scholar] [CrossRef]
- Neslušan, M.; Pitoňák, M.; Minárik, P.; Tkáč, M.; Kollár, P.; Životský, O. Influence of Domain Walls Thickness, Density and Alignment on Barkhausen Noise Emission in Low Alloyed Steels. Sci. Rep. 2023, 13, 5687. [Google Scholar] [CrossRef]
- Morcillo, M.; González-Calbet, J.M.; Jiménez, J.A.; Díaz, I.; Alcántara, J.; Chico, B.; Mazarío-Fernández, A.; Gómez-Herrero, A.; Llorente, I.; De La Fuente, D. Environmental Conditions for Akaganeite Formation in Marine Atmosphere Mild Steel Corrosion Products and Its Characterization. Corrosion 2015, 71, 872–886. [Google Scholar] [CrossRef]
- Fracchia, M.; Visibile, A.; Ahlberg, E.; Vertova, A.; Minguzzi, A.; Ghigna, P.; Rondinini, S. α- and γ-FeOOH: Stability, Reversibility, and Nature of the Active Phase under Hydrogen Evolution. ACS Appl. Energy Mater. 2018, 1, 1716–1725. [Google Scholar] [CrossRef]
- Wei, J.; Wang, C.-G.; Wei, X.; Mu, X.; He, X.-Y.; Dong, J.-H.; Ke, W. Corrosion Evolution of Steel Reinforced Concrete Under Simulated Tidal and Immersion Zones of Marine Environment. Acta Metall. Sin. (Engl. Lett.) 2019, 32, 900–912. [Google Scholar] [CrossRef]
- Xiao, K.; Dong, C.; Li, X.; Wang, F. Corrosion Products and Formation Mechanism during Initial Stage of Atmospheric Corrosion of Carbon Steel. J. Iron Steel Res. Int. 2008, 15, 42–48. [Google Scholar] [CrossRef]
- Gotoh, Y.; Hirano, H.; Nakano, M.; Fujiwara, K.; Takahashi, N. Electromagnetic Nondestructive Testing of Rust Region in Steel. IEEE Trans. Magn. 2005, 41, 3616–3618. [Google Scholar] [CrossRef]
- Dobmann, G.; Boller, C.; Herrmann, H.G.; Altpeter, I. Micromagnetic and Electromagnetic NDT for Lifetime Management by Monitoring Ageing of Structural Materials. IJMMP 2014, 9, 348. [Google Scholar] [CrossRef]









| Protection Method | Principle | Advantages | Limitations | Typical Applications | Refs. |
|---|---|---|---|---|---|
| Protective Coatings (Paint, Epoxy, Polymeric) | Creates a physical barrier between steel and environment | Cost-effective, easy to apply, customizable, wide usage | Requires regular maintenance; damaged areas expose steel; UV/salt degrade coatings over time | Ship hulls, superstructures, tanks | [5,6,7] |
| Cathodic Protection (Sacrificial Anode/Impressed Current) | Converts steel into the cathode uses sacrificial metals (e.g., zinc, aluminum) or electric currents | Highly effective for submerged/underground parts; provides continuous protection | Sacrificial anodes must be replaced; impressed current systems are costly and require monitoring | Ship hulls, ballast tanks, offshore platforms | [4,8] |
| Galvanizing (Hot-dip Zinc Coating) | Steel is dipped in molten zinc layer protects via barrier and sacrificial action | Long-lasting; good for atmospheric/marine exposure; relatively low cost | Surface prep critical; coating can be damaged; limited to certain shapes/sizes | Fasteners, pipework, structural steel, smaller components | [9,10] |
| Powder Coating | Thermoset/thermoplastic powder fused to surface, forming thick barrier | Thick coverage, durable, environmentally friendly, esthetic options | Requires careful prep, large parts may be challenging; surface damage exposes steel | Hardware, pipelines, deck fittings | [11] |
| Metallizing (Thermal Spray Al/Zn Alloy) | Sprayed molten metal forms protective metallic barrier | Suitable for complex shapes; long life (10–20 years with maintenance); effective both above and below water | Requires expert application/sealing; may need combined painting; expensive for large areas | Valves, pumps, structural members, decks | [12] |
| Petrolatum Tape/Cover System | Wraps surface in petroleum-based tape/lining plus cover (e.g., titanium) | Excellent for joints/fittings; impact/chemical-resistant (with titanium covers); easy maintenance | Limited to specific shapes/applications; can be costly for large uses | Pipe joints, pilings, pier substructures | [13,14] |
| Corrosion-resistant Steel Alloys | Steel alloyed with nickel, copper, phosphorous, etc., to improve resistance | Inherent protection, less maintenance needed | Expensive; not always effective for severe marine conditions | Specialized naval hulls, hard-to-maintain locations | [14] |
| Detection Method | Principle | Advantages | Disadvantages | Typical Applications | Refs. |
|---|---|---|---|---|---|
| Visual Inspection | Surface observation for discoloration, pitting, or rust | Low cost, simple, immediate results | Subjective—misses subsurface/internal defects | Routine maintenance, initial screening | [16] |
| Ultrasonic Thickness Measurement (UTM) | Measures change in material thickness from sound wave reflection | Non-destructive, detects internal thinning | Requires contact and calibration, cannot detect early surface corrosion | Ship hulls, pipelines, structural steel | [17] |
| Electrochemical Impedance Spectroscopy (EIS) | Evaluates electrochemical response to AC signals to estimate corrosion processes | Sensitive, provides kinetic/mechanistic info | Needs electrolyte contact, limited for in situ/large surfaces | Laboratory monitoring, coating evaluation | |
| Electrical Resistance (ER) Probes | Monitors changes in electrical resistance as metal loss occurs | Real-time, good for atmospheric and soil environments | Affected by local environment, only area-specific | Storage tanks, soil/pipeline monitoring | |
| Acoustic Emission (AE) | Detects transient sound waves from active corrosion and cracking | Detects dynamic/hidden/flaw activity, covers large areas | Noisy environments, needs expert data interpretation | Large infrastructure, pressurized vessels | [18] |
| Magnetic/Eddy Current Testing | Induces electromagnetic field detects anomalies from corrosion/pitting | Good for non-contact, rapid, through-coating inspection | Limited depth, interpretation affected by geometry | Pipelines, coated surfaces, welds | [19] |
| Computer Vision/Deep Learning | Automated corrosion detection by analyzing digital images | High-throughput, automates inspection, real-time possible | Needs large, labeled datasets, sensitive to lighting conditions | Automated monitoring, asset integrity | [16,20] |
| Elements | Fe | Mn | Si | C | P | S |
|---|---|---|---|---|---|---|
| %wt | base | 1.23 | 0.32 | 0.17 | 0.025 | 0.1 |
| Compound | Formula | Amount (g/L) |
|---|---|---|
| Sodium chloride | NaCl | 24.53 |
| Magnesium chloride | MgCl2 | 5.20 |
| Sodium sulfate | Na2SO4 | 4.09 |
| Calcium chloride | CaCl2 | 1.16 |
| Potassium chloride | KCl | 0.695 |
| Sodium bicarbonate | NaHCO3 | 0.201 |
| Potassium bromide | KBr | 0.101 |
| Boric acid | H3BO3 | 0.027 |
| Strontium chloride | SrCl2·6H2O | 0.025 |
| Sodium fluoride | NaF | 0.003 |
| Frequency Range | Frequency Band | Correlated with |
|---|---|---|
| 105 Hz to 103 Hz | High | resistance of the electrolyte |
| 103 Hz to 102 Hz | Middle | double layer formation |
| 102 Hz to 10−1 Hz | Low | steel/electrolyte interface reactions |
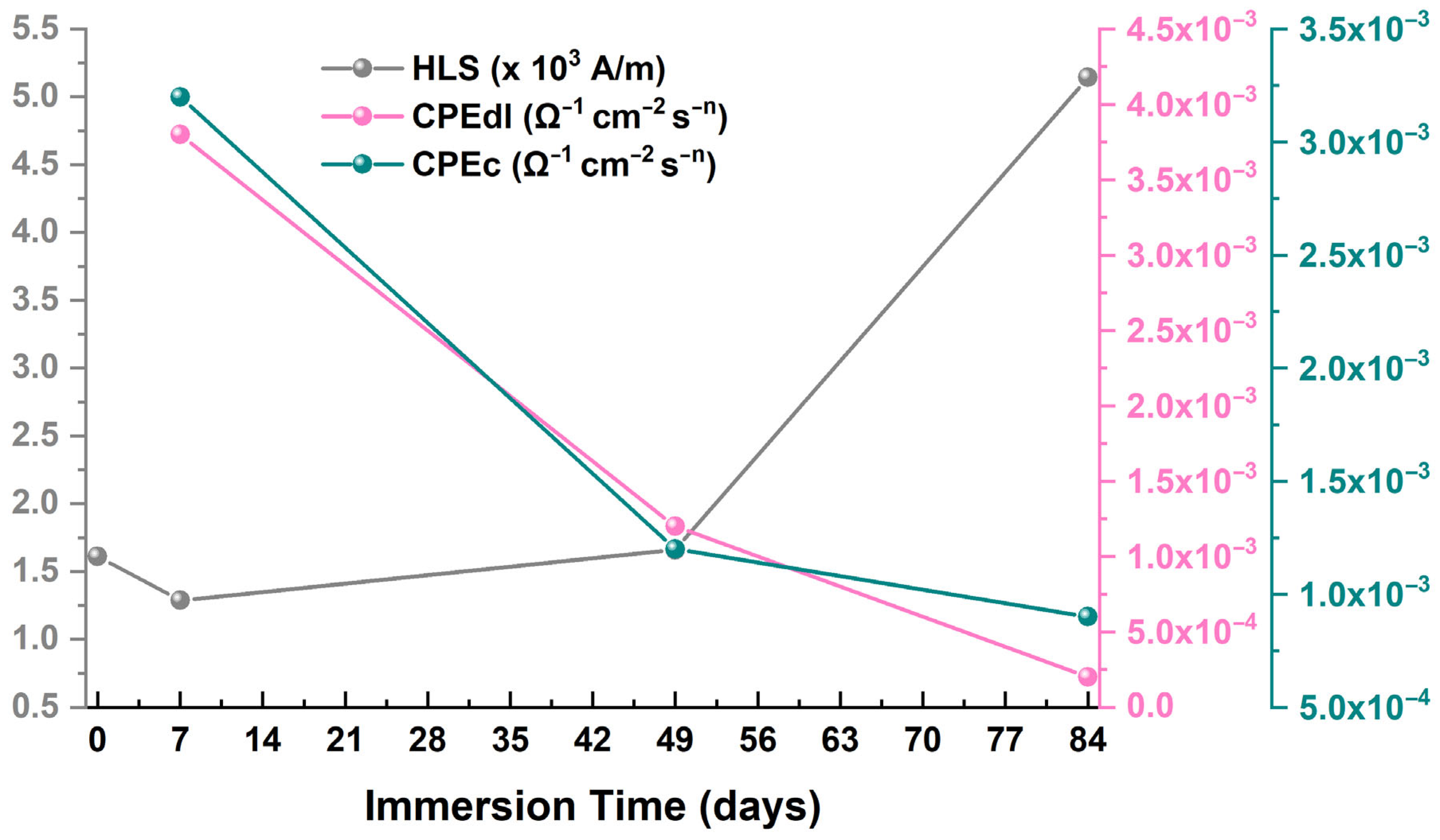
| Immersion Time (Days) | RS (Ω) | Rc (Ω) | CPEc (Ω−1 × cm−2 × sn) | nc | Rct (Ω) | CPEdl (Ω−1 × cm−2 × sn) | ndl |
|---|---|---|---|---|---|---|---|
| 0 | 8 | - | - | - | 8 | 10−4 | - |
| 7 | 10 | 1508 | 10−4 | 0.60 | 10 | 10−4 | 0.87 |
| 49 | 23 | 2489 | 10−4 | 0.75 | 141 | 10−4 | 0.93 |
| 84 | 11 | 1198 | 10−4 | 0.66 | 83 | 10−4 | 0.95 |
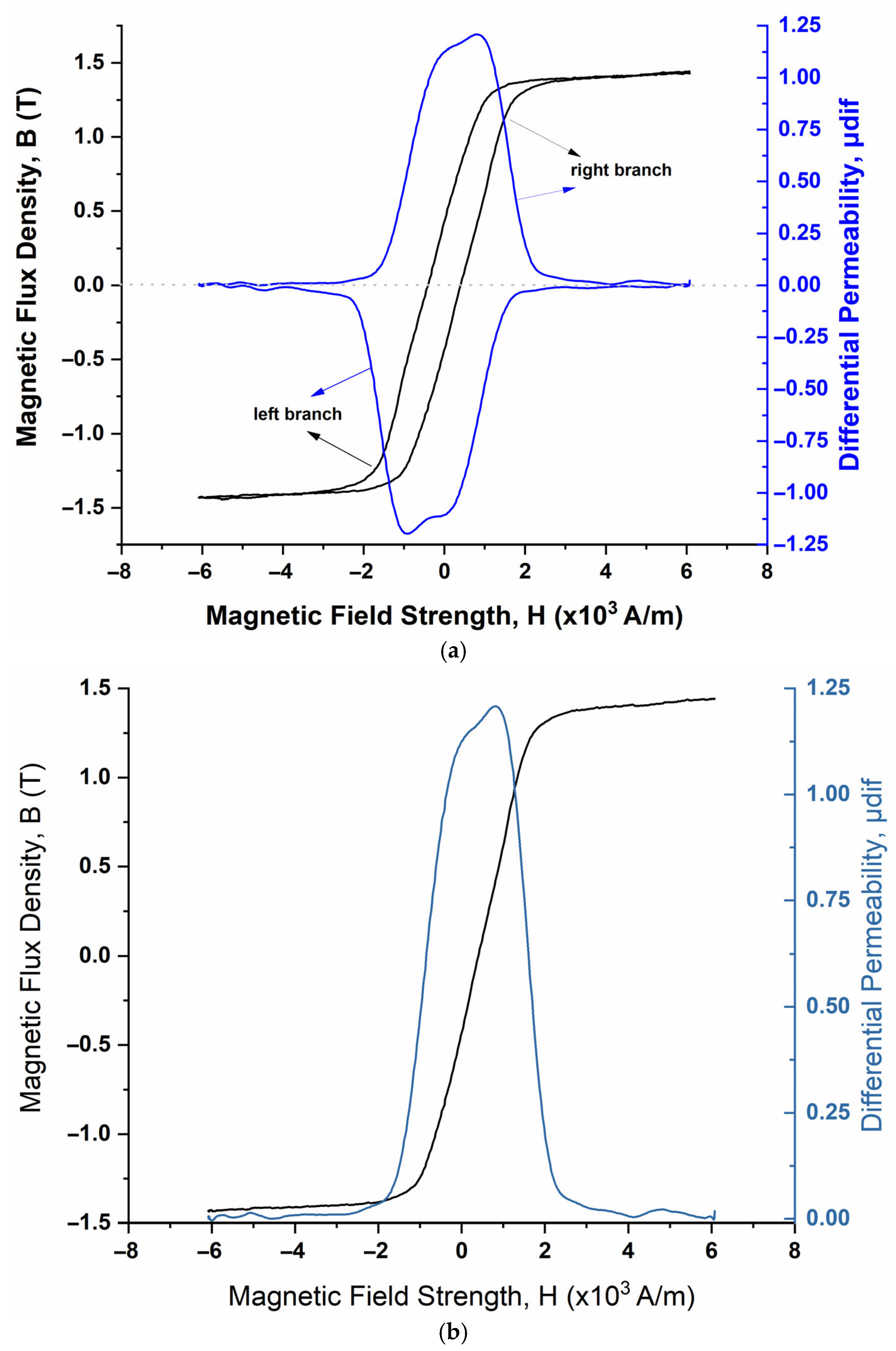
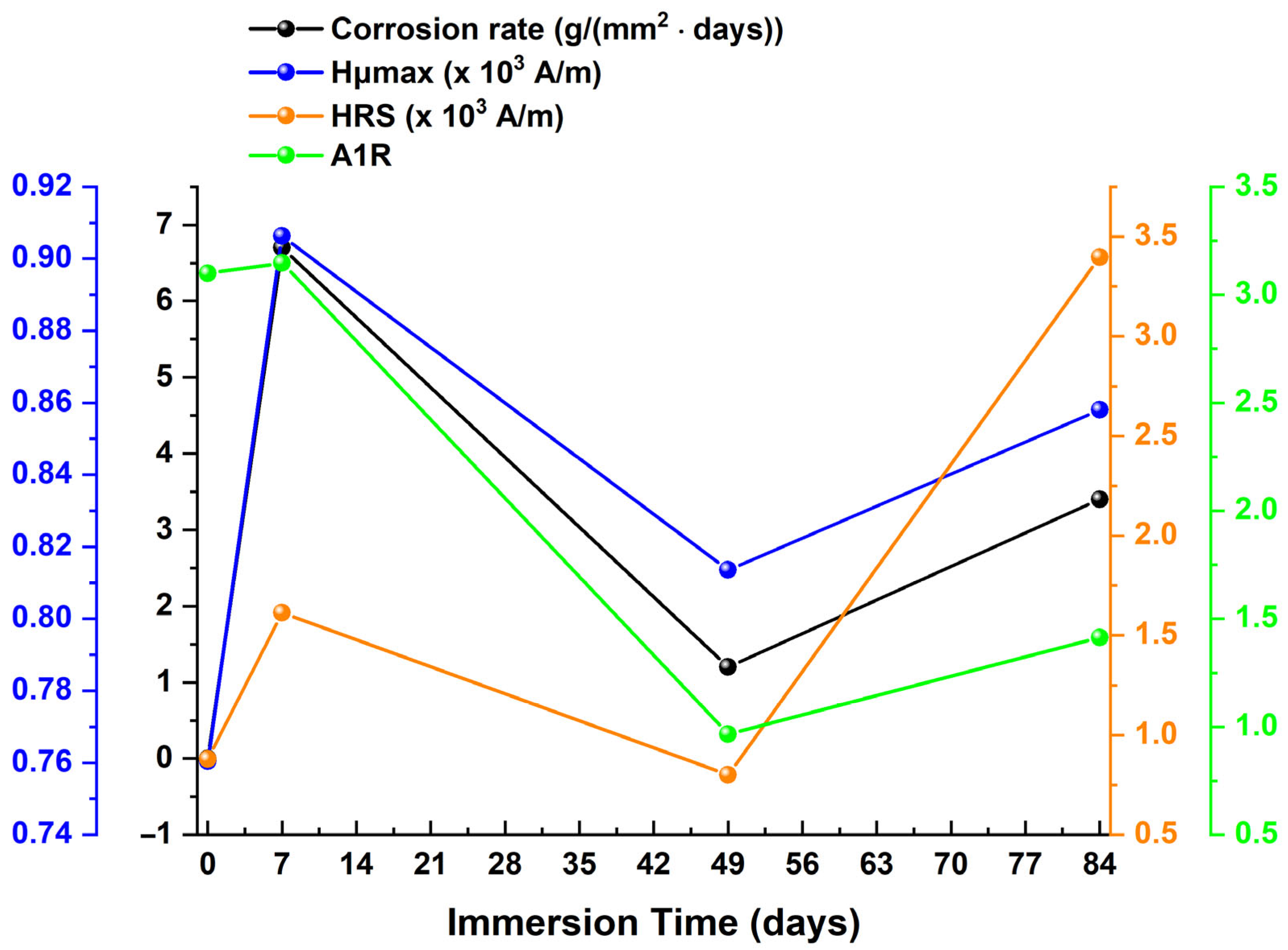
| Immersion Time (Days) | μmax 1 (a.u.) | Hμmax 2 (×103 A/m) | FWHM 3 (×103 A/m) | HLS 4 (×103 A/m) | HRS 5 (×103 A/m) | A1L 6 | A1R 7 |
|---|---|---|---|---|---|---|---|
| 0 | 3.52362 | 0.76044 | 2.46127 | 1.61173 | 0.87955 | 5.67912 | 3.09920 |
| 7 | 1.95067 | 0.90634 | 2.49008 | 1.28709 | 1.61372 | 2.51069 | 3.14784 |
| 49 | 1.20789 | 0.81358 | 2.96043 | 1.66015 | 0.80028 | 2.00528 | 0.96665 |
| 84 | 0.41621 | 0.85803 | 8.53979 | 5.14238 | 3.39741 | 2.14031 | 1.41404 |
| Effect of Corrosion | Impact on Magnetic Domains | Impact on Magnetization |
|---|---|---|
| Surface roughness | Domain walls encounter more pinning sites | Reduced magnetic permeability and remanence, lower saturation |
| Micro-cracking/pitting | Domains become smaller or fragmented | Narrower hysteresis loop |
| Chemical changes | Disruptions in ferromagnetic structure | Reduced magnetic response overall |
| Oxidation | Non-magnetic oxides form, reducing domain volume | Decrease in total magnetization |
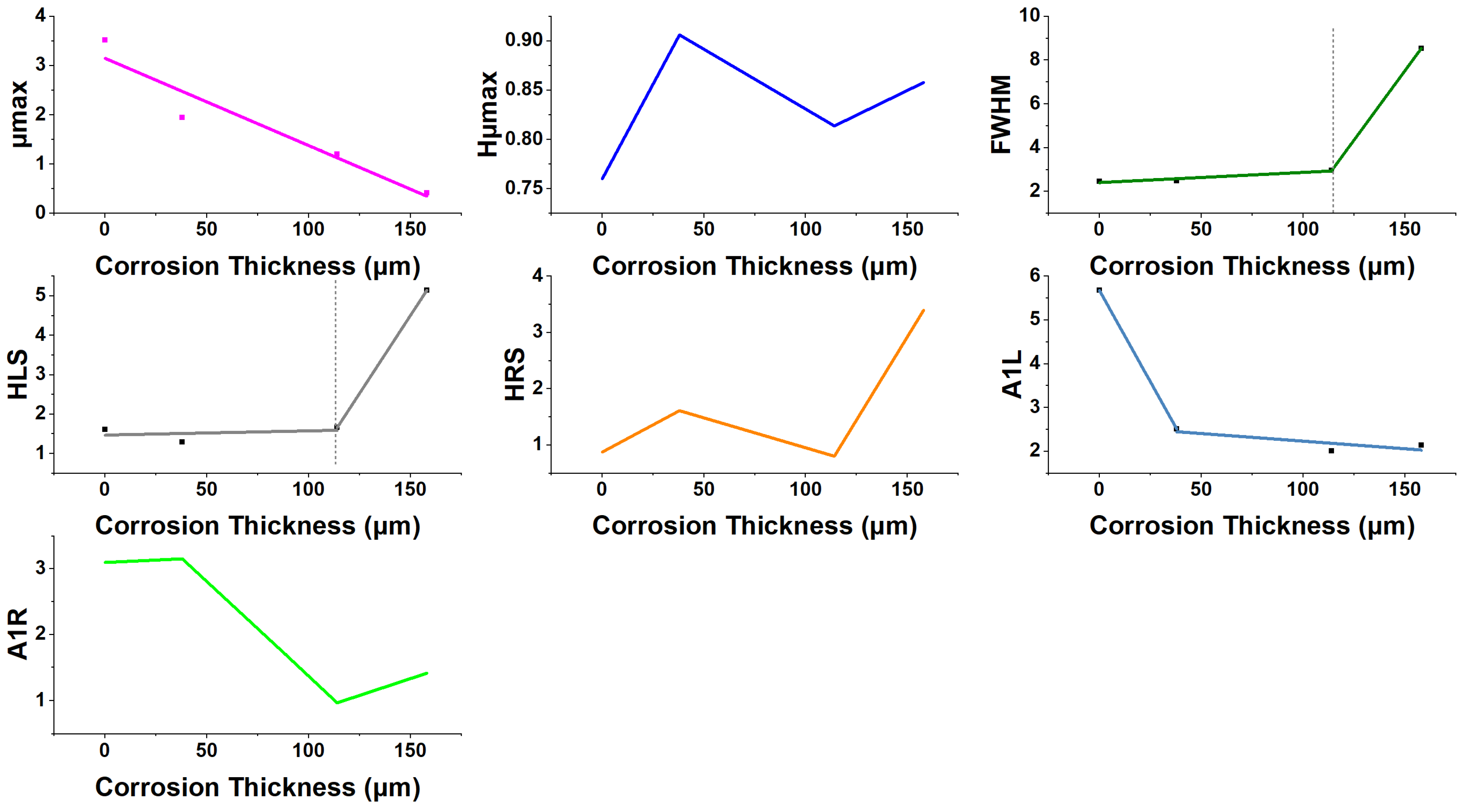
| Immersion Time (Days) | μmax | Hμmax | FWHM | HLS | HRS | A1L | A1R | CR | Corrosion Thickness | Rc | CPEc | Rct | CPEdl |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–7 | ↓ | ↑ | ↑ | ↓ | ↑ | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| 7–49 | ↓ | ↓ | ↑ | ↑ | ↓ | ↓ | ↓ | ↓ | ↑ | ↑ | ↓ | ↑ | ↓ |
| 49–84 | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↓ | ↓ | ↓ | ↓ |
| Work | Steel Type | Medium | Detection Methods | Key Findings | Refs. |
|---|---|---|---|---|---|
| Barsoukov and Macdonald | - | - | EIS, Modeling | Multi-RC-CPE circuits for complex corrosion | [37] |
| Rémazeilles et al. | Iron/Steels | Lab/Museum | XRD, Raman, SEM | Akaganeite/phase evolution in chloride | [54] |
| Xiao et al. | Carbon steel | Marine atmosphere | Rust/surface XRD SEM | Stratified corrosion, phase sequence | [78] |
| Gotoh et al. | Fe | Air/Marine | Magnetic permeability | Rust layer reduces measured permeability | [79] |
| Melchers and Jeffrey | Mild Steel | Marine/SW | Weight loss, modeling | Reaction + diffusion + microbial phases, reacceleration | [51] |
| Vukelic et al. | AH36 | Natural seawater | Weight loss, SEM | Non-linear corrosion, stabilization ~0.12mm/y | [47] |
| Present Study | DH36 | ASW (ASTM D1141) | EIS, SEM, Magnetic | Multi-phase corrosion, declining permeability | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vourna, P.; Ktena, A.; Hristoforou, E.V.; Papadopoulos, N.D. Assessment of Corrosion in Naval Steels Submerged in Artificial Seawater Utilizing a Magnetic Non-Destructive Sensor. Sensors 2025, 25, 5015. https://doi.org/10.3390/s25165015
Vourna P, Ktena A, Hristoforou EV, Papadopoulos ND. Assessment of Corrosion in Naval Steels Submerged in Artificial Seawater Utilizing a Magnetic Non-Destructive Sensor. Sensors. 2025; 25(16):5015. https://doi.org/10.3390/s25165015
Chicago/Turabian StyleVourna, Polyxeni, Aphrodite Ktena, Evangelos V. Hristoforou, and Nikolaos D. Papadopoulos. 2025. "Assessment of Corrosion in Naval Steels Submerged in Artificial Seawater Utilizing a Magnetic Non-Destructive Sensor" Sensors 25, no. 16: 5015. https://doi.org/10.3390/s25165015
APA StyleVourna, P., Ktena, A., Hristoforou, E. V., & Papadopoulos, N. D. (2025). Assessment of Corrosion in Naval Steels Submerged in Artificial Seawater Utilizing a Magnetic Non-Destructive Sensor. Sensors, 25(16), 5015. https://doi.org/10.3390/s25165015








