1. Introduction
Controlled Environment Agriculture (CEA), particularly in the form of vertical farming, offers unprecedented control over environmental parameters such as light intensity and spectrum, temperature, humidity, CO
2 concentration, and nutrient supply. This level of control enables year-round, high-efficiency production and supports the exploration of dynamic, non-traditional cultivation strategies. In contrast with conventional agriculture, CEA facilitates the deliberate modulation of environmental inputs over time to influence and optimize plant growth trajectories. While this flexibility enables novel crop strategies, it also introduces considerable system complexity. As noted in [
1], realizing the potential of dynamic cultivation in CEA will require not only advanced control architectures but also sensor technologies capable of providing real-time feedback on both environmental variables and plant responses. In this context, sensors serve as a foundational component for both scientific investigation and operational optimization, supporting data-driven control, predictive modeling, and targeted breeding efforts.
Environmental parameters such as humidity, temperature, or CO
2 concentration can be precisely monitored using existing sensor technologies without interfering with the cultivation process. These sensors provide easily interpretable data and are widely deployed for environmental monitoring and climate control [
2,
3]. In contrast, capturing plant responses presents a greater challenge. While immediate physiological responses like photosynthetic activity can be measured with specialized sensors [
4,
5], such data are often spatially heterogeneous and difficult to scale in production environments. Phenotypic traits such as morphology, leaf spectral characteristics, or leaf area index are similarly complex to quantify and interpret, particularly in real time. Despite significant advances in plant phenotyping in recent years, automated interpretation of such traits remains difficult and expensive, especially for use in closed-loop cultivation control [
6,
7].
Among plant-related variables, fresh and dry biomass (often just called weight) remain the most prevalent indicators in CEA research and practice and serve as the main comparative variables in many experimental and simulative studies [
8,
9,
10,
11,
12]. They are closely linked to plant metabolism and serve as direct proxies for growth and productivity. Consequently, they are typically one of the crucial states in plant growth models [
13,
14,
15]. Moreover, fresh biomass is a critical commercial parameter, as many crops are sold by weight or unit with target mass specifications [
12]. Compared with conventional greenhouse systems, vertical farming enables more dynamic and data-intensive cultivation strategies. These approaches depend on the continuous monitoring of relevant plant metrics—among which biomass plays a central role—as a means of assessing crop responses to variable environmental conditions. As parameters such as light quality, climate profiles, and irrigation schemes become increasingly dynamic and crop specific, real-time biomass monitoring becomes not only beneficial but essential [
1]. Ideally, fresh biomass should be measured non-destructively, enabling feedback without interrupting growth. Such measurements provide insights into plant–environment interactions and support the development and calibration of growth and response models, which in turn inform improved control and breeding strategies.
To detect subtle changes in growth dynamics—often missed by conventional snapshot-based measurements—biomass data must be collected continuously at an appropriate temporal resolution. The optimal frequency depends on crop type and cultivation regime, but in general, biomass accumulation occurs more slowly than shifts in environmental inputs [
16]. However, since fresh biomass is influenced by plant water content, which can fluctuate faster in response to environmental changes, it is important to capture this dynamic component as well [
17]. Furthermore, continuous biomass feedback is critical for advanced control strategies such as model predictive control (MPC), which rely on regular, accurate plant-state information to maintain optimal conditions. Recent studies on MPC and deep neural network-based optimal control of CEA systems assume full-state knowledge of fresh or dry biomass to design and evaluate their control strategies [
16,
18,
19]. Without direct biomass measurements or reliable proxies, these control loops lack essential input [
13]. This need becomes even more pronounced if single production systems evolve to support modular configurations involving multiple crop stages, lighting strategies, and environmental regimes. Even in tightly controlled vertical farms, spatial variability is frequently observed caused by factors such as system geometry and airflow [
20,
21]. Thus, granular, location-specific biomass monitoring is fundamental to enabling next-generation cultivation strategies and compensating for inhomogeneities through targeted, localized control.
Several approaches exist to measure or estimate fresh biomass in controlled environments. The most traditional method is manual harvesting and weighing of plants. While this provides accurate ground truth data for fresh and dry weight, it is destructive, time-consuming, and unsuitable for continuous or large-scale applications. Consequently, non-destructive alternatives have become a central focus, particularly for deployment in dynamic production settings.
One prominent approach is image-based biomass estimation, often employing computer vision (CV) and deep learning (DL) techniques [
10,
12,
22,
23,
24,
25]. These models infer biomass from image-derived features, using manually labeled data for training and validation. For instance, ref. [
12] highlighted the importance of reliable ground truth data in the Autonomous Greenhouse Challenge, where plant spacing and harvest outcomes were evaluated against fresh weight benchmarks. However, the performance of such models may degrade under changing conditions. Variations in lighting spectra, plant morphology, or environmental regimes can significantly alter crop appearance, reducing the generalizability of trained models [
25]. The potential for enhancing the detection accuracy of machine learning methods in the face of environmental variations is a promising avenue for research [
26,
27]. These approaches have the potential to facilitate biomass determination and reduce the amount of training data required.
An alternative is the use of load cells, which directly and continuously measure plant weight in a non-destructive manner. These systems require no model training or image processing and offer high temporal resolution. They serve not only as effective monitoring tools in dynamic environments but also as sources of ground truth data for validating or calibrating vision-based models. Prior research has demonstrated the utility of load cell systems primarily in greenhouse-based horticultural studies. For example, ref. [
28] developed an online monitoring system for Gerbera cultivation that linked plant weight and environmental data. Ref. [
29] introduced the product CropAssist for automated monitoring in tomato cultivation, which was later used in [
17] to generate training data for neural networks, using load cell outputs as reference values. This system is designed for individual plants that climb up a support structure like a rope (e.g., tomato, vine). Ref. [
8] presented a continuous weighing system for plant factories, emphasizing its effectiveness in tracking growth trends over time. It also measures the growth of individual plants, but is suitable for leafy greens like lettuce. Beyond academic research, commercial implementations, such as systems capable of weighing entire production racks [
30], demonstrate growing industrial interest.
Apart from real-time monitoring, load cell systems also enhance model robustness by serving as dynamic ground truth sources for CV-based biomass estimation. As demonstrated in [
8], integrating such systems enables automated training or validation of DL models in response to changing conditions in lighting, genotype, or environmental control. This integration supports the development of adaptive, scalable modeling pipelines suitable for future high-throughput CEA systems [
25].
In this paper, we propose and test a low-cost, load-cell-based biomass monitoring system with material costs of approximately EUR 40 per tray if used in a large-scale setup. The material costs for the experimental setup used in this study cost EUR 65 per tray. Details about the components can be found in
Appendix A,
Table A1. We also present data processing algorithms to compensate for noise, temperature drift, and other environmental disturbances. The system was validated against manual measurements in several experimental campaigns growing lettuce from seedlings to harvest. Unlike previously discussed approaches, the proposed system is specifically designed for integration into dynamic, large-scale vertical farming operations (see
Table 1). It operates at the level of individual growing trays—a key production unit in many shelf-based hydroponic systems, where light and other inputs can typically be controlled per tray. This granularity enables localized diagnostics (e.g., anomaly detection) and targeted control strategies, such as layer-specific lighting. A central goal of the system is affordability and ease of integration into existing infrastructure, addressing key barriers to widespread adoption. Although demonstrated in a small-scale vertical farm, the simplicity of the underlying principle makes the system broadly adaptable to most shelf-based hydroponic setups. This is particularly relevant for both commercial producers and research facilities, where budgets and retrofitting flexibility often limit the implementation of additional sensors. Scaling low-cost load cell deployment introduces challenges such as mechanical noise, thermal drift, and sensitivity to vibrations. Inexpensive sensors often lack built-in temperature compensation or self-calibration. We addressed these issues at the data processing level, developing compensation algorithms that incorporate existing environmental measurements already available in most vertical farms. Designing robust calibration, filtering, and compensation strategies is therefore a key contribution of this work.
The rest of the paper is organized as follows:
Section 2 outlines the materials and methods, including the experimental setup, data processing, and all experimental steps before and during the growth experiments.
Section 3 presents the experimental results achieved with the proposed low-cost setup. The results are discussed in
Section 4, along with necessary adjustments for larger implementation and future directions. Finally,
Section 5 provides the conclusion of this paper.
2. Materials and Methods
2.1. Experimental Setup
This study involved growth experiments conducted in a laboratory-scale hydroponic indoor vertical farming (IVF) system, as described in
Section 2.1.1. An integrated system for sectional weighing of multiple plants was incorporated into this setup and is detailed in
Section 2.1.2.
2.1.1. Vertical Farm Setup
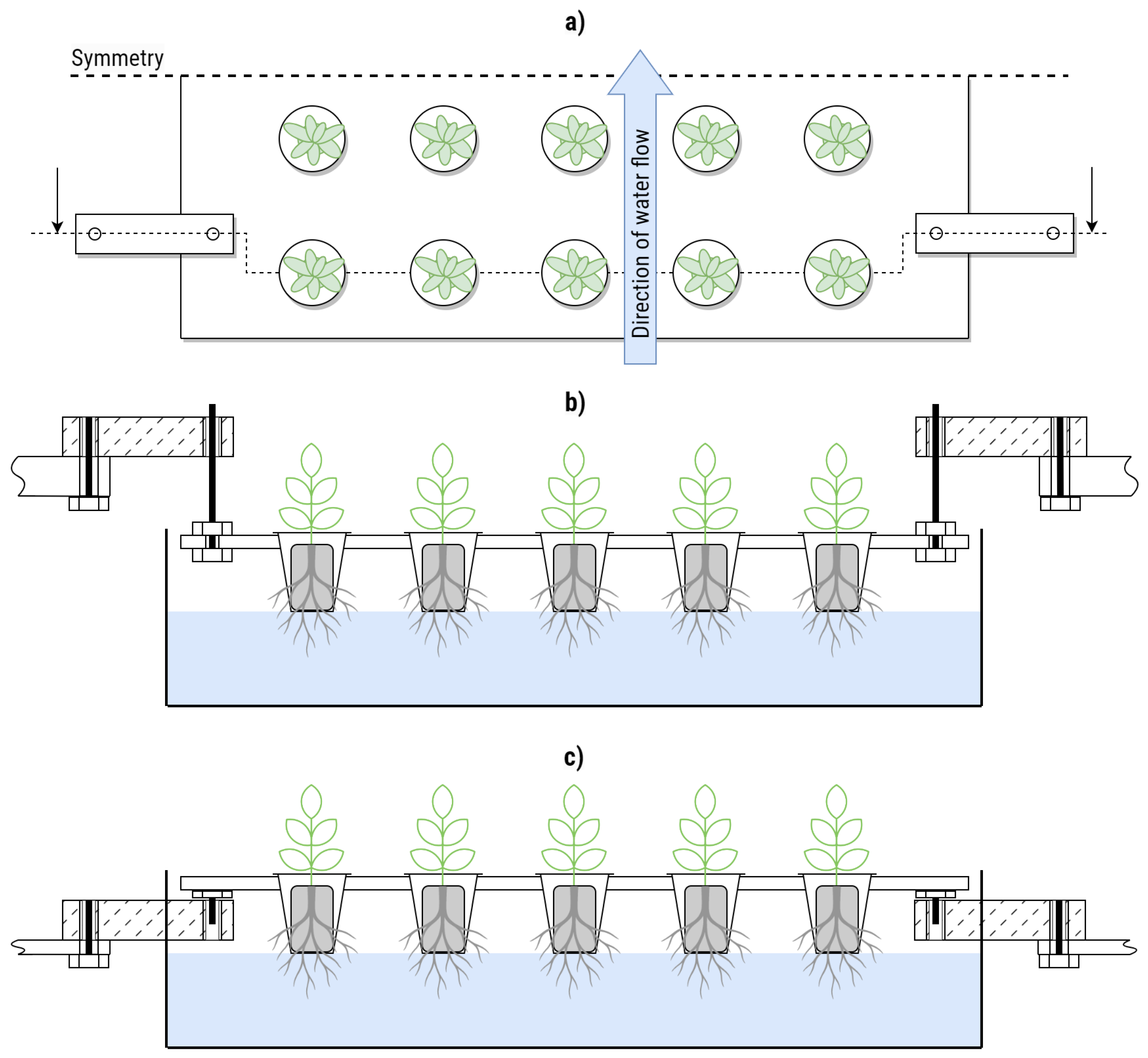
The vertical farming system used in this study is the GreenResearcher (greenhub solutions GmbH, Leipzig, Germany), a two-tier cultivation unit. Each tier consists of three adjacent cultivation trays, each measuring 40 cm by 60 cm. In the configuration used here, each tray accommodates 20 net pots for growing lettuce plants. The plants are cultivated in rockwool substrate (Rockwool B.V. GRODAN, Roermond, The Netherlands). During normal operation, the trays rest on the edges of a basin through which a hydroponic nutrient solution flows. The water level reaches just below the net pots and maintains a depth of approximately 4.5 cm. The system controls pH and nutrients by automatically mixing stock solutions based on pH and electrical conductivity (EC) measurements. The GreenResearcher system is housed within a climate-controlled growth tent (Mars Hydro, Shenzhen, China) measuring 1.5 m × 1.5 m × 2.5 m. Temperature and humidity are monitored at multiple locations inside the growth tent. Climate control is provided by a portable air conditioning unit (KLIMATRONIC TRANSFORM 12000 Eco R290, Suntec Wellness GmbH, Berlin, Germany).
2.1.2. Load Cell Setup
A total of four bending beam load cells were installed per tray. They were anchored on steel brackets on the fixed side and connected to the trays with custom cut threaded rods on the weighing side.
Figure 1 shows the setup per tray. The threaded rods were used to adjust the height during initial installation so that the tray would float above its usual mounting surface. This ensured that all force would be directed through the load cells. The same setup was installed for the three trays spanning one level of the experimental IVF (see
Figure 2).
2.2. Load Cell
Load cells are devices used to measure force and torque and are widely employed in various applications, most commonly for weight measurement [
32,
33,
34]. They are low-cost sensors with a simple mechanical structure and can provide accurate force readings when properly designed and calibrated. There are several types of load cells, including strain gauge, hydraulic, and pneumatic load cells [
35] (Chapter 7). While their implementations differ, they all operate on the same fundamental principle: the conversion of mechanical force into a detectable electrical signal that can be measured.
One of the most commonly used types—also used in this work—is the strain gauge load cell. When a load is applied to the load cell, it induces a slight deformation in its metal structure. Four strain gauges bonded to the structure experience this deformation: some undergo tension (elongation), while others undergo compression, leading to a change in their electrical resistance. These strain gauges are connected in a Wheatstone bridge configuration, which translates the resistance changes into a measurable output voltage given by
where
is a known excitation voltage,
R is the nominal resistance of the strain gauges, and
to
represent the resistance changes of the four strain gauges due to deformation.
For small changes in resistance (i.e.,
), the output voltage can be approximated using a linear equation. Assuming a symmetric configuration where
and
, Equation (
1) simplifies to
Under no-load conditions, the strain gauges maintain their nominal resistance, resulting in zero output voltage. When a load is applied within the linear operating region of the sensor, the change in resistance—and hence the output voltage—increases linearly with the applied force. This output voltage is then processed to determine the magnitude of the weight, allowing for an accurate measurement of the applied load.
Load Cell Calibration
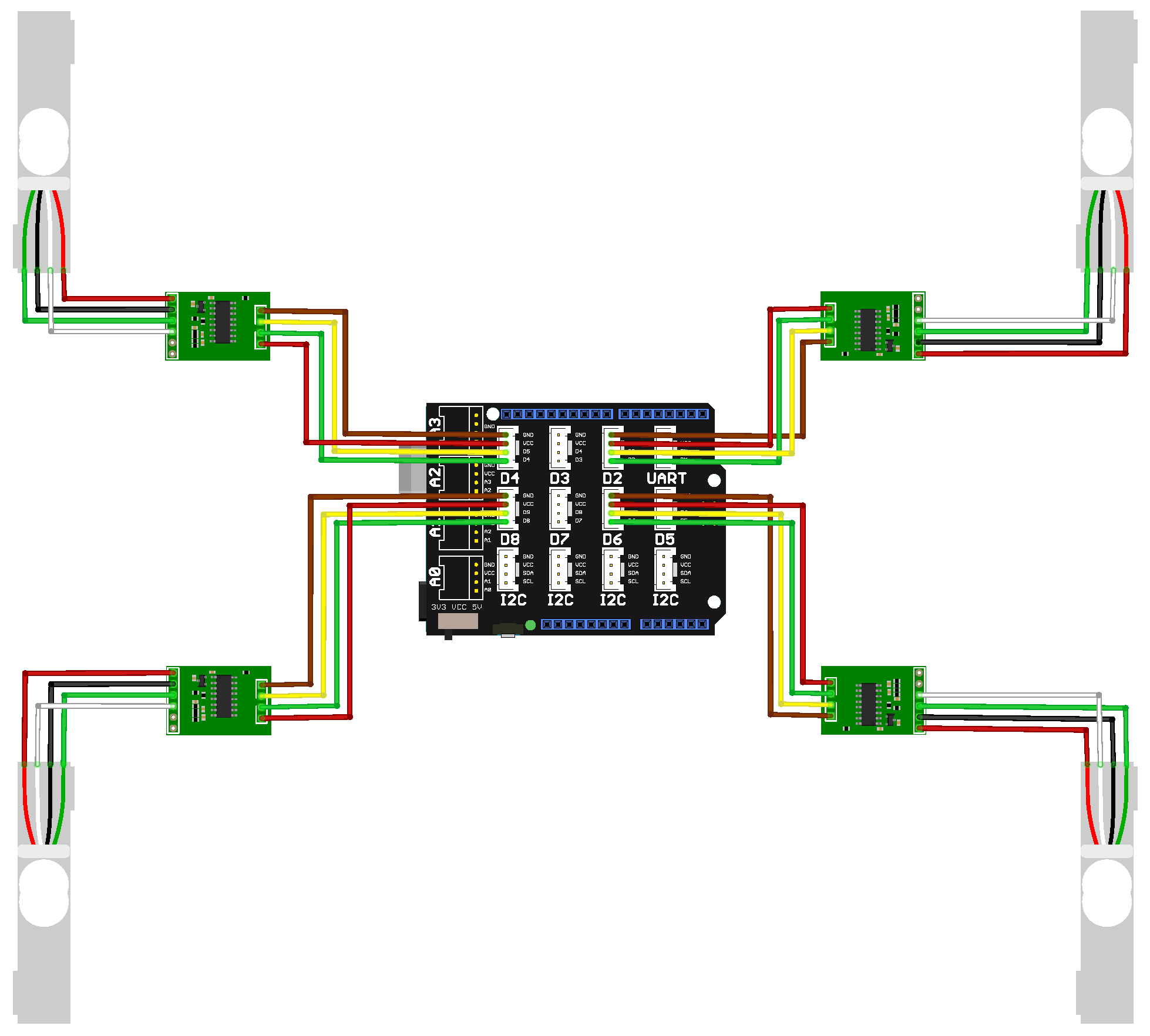
Each load cell was interfaced with the HX711 (Joy-IT (Simac Electronics GmbH), Kürten, Germany)analog-to-digital converter module, which was in turn connected to an Arduino Uno (Arduino, Monza, Italy) equipped with a Grove Base Shield(Seeed Technology Co., Ltd. (Seeed Studio), Shenzhen, Guangdong, China). The Grove Base Shield facilitates easy integration of various sensors and provides a distributed power supply.
Calibration of the load cells is necessary to ensure accurate weight measurements. The calibration procedure involves finding a calibration factor to convert the measured voltage difference into weight in . This involves placing a known weight, preferably half of the maximum payload specified by the sensor, on each load cell and recording the corresponding output to determine the sensitivity of the sensor. This process was carried out individually for each load cell, and the calibration factors were collected accordingly. After the calibration factor is set, zero/tare calibration must be performed on the final mounted setup.
In the hydroponic farm setup, a group of four load cells was connected, as shown in
Figure 3. A total of three load cell setups were integrated into the vertical farming system, as illustrated in
Figure 2. Each setup consisted of four calibrated load cells supporting an empty plant tray. Since the trays have their own inherent weight, a zero-offset calibration was performed for each setup. This involved recording the measured output from each load cell while the tray was empty and storing that value as a baseline. During the experiment, these baseline values were subtracted from the real-time readings to ensure that only the weight of the crops was measured.
2.3. Data Processing
2.3.1. Data Collection
The values of the four load cells
were recorded at 15 s intervals and stored in a database. Given the relatively slow rate of biomass growth, a measurement frequency of approximately 60 min for a biomass growth sensor is usually sufficient. However, a significantly higher measurement frequency enables the identification and compensation of undesired effects during operation and disturbances without imposing any drawbacks, since measurements with the proposed setup are very cheap. The raw sampling interval is chosen to be significantly higher than the biomass growth rate in order to detect and compensate for short-term disturbances. The design criteria for post-processing filter frequency are discussed in
Section 2.3.5, with a quantitative justification provided in
Section 3.1.
2.3.2. Data Cleaning and Outlier Detection
There were outliers in the recorded data as a result of voltage fluctuations or short-term loads on the system, such as when manually measuring the plants or during maintenance of the IVF. These outliers were cleaned using a Hampel Filter [
36] with a window of 150 s.
2.3.3. Temperature Correction
Load cells demonstrate a certain degree of temperature dependency [
37]. Despite manufacturers’ attempts to mitigate temperature effects through load cell compensation across a temperature range, recalibration remains a necessity, particularly for cost-effective models [
38]. Since the temperature calibration is conducted after the load cells are installed, it is also possible to compensate for the effects of temperature on the suspension and trays. Preliminary tests have shown that linear compensation of the sensors is sufficient. The temperature-corrected sensor value is given by
where
is the sensor output,
is the temperature-corrected output,
is the reference temperature at the time of the zero calibration, and
and
are the parameters of the correction model. The variables are summarized in
Table 2.
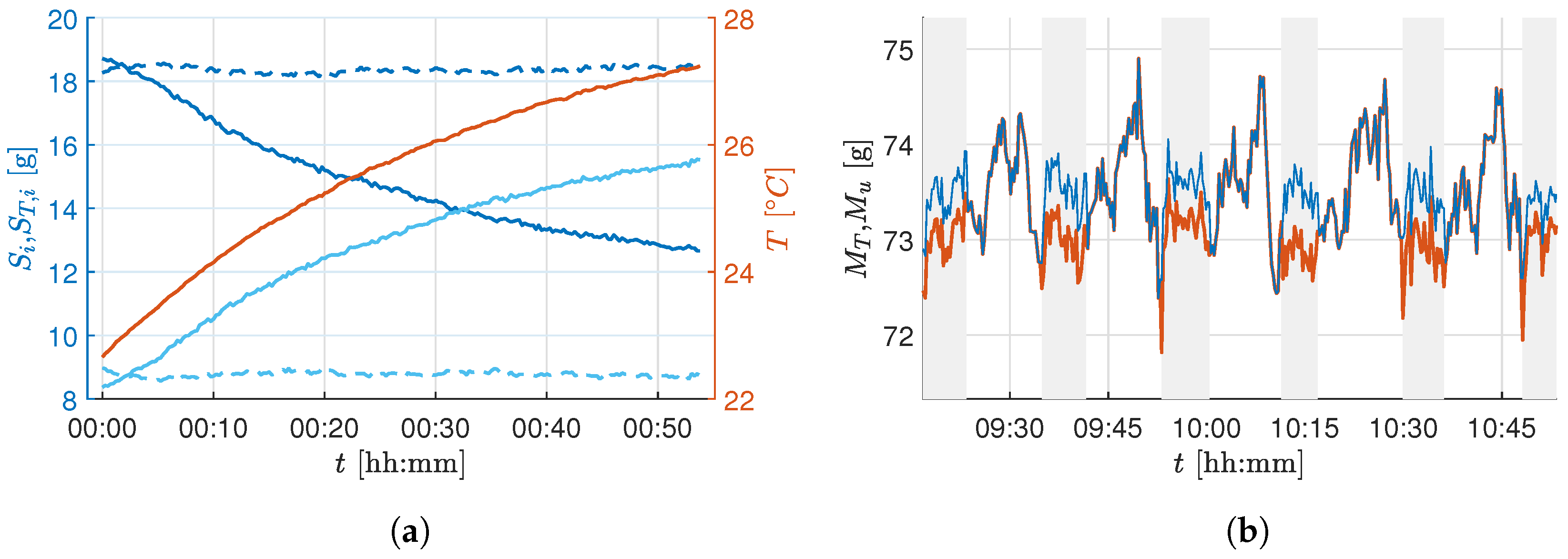
To estimate the necessary parameters, a calibration procedure was performed in which a series of load cells with a constant mass were taken over the temperature curve at which the growth chamber operates. The parameters
and
were estimated by fitting a linear regression model, resulting in an
for the first load cell.
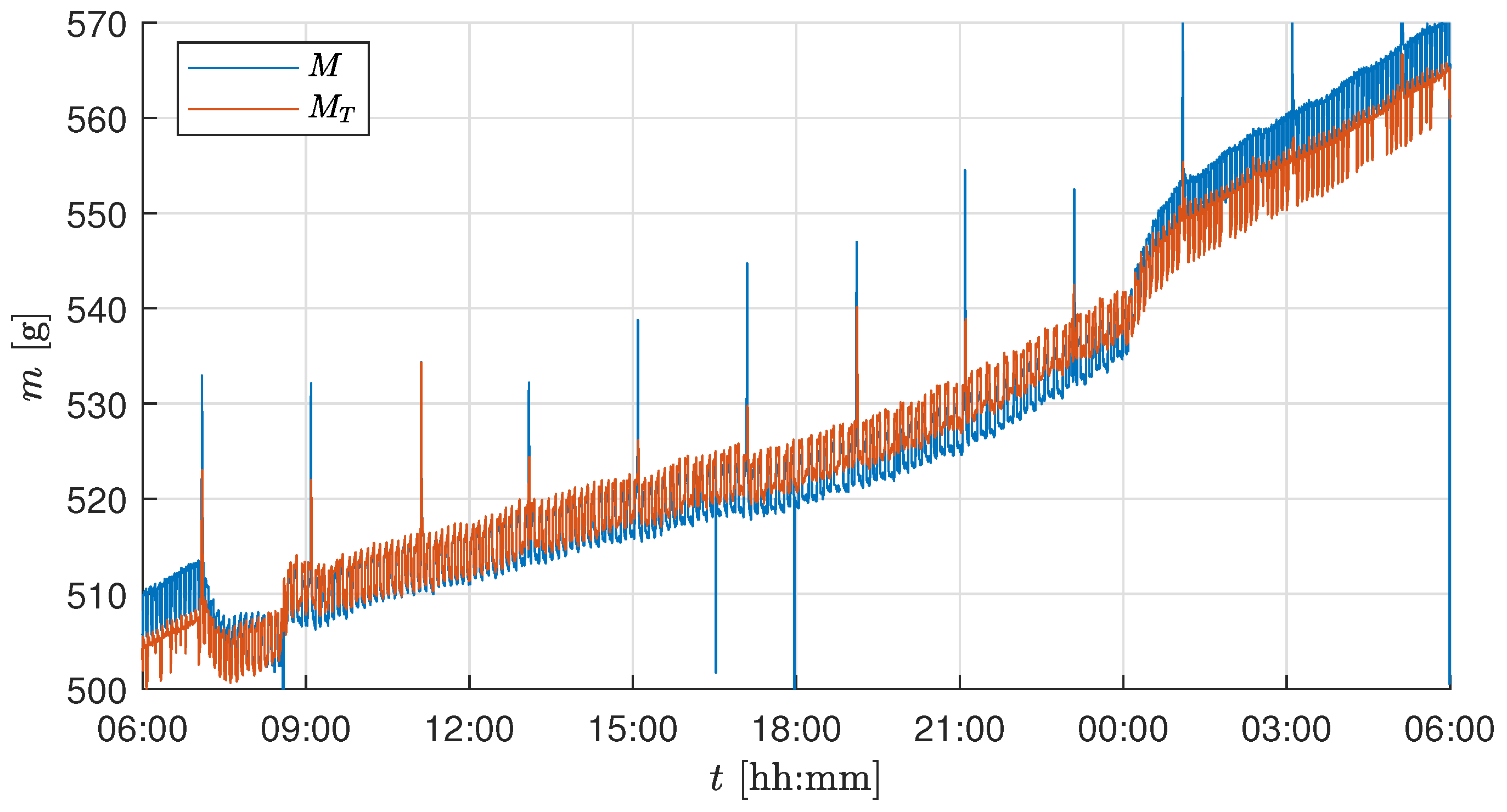
Figure 4a shows the change of two load cell outputs during this experiment. This drift can be corrected by applying the temperature correction in (
3). The dashed lines show the corrected values
. The mass of the whole tray is the sum of all load cell outputs
.
2.3.4. Actuator Compensation
Actuators such as pumps, fans, or the climate control system can interfere with sensors due to vibrations or pressure changes. If an actuator is recorded, a correlation analysis between the actuator signal
u and the load cell measurements
M can reveal such a relationship. The correlation coefficient is calculated as
where
and
are the respective average values.
ranges from −1 to 1, with positive values denoting positive correlation and negative values denoting negative correlation. In the setup under consideration, there is a correlation coefficient of
between the activity of the climate control system and the sensor output.
The change in the sensor value between active
and inactive
climate control systems is calculated by
which yields the corrected sensor value
After the actuator correction, the correlation coefficient reduces to
, which shows a successful compensation.
Figure 4b shows the sensor output before and after the output correction. Note that, with active climate control systems, the biomass of the plants is measured too low. Since disturbances in the setup, such as a harvest or changes to the actuators, can change
, an automatic estimate of this parameter is carried out regularly during operation.
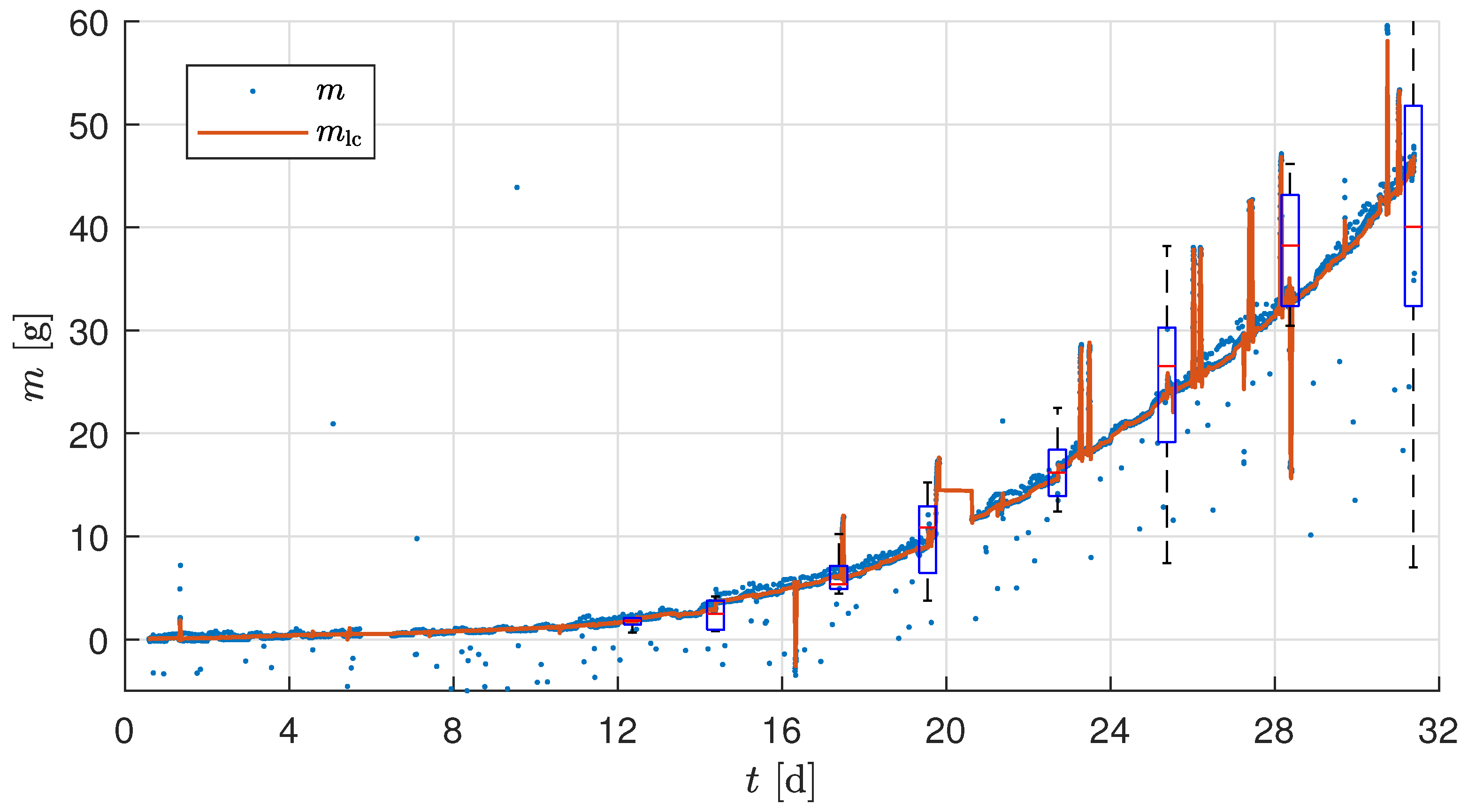
2.3.5. Measurement Noise Compensation
The temperature- and actuator-corrected mass
is corrupted by high-frequency measurement noise, as seen in
Figure 4. To handle this, a low-pass Butterworth filter of order four was chosen. The frequency response of a small segment of the raw experimental sensor value was analyzed based on the amplitude spectrum of the compensated sensor value to identify the cutoff frequency for the filter. The choice of cutoff frequency depends on the minimum detectable growth increment relevant to the crop growth being measured and the attainable accuracy through the installed load cells. The installed load cells’ measurement range is
with an absolute accuracy of
of full scale. Thus, the accuracy of a single load cell is
, and the summed accuracy of the four load cells in the developed biomass sensor setup is
.
Figure 5 shows the raw sensor value
M, the temperature and actuator-compensated sensor value
, and the final filtered sensor value
using an low-pass filter (LPF) with a cutoff frequency of 0.025 min
−1 for a constant known weight being applied on the tray.
The selected cutoff frequency of 0.025 min
−1 (corresponding to a 40 min time constant) ensures that biomass changes exceeding the accuracy limit of the load cell subsystem (
for the whole tray) can be reliably distinguished from high-frequency measurement noise. The accuracy of
refers solely to the load cells’ combined measurement of the raw tray-level mass. It does not account for additional uncertainties introduced by temperature and actuator compensation, substrate correction, and division by plant count. The fixed cutoff ensures sensitivity to relevant changes without amplifying noise. A more detailed explanation of the relationship between load cell accuracy, the number of plants, and filter selection is provided in
Section 3.1, while the harvesting strategy used during the experiments is described in
Section 2.6.
2.3.6. Calculating the Mass per Plant
The wet rockwool plugs constitute part of the mass measured by the load cells. However, an initial compensation is insufficient because the number of plants, and therefore the amount of substrate, can change over the course of the growing period. For example, diseased plants may be removed, or plants may be thinned out for space reasons. At the start of a trial, the average mass of a wet rockwool plug
is calculated by dividing the load cell measurement by the number of plants. The mass of the seedlings can be neglected. As the mass of substrate per plant is now known, a correction can still be applied if the number of plants is changed during a harvest. However, the number of plants must be recorded. The mass per plant is then calculated as
2.4. Installation and Calibration Procedure of the Biomass Sensor
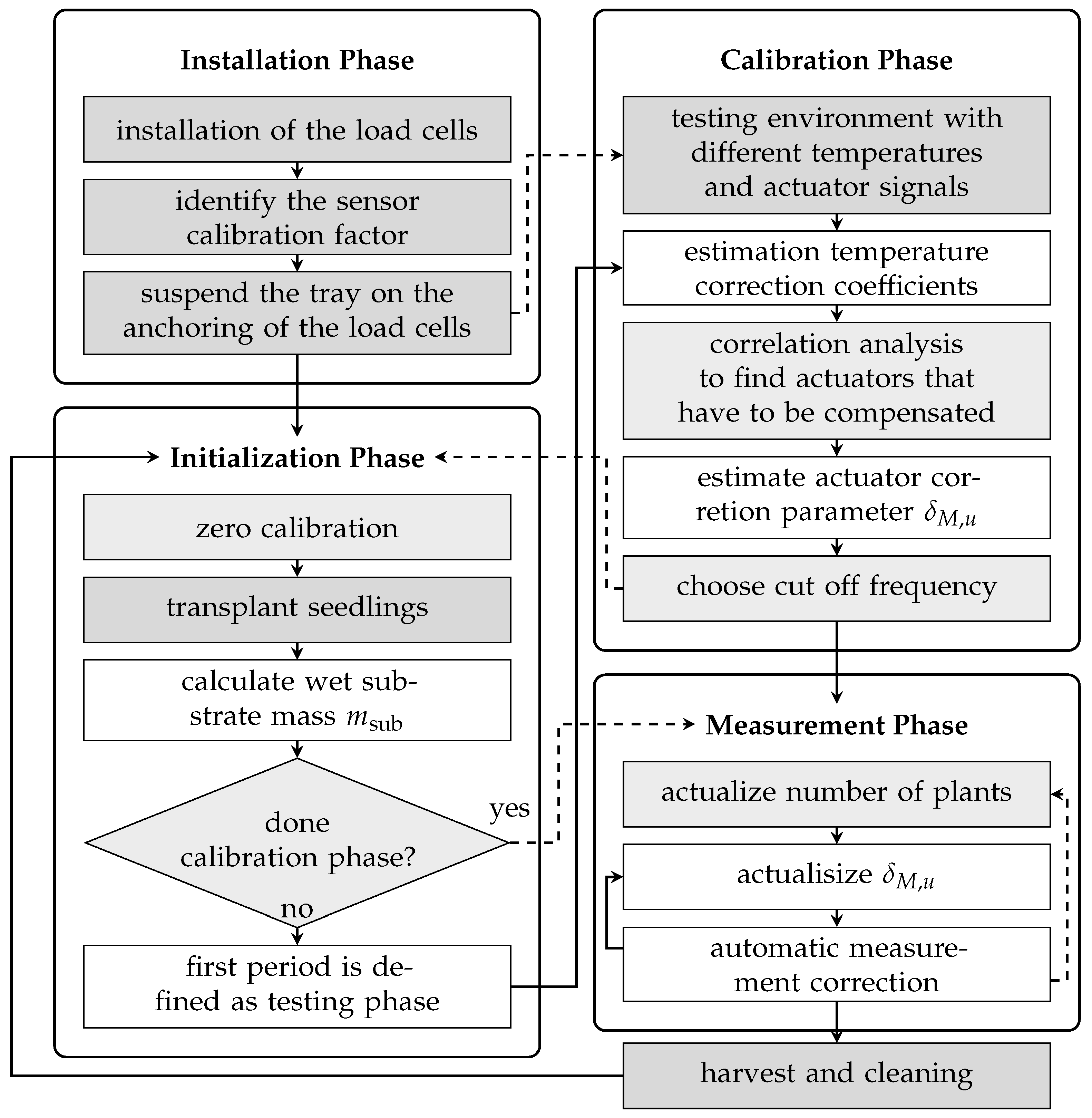
The installation and operation procedure of the biomass sensor setup in any hydroponic-based vertical farm system is shown in
Figure 6. It can be divided into four phases. The installation phase is a one-time undertaking encompassing the mechanical and electrical configuration of the system, as well as the sensor calibration with known weights, as elaborated in
Section 2.1.2 and
Section 2.2. For each new trial, the setup configuration steps are carried out, as described in
Section 2.3. The subsequent phase, initialization, entails the transplantation of the plants and as the estimation of the masses of non-plant components, a prerequisite for precise biomass determination. In the third phase, calibration is performed to estimate parameters for temperature compensation and to identify actuators that may introduce disturbances to the load cell output. It is also possible to execute this calibration step before the initialization phase using a constant weight and a calibration routine with different temperature and actuator trajectories (dashed arrows in the flow diagram). During continuous operation, measurements are recorded alongside automated recalibration routines that compensate for actuator-induced disturbances. In order to calculate the mass per plant and to ensure that the substrate mass is appropriately compensated, it is necessary to record the number of plants. A manual log of the harvests was kept for this purpose. After the successful installation, calibration, and software compensation of the sensor, continuous measurements of biomass growth are possible.
2.5. Cultivation Conditions and Manual Mass Measurement
This section describes the cultivation conditions and execution of manual reference measurements for experiments testing and validating the presented method. For all experiments, seeds from the green oak-leaf lettuce variety Salanova® Cousteau (Lactuca sativa) were used (Rijk Zwaan Welver GmbH, Welver, Germany). The seeds were planted into the pre-treated rockwool plugs in plug trays and then incubated in a separate growth tent (Secret Jardin, Manage, Belgium) for 10 days. Each day within the growth tent, there was a 16 h light period and 8 h dark period. As the growth tent was used for seed germination for multiple experiments, many of which ran in parallel and studied different plant species, the photoperiod of the growth tent was selected to balance the disparate needs of differing plant species. As the germination period was not the focus of this work and changes in biomass were not monitored during germination, the variation in the photoperiods between the growth tent and the IVF had a negligible impact on the results. The temperature ranged between 21.9 °c and 25.9 °C with a range in relative humidity from to . The rockwool was kept moist with tap water manually.
After 10 days in the growth tent, the germinated seeds in their rockwool plugs were placed in net pots and then transplanted to the hydroponic IVF (greenhub solutions GmbH, Leipzig, Germany). A growth level consists of 3 trays, each containing 20 slots. For all trials, the temperature was maintained at 20 °C and the pH value was kept at 6.0 using Terra Aquatica pH- (Terra Aquatica, Fleurance, France) diluted with DI water in a 50:50 ratio. The daily light period during the trials was 17 h on, with a constant photosynthetic photon flux density of 270 μmol m−2 s−1, and 7 h off. Trials were ended and all samples were harvested after the plants had spent 32 days in the units, for a total growth period of at least 42 days, including the germination period.
During each trial, up to 15% of the plants were randomly selected to be manually weighed once per day throughout the duration of the trial. For these measurements, the plant and the rockwool plug were weighed inside their net pot (Pöppelmann GmbH & Co. KG, Lohne, Germany) in order to prevent root damage, and placed back in the hydroponic system after the measurement. These instances of measurements were referred to as manual measurement points. At the harvest points, the wet biomass of the whole plant with and without the rockwool plug was recorded. Subsequently, the roots were separated from the shoot system, and the wet biomass of the root and shoot systems was then recorded separately. For selected samples, the dry weight was also recorded. These samples were placed in a drying oven (BINDER GmbH, Tuttlingen, Germany) at 65 °C for 24 h before being weighed. For each of the selected samples, the dry weight was recorded for the whole plant without the rock wool plug, the shoot system, and the roots.
2.6. Experiment Trial Design
As discussed in
Section 2.4, the biomass sensor was installed, calibrated, and software-compensated to measure the biomass growth continuously. To test and validate the proposed biomass sensor, experiments were conducted to measure the biomass of the plants manually as explained in
Section 2.5 from Day 1 to Day 32 in the vertical farming system. Here, the germination growth period (the initial 10 days of the total 42 days) is of no interest, as biomass is measured only after transplantation in the vertical farming units.
Two experiments were performed. In the first, called the test experiment, two plants were harvested every 2 to 3 days from each tray, starting from the 12th day in the IVF until the end of the growth cycle (see
Table 3). This was performed to obtain regular wet and dry biomass measurements throughout the trial for the comparison of the load cell output with the actual mass. Additionally, two plants were selected in each tray for daily weighing. The plants that were weighed daily were not harvested until the end of the trial. Due to the stress induced by frequent harvests in addition to the daily manual measurements, the growth of all plants in the tray was not uniform during the test experiment.
Therefore, a second experiment, called the validation experiment, was performed on the same level of the IVF as in the test experiment, in which the plants were harvested less often in order to minimize stress effects on the plants. As during the test experiment, two plants per tray were randomly selected for daily manual measurement. However, the frequency of mid-trial harvests was drastically reduced. Only 10 plants were removed on Day 18 (mid-growth period) to create space for the remaining 10 plants to grow properly. The intermediate harvest also allowed for an evaluation of the dry mass for a larger number of plants after half of the cultivation time. As the manual measurements and the harvested plant measurements were recorded for individual plants, the testing and validation of the results were performed on the mass per plant
and
. Although the entire plant was weighed, only the leaf biomass was of interest. The masses of the substrate
(measured at the start of the growth cycle) and the basket
were subtracted, but the mass of the roots could not be determined. To estimate the leaf mass of the manual measurements, a linear factor
was estimated based on the harvest measurements of the test experiment. This factor represents the ratio of the leaf mass to the total mass of a plant, and is given by
where
is the mean of the leaf mass and
is the mean of the root mass of the harvested plants. Additionally, the sample size of the manual measurements was too small to draw conclusions about the entire tray, as the masses of the individual plants varied greatly. The ratio of the plant mass of the sample to the average total mass per plant was calculated on the harvest of the validation trial as follows:
where
is the mean of the manual measurement at the harvest time. Using the above factor, the wet leaf biomass was estimated for the manual measurement
, resulting in the adjusted manual measurement