A Cost-Effective 3D-Printed Conductive Phantom for EEG Sensing System Validation: Development, Performance Evaluation, and Comparison with State-of-the-Art Technologies
Abstract
1. Introduction
- Systematic development and validation of a 3D-printed phantom design demonstrating consistent electrical properties suitable for standardized EEG electrode testing, achieving 85% cost reduction and fabrication time reduction from weeks to under 48 h compared to commercial alternatives
- Comprehensive electrical characterization using multi-tier validation: DC resistance measurements (821–1502 ), complex impedance spectroscopy at 100 Hz revealing spatial variation (3.01–6.4 k) and capacitive behavior (phase angles −53° to −67°), and clinical EEG system compatibility demonstration (5–11 k electrode-phantom interface impedance)
- Comparative analysis of six distinct phantom technologies for EEG validation applications, evaluating electrical properties, fabrication requirements, costs, and accessibility limitations to provide evidence-based selection guidance for different research contexts
- Practical demonstration of accessible validation methodologies that enable broader participation in EEG electrode development, particularly benefiting resource-constrained research environments and educational applications
2. State-of-the-Art Analysis: Phantom Technologies for EEG Sensing System Validation
2.1. Commercial Injection-Molded Phantoms
2.2. Saline and Electrolyte Solutions
2.3. Gelatin and Hydrogel-Based Phantoms
2.4. 3D-Printed Conductive Phantoms
2.5. Silicone and Elastomer-Based Phantoms
2.6. Multi-Material Phantoms
2.7. Emerging Textile-Based Phantoms
2.8. Comparative Analysis and Identification of Key Limitations
3. 3D-Printed Conductive Phantom Implementation
3.1. Design and Fabrication Approach
3.1.1. Phantom Architecture and Model Development
3.1.2. Material Selection and Characterization
3.1.3. Manufacturing System Implementation
3.1.4. Process Parameter Optimization
3.1.5. Production Timeline and Resource Requirements
3.2. Electrical Characterization and Validation
3.2.1. Resistance Measurement Protocol
3.2.2. Electrical Performance Results
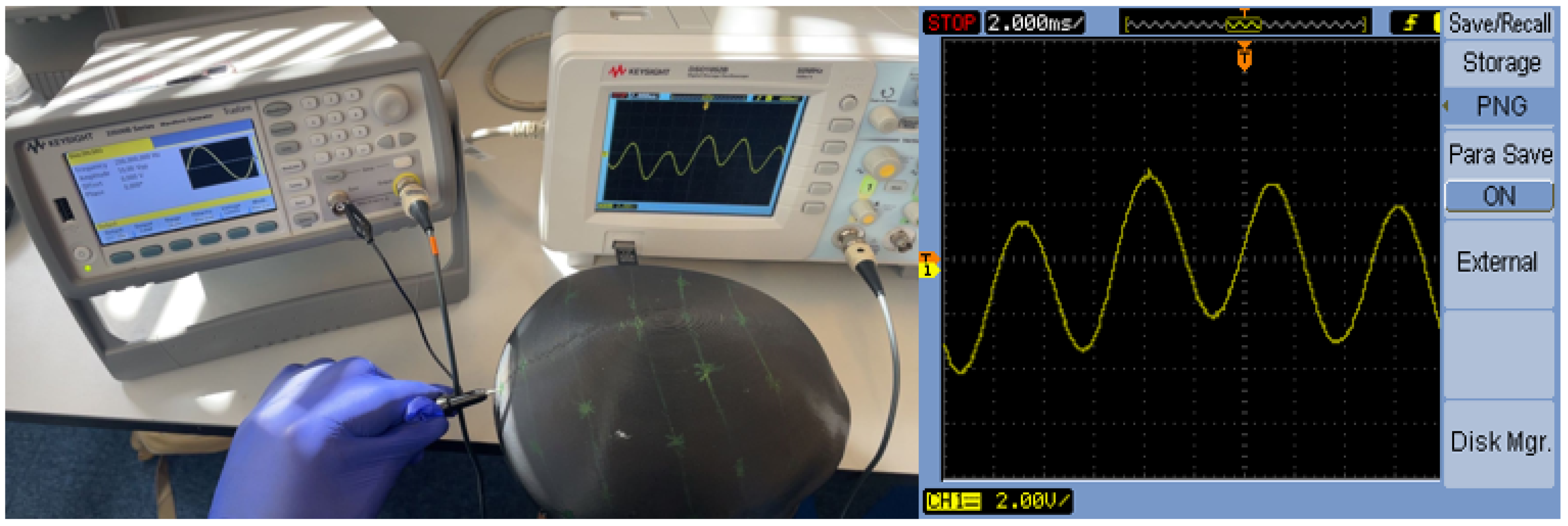
3.2.3. Signal Transmission and Frequency Response Validation
3.3. Prototype Validation and Quality Control
3.3.1. Scale Model Verification
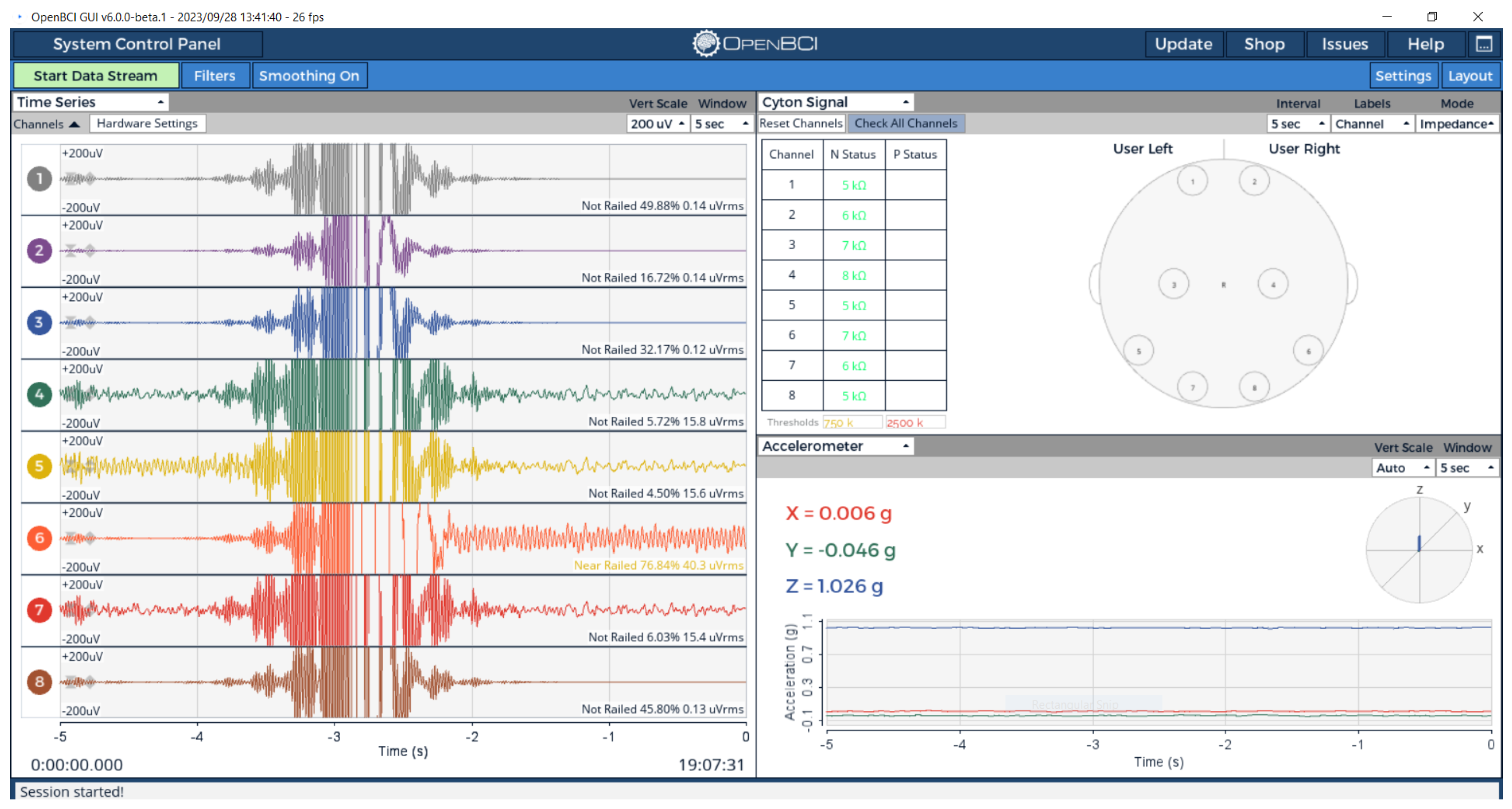
3.3.2. Multi-Channel EEG System Validation
Experimental Setup and Configuration
Electrode-Phantom Interface Characterization
Spatial Signal Localization
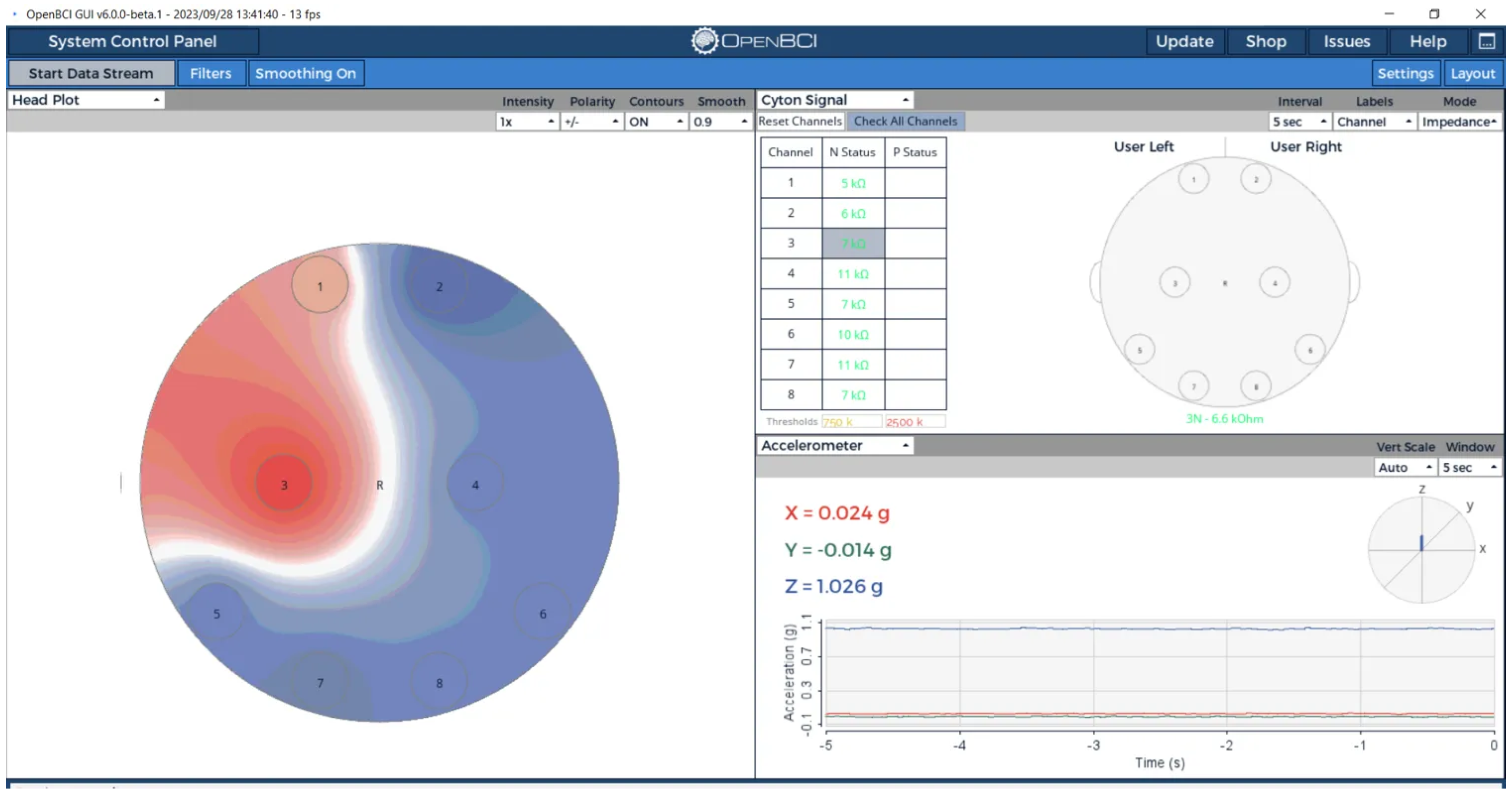
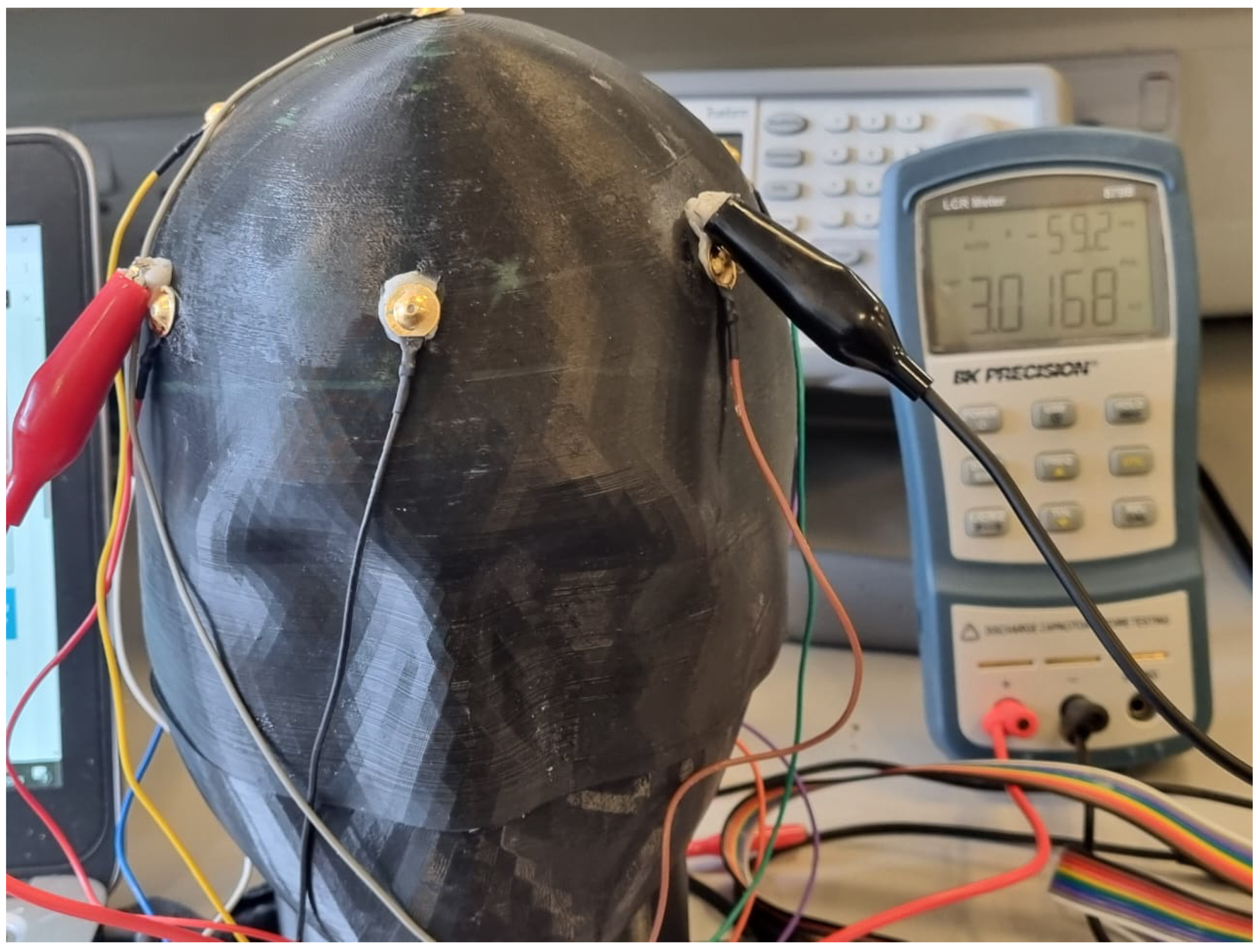
3.3.3. Complex Impedance Spectroscopy Analysis
Regional Impedance Mapping Protocol
Multi-Method Electrical Validation Approach
Complex Impedance Results
Measurement Method Considerations
Complex Impedance Visualization
4. Discussion
4.1. Democratizing Access to EEG Validation Technologies
4.2. Technical Performance and Validation Capabilities
4.3. Strategic Technology Selection Framework
4.4. Economic Impact and Resource Optimization
4.5. Innovation Impact on EEG Sensing System Development
4.6. Future Technological Evolution and Research Directions
4.7. Broader Impact on Biomedical Engineering Education and Research
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABS | Acrylonitrile Butadiene Styrene |
| CAD | Computer-Aided Design |
| CSF | Cerebrospinal Fluid |
| CT | Computed Tomography |
| ECG | Electrocardiogram |
| EEG | Electroencephalography |
| FDM | Fused Deposition Modeling |
| GelMA | Methacrylate Gelatin |
| KCl | Potassium Chloride |
| LCR | Inductance, Capacitance, Resistance |
| MR | Magnetic Resonance |
| MRI | Magnetic Resonance Imaging |
| NaCl | Sodium Chloride |
| PAA | Polyacrylamide |
| PDMS | Polydimethylsiloxane |
| PET | Positron Emission Tomography |
| PLA | Polylactic Acid |
| PVA | Polyvinyl Alcohol |
| PVP | Polyvinylpyrrolidone |
| RF | Radio Frequency |
| TPE | Thermoplastic Elastomer |
| TPU | Thermoplastic Polyurethane |
References
- World Health Organization. Epilepsy. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/epilepsy (accessed on 7 February 2024).
- Ramakrishnan, S.; Asuncion, R.M.D.; Rayi, A. Localization-Related Epilepsies on EEG. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Niso, G.; Romero, E.; Moreau, J.T.; Araujo, A.; Krol, L.R. Wireless EEG: A survey of systems and studies. NeuroImage 2023, 269, 119774. [Google Scholar] [CrossRef]
- Collier, T.; Kynor, D.; Bieszczad, J.; Audette, W.; Kobylarz, E.; Diamond, S. Creation of a human head phantom for testing of electroencephalography equipment and techniques. IEEE Trans. Biomed. Eng. 2012, 59, 2628–2634. [Google Scholar] [CrossRef]
- Audette, W.; Bieszczad, J.; Allen, L.; Diamond, S.; Kynor, D. Design and Demonstration of a Head Phantom for Testing of Electroencephalography (EEG) Equipment; Technical Report; Creare LLC.: Hanover, NH, USA, 2020. [Google Scholar]
- Formlabs. How to Estimate Injection Molding Cost? 2023. Available online: https://formlabs.com/blog/injection-molding-cost/ (accessed on 15 August 2023).
- Tseghai, G.B.; Malengier, B.; Fante, K.A.; Van Langenhove, L. A Long-Lasting Textile-Based Anatomically Realistic Head Phantom for Validation of EEG Electrodes. Sensors 2021, 21, 4658. [Google Scholar] [CrossRef]
- Tack, P.; Victor, J.; Gemmel, P.; Annemans, L. 3D Printing Techniques in Medical Application and the Challenges. Bioprinting 2016, 5, 9–21. [Google Scholar] [CrossRef]
- Gabriel, C.; Peyman, A.; Grant, E. Electrical conductivity of tissue at frequencies below 1 MHz. Phys. Med. Biol. 2009, 54, 4863–4878. [Google Scholar] [CrossRef]
- Gabriel, C.; Gabriel, S.; Corthout, E. The dielectric properties of biological tissues: I. Literature survey. Phys. Med. Biol. 1996, 41, 2231–2249. [Google Scholar] [CrossRef]
- Schmid & Partner Engineering AG. SAM-V4.5 Anthropomorphic Head Phantom (Shell Only) for OTA Evaluation of Antennas Implanted Inside the Head or SAR Measurements of Head-Worn Devices; Product Specification Sheet; Schmid & Partner Engineering AG: Zurich, Switzerland, 2025. [Google Scholar]
- Abedi, S. Standard Phantoms for EM Device Testing; Electromagnetism, Sorbonne Université: Paris, France, 2022; NNT: 2022SORUS004. [Google Scholar]
- Sheng, S.; Nalleballe, K.; Yadala, S. EEG Benign Variants. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Casson, A.J. Wearable EEG and beyond. Biomed. Eng. Lett. 2019, 9, 53–71. [Google Scholar] [CrossRef]
- Omar, H.; Ahmad, A.; Hayashi, N.; Idris, Z.; Abdullah, J. Magnetoencephalography Phantom Comparison and Validation: Hospital Universiti Sains Malaysia (HUSM) Requisite. Malays. J. Med. Sci. 2015, 22, 20–28. [Google Scholar]
- Iravanian, S.; Langberg, J.J. A review of bioelectrodes for clinical electrophysiologists. Heart Rhythm 2019, 16, 460–469. [Google Scholar] [CrossRef]
- Costanzo, S.; Cioffi, V.; Qureshi, A.; Borgia, A. Gel-Like Human Mimicking Phantoms: Realization Procedure, Dielectric Characterization and Experimental Validations on Microwave Wearable Body Sensors. Biosensors 2021, 11, 111. [Google Scholar] [CrossRef]
- Hunold, A.; Machts, R.; Haueisen, J. Head phantoms for bioelectromagnetic applications: A material study. Biomed. Eng. Online 2020, 19, 87. [Google Scholar] [CrossRef]
- Bennett, D. NaCl doping and the conductivity of agar phantoms. Mater. Sci. Eng. C 2011, 31, 494–498. [Google Scholar] [CrossRef]
- Ianniello, C.; de Zwart, J.; Duan, Q.; Deniz, C.; Alon, L.; Lee, J.; Lattanzi, R.; Brown, R. Synthesized tissue-equivalent dielectric phantoms using salt and polyvinylpyrrolidone solutions. Magn. Reson. Med. 2019, 82, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Anand, G.; Lowe, A.; Al-Jumaily, A. Tissue phantoms to mimic the dielectric properties of human forearm section for multi-frequency bioimpedance analysis at low frequencies. Mater. Sci. Eng. C 2019, 96, 496–508. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Sohrabpour, A.; Brown, E.; Liu, Z. Electrophysiological Source Imaging: A Noninvasive Window to Brain Dynamics. Annu. Rev. Biomed. Eng. 2018, 20, 171–196. [Google Scholar] [CrossRef] [PubMed]
- Shephard, C.; Jochum, T.; Abzug, Z.; Wolf, P. Planar Saline Bath Phantom of the Rush Head Model. In Proceedings of the IEEE Engineering in Medicine and Biology Society Annual International Conference, Boston, MA, USA, 23–27 June 2019; pp. 5794–5797. [Google Scholar]
- Chandra, A.; Bagchi, B. Frequency dependence of ionic conductivity of electrolyte solutions. Chin. J. Chem. Phys. 2000, 112, 1876–1886. [Google Scholar] [CrossRef]
- Caine, M.; Bian, S.; Tang, Y.; Garcia, P.; Henman, A.; Dreher, M.; Daly, D.; Carlisle, R.; Stride, E.; Willis, S.L.; et al. In situ evaluation of spatiotemporal distribution of doxorubicin from Drug-eluting Beads in a tissue mimicking phantom. Eur. J. Pharm. Sci. 2021, 160, 105772. [Google Scholar] [CrossRef]
- Vocciante, M.; Dovì, V.; Ferro, S. Sustainability in ElectroKinetic Remediation Processes: A Critical Analysis. Sustainability 2021, 13, 770. [Google Scholar] [CrossRef]
- Woods, A.; Antal, A.; Bikson, M.; Boggio, P.; Brunoni, A.; Celnik, P.; Cohen, L.; Fregni, F.; Herrmann, C.; Kappenman, E.; et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol. 2016, 127, 1031–1048. [Google Scholar] [CrossRef]
- Nascimento, A.; Duhaime, E.; Kapur, S.; Karakis, I.; Ng, M.; Herlopian, A.; Lam, A.; Maus, D.; Halford, J.J.; Cash, S.; et al. On-demand EEG education through competition—A novel, app-based approach to learning to identify interictal epileptiform discharges. Clin. Neurophysiol. Pract. 2023, 8, 177–186. [Google Scholar] [CrossRef]
- Sadleir, R.; Argibay, A. Modeling skull electrical properties. Ann. Biomed. Eng. 2007, 35, 1699–1712. [Google Scholar] [CrossRef]
- Xu, Z.; Lin, S.; Yin, Y.; Gu, X. Synergy between gelatin hydrogels and electrodes for adjustable and thermally stable ionic thermoelectric supercapacitors. Chem. Eng. J. 2024, 493, 152734. [Google Scholar] [CrossRef]
- Jonasson, H.; Anderson, C.; Saager, R. Water and hemoglobin modulated gelatin-based phantoms to spectrally mimic inflamed tissue in the validation of biomedical techniques and the modeling of microdialysis data. J. Biomed. Opt. 2022, 27, 074712. [Google Scholar] [CrossRef] [PubMed]
- Yusuff, H.; Chatelin, S.; Dillenseger, J.P. Narrative review of tissue-mimicking materials for MRI phantoms: Composition, fabrication, and relaxation properties. Radiography 2024, 30, 1655–1668. [Google Scholar] [CrossRef] [PubMed]
- Gorth, D.; Webster, T. 10—Matrices for tissue engineering and regenerative medicine. In Biomaterials for Artificial Organs; Lysaght, M., Webster, T.J., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2011; pp. 270–286. [Google Scholar] [CrossRef]
- Richardson, C.; Bernard, S.; Dinh, V. A Cost-effective, Gelatin-Based Phantom Model for Learning Ultrasound-Guided Fine-Needle Aspiration Procedures of the Head and Neck. J. Ultrasound Med. 2015, 34, 1479–1484. [Google Scholar] [CrossRef]
- Marchal, C.; Nadi, M.; Tosser, A.; Roussey, C.; Gaulard, M. Dielectric properties of gelatine phantoms used for simulations of biological tissues between 10 and 50 MHz. Int. J. Hyperth. 1989, 5, 725–732. [Google Scholar] [CrossRef]
- Tejo-Otero, A.; Fenollosa-Artés, F.; Achaerandio, I.; Rey-Vinolas, S.; Buj-Corral, I.; Mateos-Timoneda, M.Á.; Engel, E. Soft-tissue-mimicking using hydrogels for the development of phantoms. Materials 2020, 13, 3484. [Google Scholar] [CrossRef]
- Hariri, A.; Palma-Chavez, J.; Wear, K.A.; Pfefer, T.J.; Jokerst, J.V.; Vogt, W.C. Polyacrylamide hydrogel phantoms for performance evaluation of multispectral photoacoustic imaging systems. Biomed. Opt. Express 2020, 11, 2130–2145. [Google Scholar] [CrossRef]
- Choi, S.; Guan, W.; Chung, K. Basic principles of hydrogel-based tissue transformation technologies and their applications. Cell 2021, 184, 4115–4136. [Google Scholar] [CrossRef]
- El-Sherbiny, I.; Yacoub, M. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 316–342. [Google Scholar] [CrossRef]
- Tamminen, A.; Baggio, M.; Nefedova, I.I.; Sun, Q.; Presnyakov, S.A.; Ala-Laurinaho, J.; Brown, E.R.; Wallace, V.P.; Pickwell-MacPherson, E.; Maloney, T.; et al. Extraction of thickness and water-content gradients in hydrogel-based water-backed corneal phantoms via submillimeter-wave reflectometry. IEEE Trans. Terahertz Sci. Technol. 2021, 11, 643–659. [Google Scholar] [CrossRef]
- Cao, Y.; Li, G.Y.; Zhang, X.; Liu, Y.L. Tissue-mimicking materials for elastography phantoms: A review. Extrem. Mech. Lett. 2017, 17, 62–70. [Google Scholar] [CrossRef]
- Uhrhan, K.; Schwindt, E.; Witte, H. Fabrication and Dielectric Validation of an Arm Phantom for Electromyostimulation. Bioengineering 2024, 11, 724. [Google Scholar] [CrossRef] [PubMed]
- Luong, P.; Browning, M.; Bixler, R.; Cosgriff-Hernandez, E. Drying and Storage Effects on Poly(ethylene glycol) Hydrogel Mechanical Properties and Bioactivity. J. Biomed. Mater. Res. Part A 2014, 102, 3066–3076. [Google Scholar] [CrossRef]
- Althumayri, M.; Das, R.; Banavath, R.; Beker, L.; Achim, A.; Ceylan Koydemir, H. Recent Advances in Transparent Electrodes and Their Multimodal Sensing Applications. Adv. Sci. 2024, 11, 2405099. [Google Scholar] [CrossRef]
- Ntombela, L.; Adeleye, B.; Chetty, N. Low-cost fabrication of optical tissue phantoms for use in biomedical imaging. Heliyon 2020, 6, e03602. [Google Scholar] [CrossRef]
- Madamesila, J.; McGeachy, P.; Villarreal-Barajas, J.; Khan, R. Characterizing 3D printing in the fabrication of variable density phantoms for quality assurance of radiotherapy. Phys. Medica 2015, 32, 242–247. [Google Scholar] [CrossRef]
- Savi, M.; Bertoncini Andrade, M.; Potiens, M. Commercial filament testing for use in 3D printed phantoms. Radiat. Phys. Chem. 2020, 174, 108906. [Google Scholar] [CrossRef]
- Park, H.; Wilson, A. Cost analysis of 3D-printed phantoms for neurophysiological research. J. Med. Devices 2020, 14, 034501. [Google Scholar]
- Ramírez, J.; Sullivan, M. Electrical characterization of carbon-loaded thermoplastics for EEG phantom development. IEEE Trans. Biomed. Eng. 2023, 70, 689–697. [Google Scholar]
- Zhang, J.; Yang, B.; Li, H.; Fu, F.; Shi, X.; Dong, X.; Dai, M. A novel 3D-printed head phantom with anatomically realistic geometry and continuously varying skull resistivity distribution for electrical impedance tomography. Sci. Rep. 2017, 7, 4608. [Google Scholar] [CrossRef]
- Creegan, A.; Nielsen, P.M.F.; Tawhai, M.H. A novel two-dimensional phantom for electrical impedance tomography using 3D printing. Sci. Rep. 2022, 12, 1–12. [Google Scholar] [CrossRef]
- Johnson, K.; Lee, S. Comparative analysis of electrical properties in 3D-printed versus traditional head phantoms. Clin. Neurophysiol. 2020, 131, 2147–2156. [Google Scholar]
- Tanaka, H.; Miller, P. Design flexibility in 3D-printed head phantoms for neurophysiological testing. J. Neural Eng. 2022, 19, 046014. [Google Scholar]
- Faenger, B.; Ley, S.; Helbig, M.; Sachs, J.; Hilger, I. Breast phantom with a conductive skin layer and conductive 3D-printed anatomical structures for microwave imaging. IEEE J. Electromagn. Microwaves Med. Biol. 2021, 5, 184–192. [Google Scholar]
- Webb, A.; Patel, N. Durability assessment of various phantom technologies for longitudinal studies. Med. Eng. Phys. 2023, 105, 103842. [Google Scholar]
- Higgins, M.; Leung, S.; Radacsi, N. Applications of 3D-printed phantoms in medical education and training. J. Med. Educ. Curric. Dev. 2022, 9, 23821205221074650. [Google Scholar]
- Sato, K.; Anderson, R. Anisotropic conductivity in 3D-printed phantom materials: Measurement and implications. IEEE Sens. J. 2021, 21, 16892–16901. [Google Scholar]
- Nguyen, T.; Williams, J. Mechanical integrity of layer-based additive manufacturing for biomedical phantoms. J. Mech. Behav. Biomed. Mater. 2020, 112, 104041. [Google Scholar]
- Monea, B.; Vervier, A.; Egan, W.; Murphy, C.; Dempsey, M. The future of 3D printing in conductive phantom development: Challenges and opportunities. Annu. Rev. Biomed. Eng. 2022, 24, 219–241. [Google Scholar]
- Bhusal, A.; Farahmand, M.; Hasan, M.; Vasudevan, S.; Vogt, W.; Ibarra, B.; Weininger, S.; Scully, C.; Frank Zhang, X.; Chen, Y.; et al. Development and characterization of silicone-based tissue phantoms for pulse oximeter performance testing. J. Biomed. Opt. 2024, 29, S33314. [Google Scholar] [CrossRef]
- Gao, S.; Yang, Y.; Falchevskaya, A.; Vinogradov, V.; Yuan, B.; Liu, J.; Sun, X. Phase Transition Liquid Metal Enabled Emerging Biomedical Technologies and Applications. Adv. Sci. 2024, 11, 2306692. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhu, S.; Liu, M.; Zhong, B.; Chen, Y.; Luo, Y.; Liu, F.; Jia, Z.; Jia, D. A high-performance, thermal and electrical conductive elastomer composite based on Ti3C2 MXene. Compos. Part A Appl. Sci. Manuf. 2021, 145, 106292. [Google Scholar] [CrossRef]
- Alarifi, I.M. A comprehensive review on advancements of elastomers for engineering applications. Adv. Ind. Eng. Polym. Res. 2023, 6, 451–464. [Google Scholar] [CrossRef]
- Hatamikia, S.; Jaksa, L.; Kronreif, G.; Birkfellner, W.; Kettenbach, J.; Buschmann, M.; Lorenz, A. Silicone phantoms fabricated with multi-material extrusion 3D printing technology mimicking imaging properties of soft tissues in CT. Phys. Med. Biol. 2023, 68, 125009. [Google Scholar] [CrossRef] [PubMed]
- Nikawa, Y.; Chino, M.; Kikuchi, K. Soft and dry phantom modeling material using silicone rubber with carbon fiber. IEEE Trans. Microw. Theory Tech. 1996, 44, 1949–1953. [Google Scholar] [CrossRef]
- Wagner, M.; Fugger, O.; Foschum, F.; Kienle, A. Silicone-based evaluation phantoms for biomedical optics from 400 to 1550 nm. Biomed. Opt. Express 2024, 15, 132–141. [Google Scholar] [CrossRef]
- Motovilova, E.; Aronowitz, E.; Vincent, J.; Shin, J.; Tan, E.; Robb, F.; Taracila, V.; Sneag, D.; Dyke, J.; Winkler, S. Silicone-based materials with tailored MR relaxation characteristics for use in reduced coil visibility and in tissue-mimicking phantom design. Med. Phys. 2023, 50, 3498–3510. [Google Scholar] [CrossRef]
- Trotta, A.; Ní Annaidh, A. Mechanical characterisation of human and porcine scalp tissue at dynamic strain rates. J. Mech. Behav. Biomed. Mater. 2019, 100, 103381. [Google Scholar] [CrossRef]
- Maggi, L.E.; von Krüger, M.A.; Pereira, W.C.A.; Monteiro, E.E.C. Development of silicon-based materials for ultrasound biological phantoms. In Proceedings of the IEEE International Ultrasonics Symposium, Rome, Italy, 20–23 September 2009; pp. 1962–1965. [Google Scholar]
- Ayers, F.; Grant, A.; Kuo, D.; Cuccia, D.J.; Durkin, A.J. Fabrication and characterization of silicone-based tissue phantoms with tunable optical properties in the visible and near infrared domain. In Proceedings of the SPIE, San Jose, CA, USA, 19–24 January 2008; Volume 6870. [Google Scholar]
- Dąbrowska, A.; Rotaru, G.M.; Derler, S.; Spano, F.; Camenzind, M.; Annaheim, S.; Stämpfli, R.; Schmid, M.; Rossi, R. Materials used to simulate physical properties of human skin. Ski. Res. Technol. 2016, 22, 3–14. [Google Scholar] [CrossRef]
- Liao, L.D.; Wang, I.J.; Chen, S.F.; Chang, J.Y.; Lin, C.T. Design, Fabrication and Experimental Validation of a Novel Dry-Contact Sensor for Measuring Electroencephalography Signals without Skin Preparation. Sensors 2011, 11, 5819–5834. [Google Scholar] [CrossRef]
- Duran, M.M.; Moro, G.; Zhang, Y.; Islam, A. 3D printing of silicone and polyurethane elastomers for medical device application: A review. Adv. Ind. Manuf. Eng. 2023, 7, 100125. [Google Scholar] [CrossRef]
- Jerome, N.; Papoutsaki, M.V.; Orton, M.; Parkes, H.; Winfield, J.; Boss, M.; Leach, M.; deSouza, N.; Collins, D. Development of a temperature-controlled phantom for magnetic resonance quality assurance of diffusion, dynamic, and relaxometry measurements. Med. Phys. 2016, 43, 2998–3007. [Google Scholar] [CrossRef]
- Yin, J.; Li, M.; Dai, G.; Zhou, H.; Ma, L.; Zheng, Y. 3D Printed Multi-material Medical Phantoms for Needle-tissue Interaction Modelling of Heterogeneous Structures. J. Bionic Eng. 2021, 18, 346–360. [Google Scholar] [CrossRef]
- Chen, A.; Balter, M.; Chen, M.; Gross, D.; Alam, S.; Maguire, T.; Yarmush, M. Multilayered tissue mimicking skin and vessel phantoms with tunable mechanical, optical, and acoustic properties. Med. Phys. 2016, 43, 3117–3131. [Google Scholar] [CrossRef]
- Mobashsher, A.; Abbosh, A. Artificial human phantoms: Human proxy in testing microwave apparatuses that have electromagnetic interaction with the human body. IEEE Microw. Mag. 2020, 16, 42–62. [Google Scholar] [CrossRef]
- Huber, J.; Peng, Q.; Moses, W. Multi-Modality Phantom Development. IEEE Trans. Nucl. Sci. 2019, 66, 279–286. [Google Scholar]
- Thomas, Q.; Weller, T. Multi-Layer RF Tissue Phantoms for Mimicking a Human Core. IEEE Trans. Microw. Theory Tech. 2018, 66, 3495–3503. [Google Scholar]
- Lacana, F.; Johnston, R.; Carrington, R.; Spezi, E.; Theobald, P. Towards using a multi-material, pellet-fed additive manufacturing platform to fabricate novel imaging phantoms. J. Med. Phys. 2022, 49, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lowe, A.; Anand, G.; Kalra, A. Tissue phantom to mimic the dielectric properties of human muscle within 20 Hz and 100 kHz for biopotential sensing applications. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Berlin, Germany, 23–27 July 2019; pp. 6490–6493. [Google Scholar] [CrossRef]
- Hansen, S.T.; Hauberg, S.; Hansen, L.K. Data-driven forward model inference for EEG brain imaging. NeuroImage 2016, 139, 249–258. [Google Scholar] [CrossRef]
- Manohar, S.; Sechopoulos, I.; Anastasio, M.; Maier-Hein, L.; Gupta, R. Super phantoms: Advanced models for testing medical imaging technologies. Commun. Eng. 2024, 3, 73. [Google Scholar] [CrossRef]
- Piastra, M.; Oostenveld, R.; Homölle, S.; Han, B.; Chen, Q.; Oostendorp, T. How to assess the accuracy of volume conduction models? A validation study with stereotactic EEG data. Front. Hum. Neurosci. 2024, 18, 1279183. [Google Scholar] [CrossRef]
- Ragunathan, R.; Mireles, M.; Xu, E.; Lewis, A.; Vanegas, M.; Fang, Q. Direct 3-D printing of complex optical phantoms using dynamic filament mixing. Sci. Rep. 2025, 15, 9705. [Google Scholar] [CrossRef]
- Nakamura, T.; Patel, K. Interface effects in multi-material head phantoms: Implications for EEG testing. IEEE J. Electromagn. Microwaves Med. Biol. 2020, 4, 184–192. [Google Scholar]
- Kaveh, R.; Tetreault, N.; Gopalan, K.; Maravilla, J.; Lustig, M.; Muller, R.; Arias, A. Rapid and Scalable Fabrication of Low Impedance, 3D Dry Electrodes for Physiological Sensing. Adv. Mater. Technol. 2022, 7, 2200342. [Google Scholar] [CrossRef]
- Yu, A.B.; Hairston, W.D. Open EEG Phantom. 2021. Available online: https://osf.io/qrka2/ (accessed on 4 January 2021).
- ProtoPlant Inc. Conductive PLA Technical Data Sheet; Technical Report; Protopasta: Vancouver, WA, USA, 2020. [Google Scholar]
- Ferree, T.; Luu, P.; Russell, G.; Tucker, D. Scalp electrode impedance, infection risk, and EEG data quality. Clin. Neurophysiol. 2001, 112, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Owda, A.Y.; Casson, A.J. Investigating Gelatine Based Head Phantoms for Electroencephalography Compared to Electrical and Ex Vivo Porcine Skin Models. IEEE Access 2021, 9, 96722–96738. [Google Scholar] [CrossRef]
- Mathewson, K.E.; Harrison, T.J.L.; Kizuk, S.A.D. High and dry? Comparing active dry EEG electrodes to active and passive wet electrodes. Psychophysiology 2017, 54, 74–82. [Google Scholar] [CrossRef]
- Christie, L.B.; Zheng, W.; Johnson, W.; Marecki, E.K.; Heidrich, J.; Xia, J.; Oh, K.W. Review of imaging test phantoms. J. Biomed. Opt. 2023, 28, 080903. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
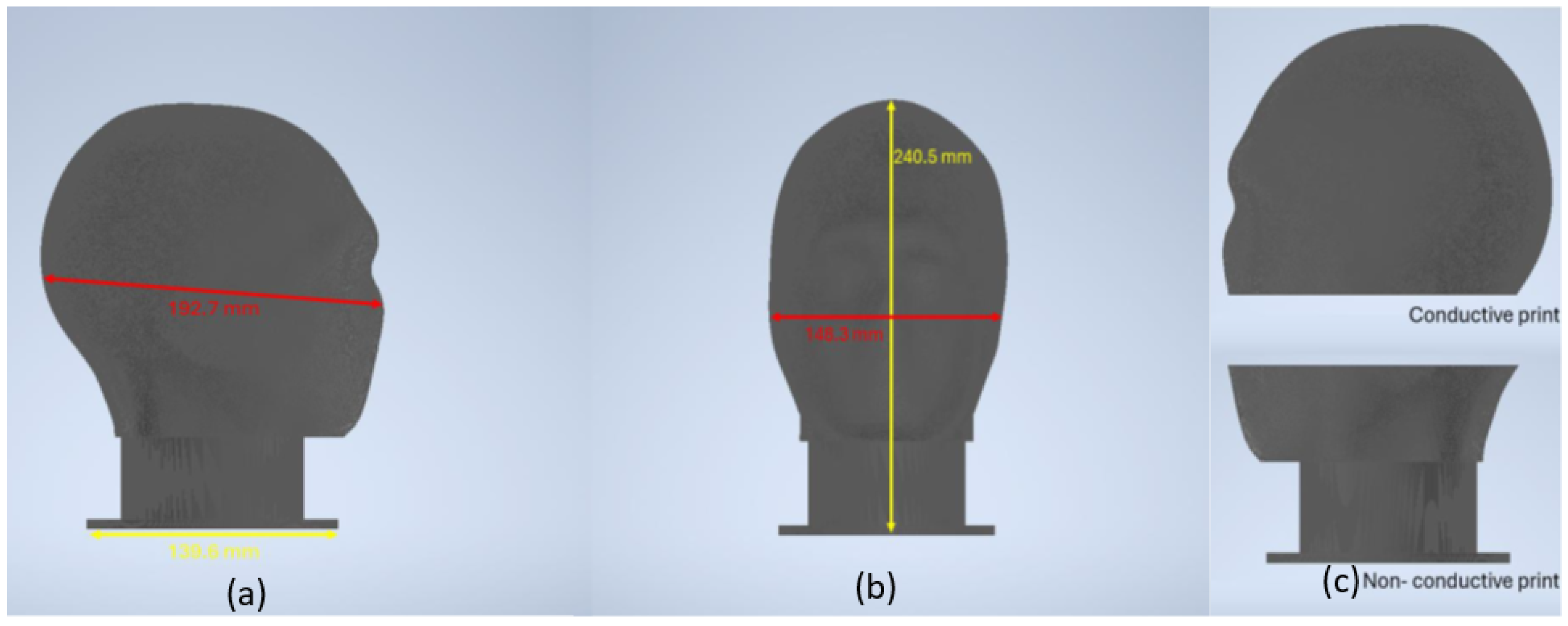
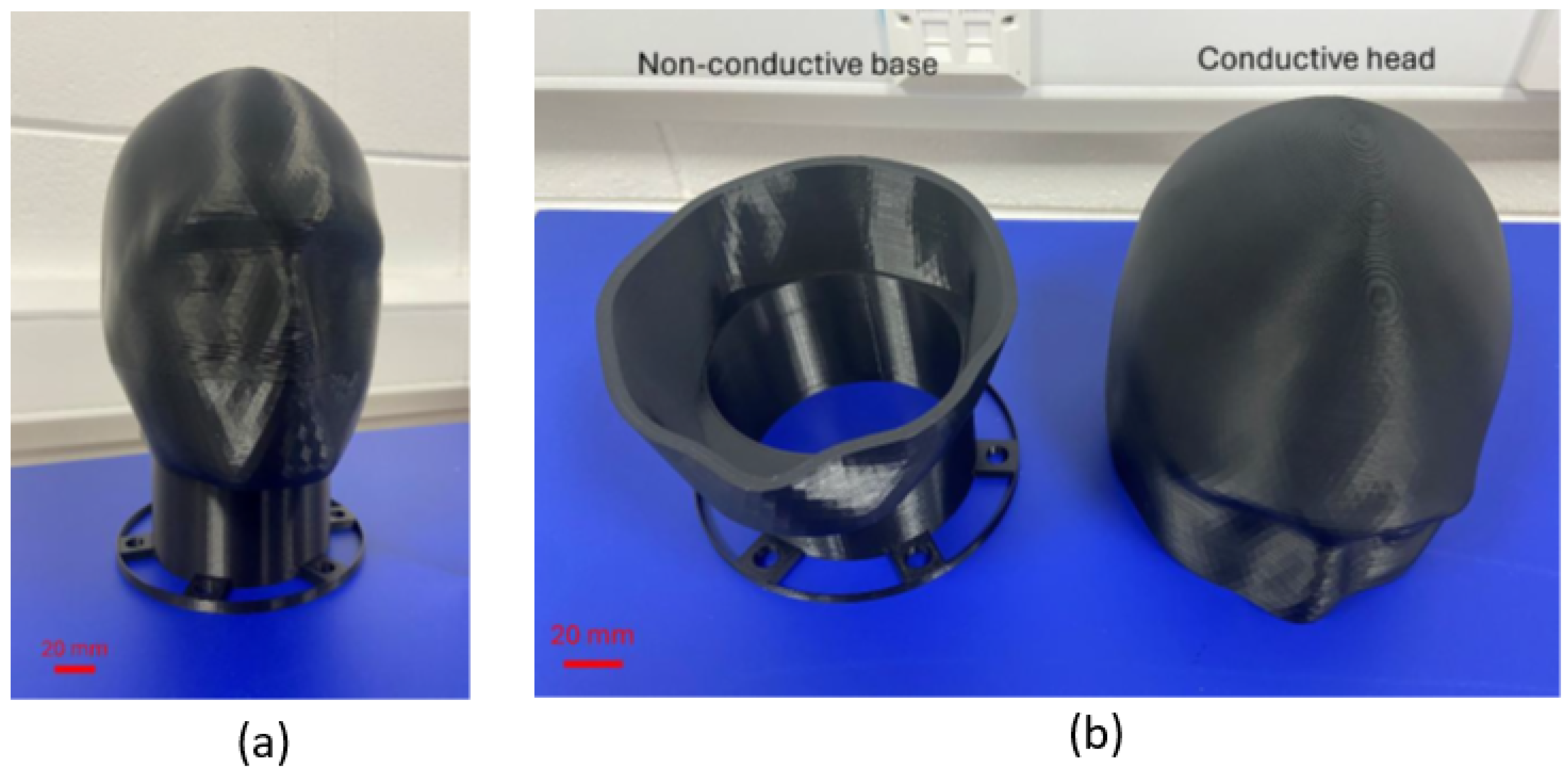
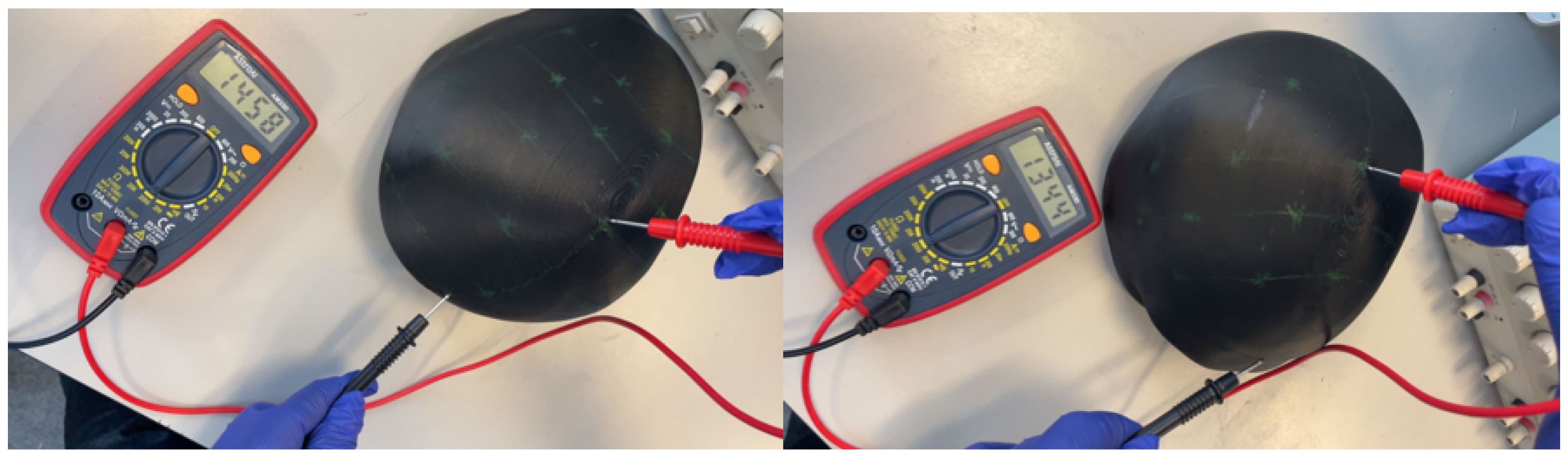
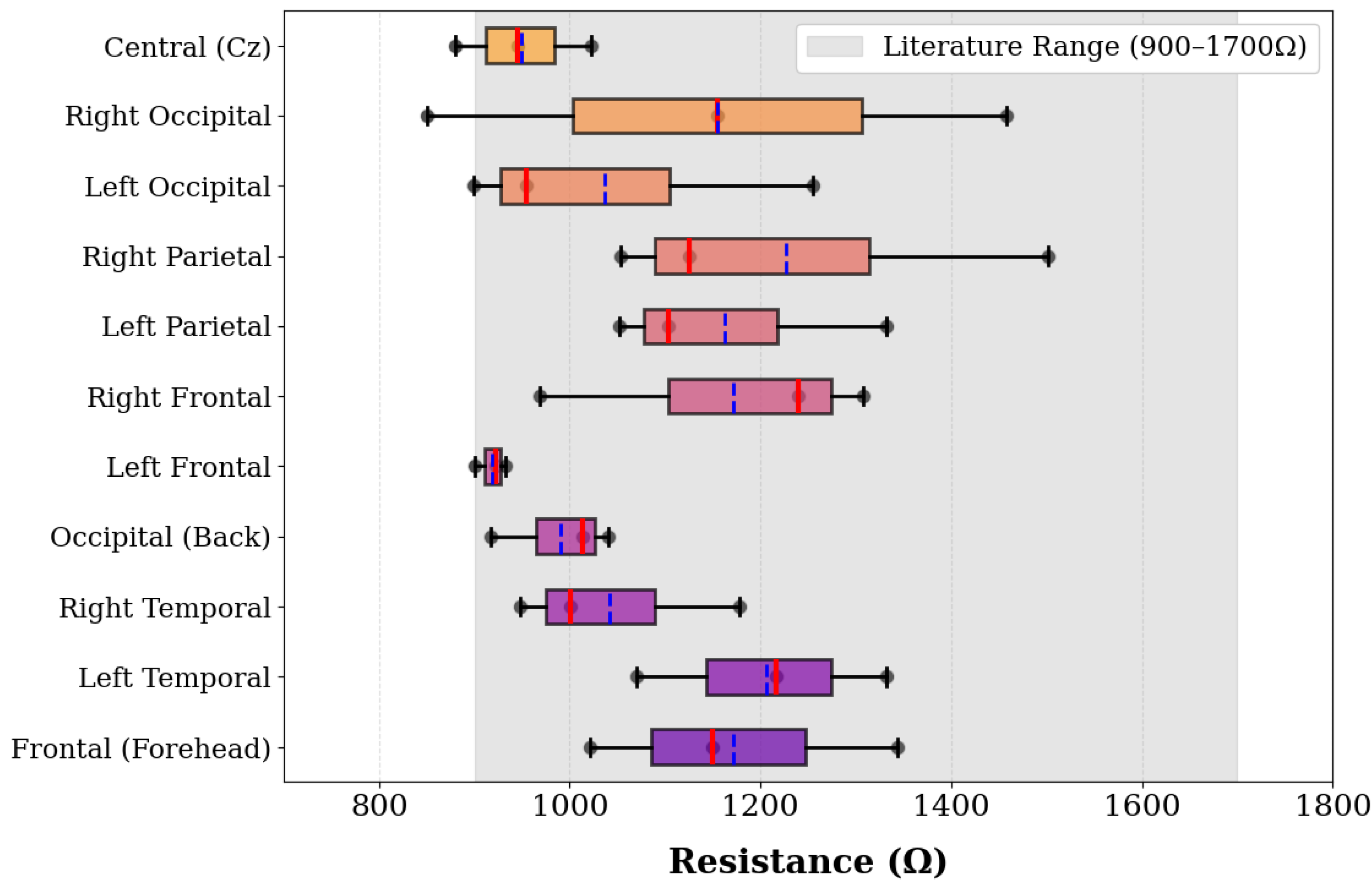

| Phantom Type | Material Cost | Equipment Cost | Production Time | Electrical Properties | Operational Lifespan |
|---|---|---|---|---|---|
| Injection-Molded [6,10] | £300–500/unit | £5k–20k tooling | Weeks | Consistent, 10–20 cm | Years |
| Saline Solutions [17,87] | £10–30 | Minimal | Hours | Adjustable, ionic | Days–weeks |
| Gelatin/Hydrogel [34,35] | £30–80 | Minimal | 1–2 days | 2–5 cm, adjustable | Weeks |
| 3D-Printed [48,52] | £40–150 | 3D printer | 1–3 days | 15–100 cm | Years [55] |
| Silicone-based [63,67] | £100–300 | Molds required | 2–3 days | 5–15 cm | Years |
| Multi-Material [77] | £200–600+ | Various | 3–7 days | Heterogeneous | Years |
| Textile-based [7] | £80–200 | 3D printer + textiles | 2–3 days | 1.8–2.3 k | Years |
| Parameter | Conductive Section | Non-Conductive Base |
|---|---|---|
| Print speed [mm/s] | 75 | 80 |
| Printing temperature [°C] | 230 | 225 |
| Build plate temperature [°C] | 60 | 60 |
| Layer height [mm] | 0.2 | 0.2 |
| Line width [mm] | 0.4 | 0.4 |
| Infill density [%] | 100 | 20 |
| Infill pattern | Zig-zag | Zig-zag |
| Wall thickness [mm] | 0.8 | 0.8 |
| Support material | White breakaway | White breakaway |
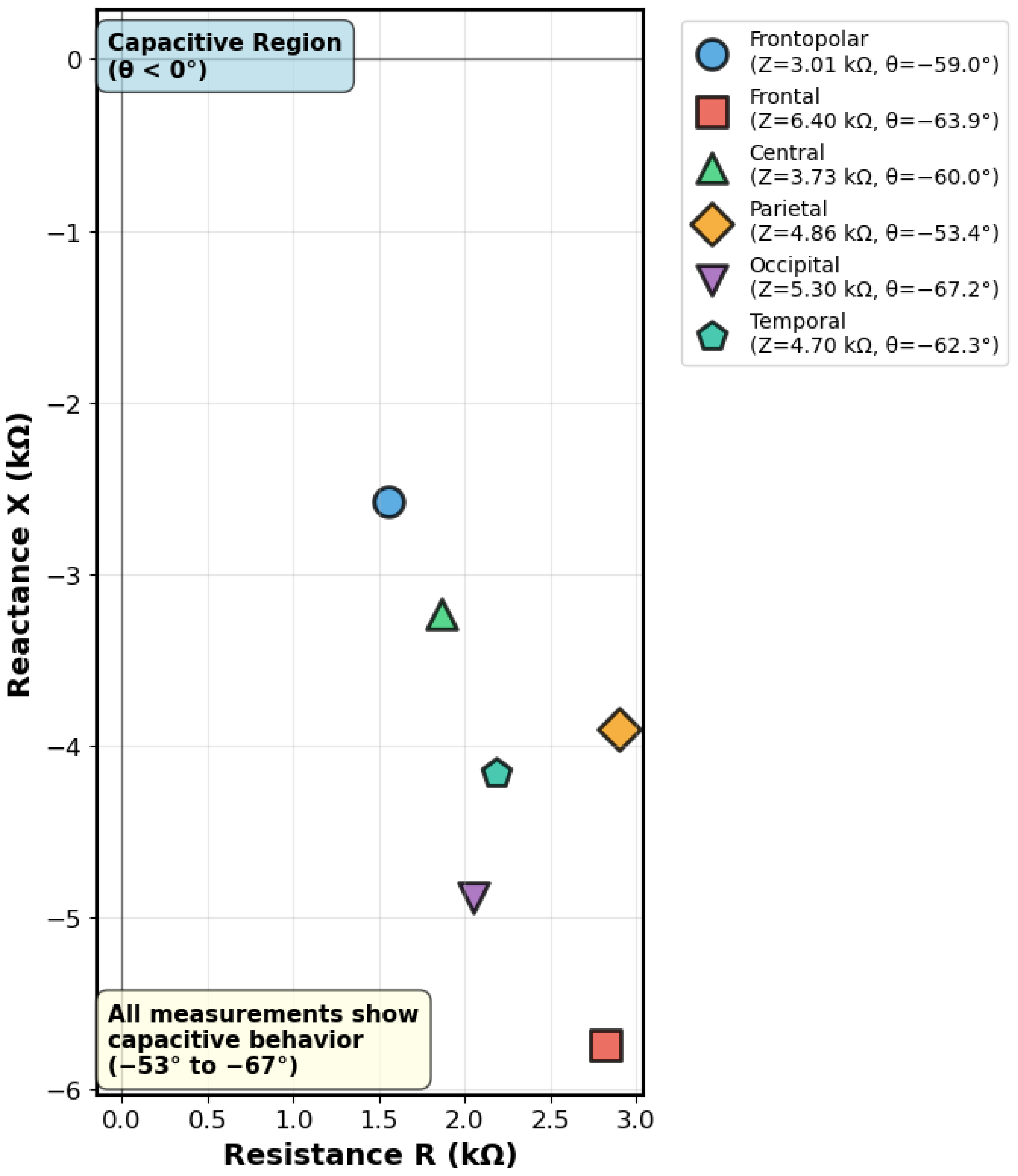
| Anatomical Region | Electrode Pair | |Z| (k) | (°) | R (k) | X (k) |
|---|---|---|---|---|---|
| Frontopolar | Fp1-Fp2 | 3.01 | −59.0 | 1.55 | −2.58 |
| Frontal | F3-F4 | 6.40 | −63.9 | 2.83 | −5.74 |
| Central | C3-C4 | 3.73 | −60.0 | 1.87 | −3.23 |
| Parietal | P3-P4 | 4.86 | −53.4 | 2.89 | −3.91 |
| Occipital | O1-O2 | 5.30 | −67.2 | 2.06 | −4.89 |
| Temporal | T7-T8 | 4.70 | −62.3 | 2.19 | −4.16 |
| Mean ± SD | 4.67 ± 1.36 | −61.0 ± 4.6 | 2.23 ± 0.53 | −4.09 ± 1.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akor, P.; Enemali, G.; Muhammad, U.; Crowley, J.; Desmulliez, M.; Larijani, H. A Cost-Effective 3D-Printed Conductive Phantom for EEG Sensing System Validation: Development, Performance Evaluation, and Comparison with State-of-the-Art Technologies. Sensors 2025, 25, 4974. https://doi.org/10.3390/s25164974
Akor P, Enemali G, Muhammad U, Crowley J, Desmulliez M, Larijani H. A Cost-Effective 3D-Printed Conductive Phantom for EEG Sensing System Validation: Development, Performance Evaluation, and Comparison with State-of-the-Art Technologies. Sensors. 2025; 25(16):4974. https://doi.org/10.3390/s25164974
Chicago/Turabian StyleAkor, Peter, Godwin Enemali, Usman Muhammad, Jane Crowley, Marc Desmulliez, and Hadi Larijani. 2025. "A Cost-Effective 3D-Printed Conductive Phantom for EEG Sensing System Validation: Development, Performance Evaluation, and Comparison with State-of-the-Art Technologies" Sensors 25, no. 16: 4974. https://doi.org/10.3390/s25164974
APA StyleAkor, P., Enemali, G., Muhammad, U., Crowley, J., Desmulliez, M., & Larijani, H. (2025). A Cost-Effective 3D-Printed Conductive Phantom for EEG Sensing System Validation: Development, Performance Evaluation, and Comparison with State-of-the-Art Technologies. Sensors, 25(16), 4974. https://doi.org/10.3390/s25164974







