Magnetic Induction Spectroscopy-Based Non-Contact Assessment of Avocado Fruit Condition
Abstract
1. Introduction
2. Methodology
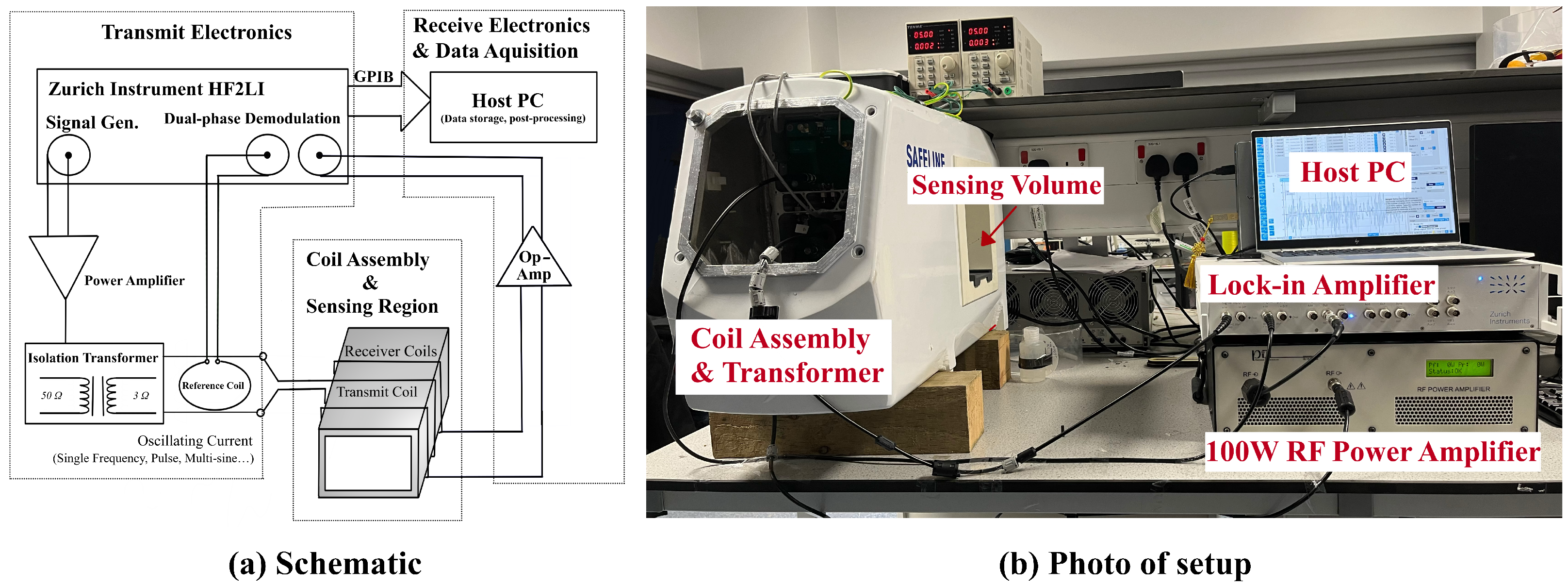
2.1. Magnetic Induction Spectroscopy System
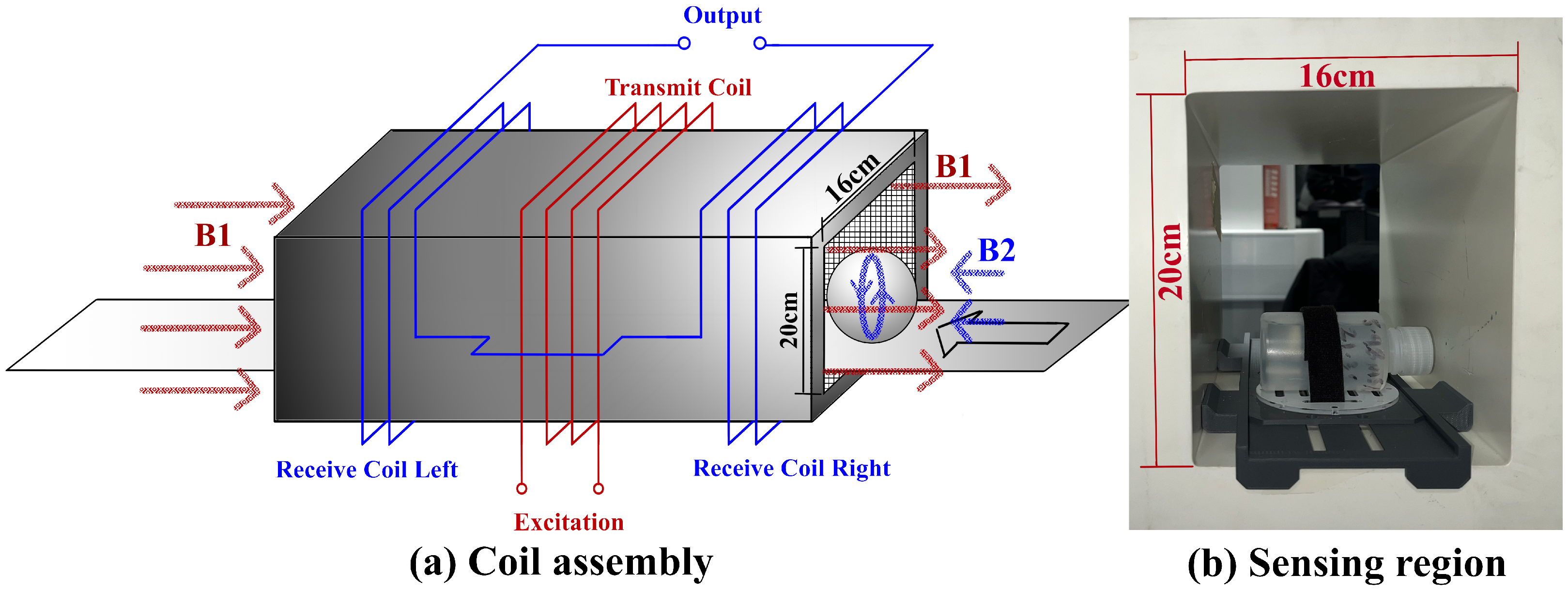
2.1.1. Coil Assembly
2.1.2. Transmit Electronics
2.1.3. Receive Electronics and Data Acquisition
2.2. Background Theory
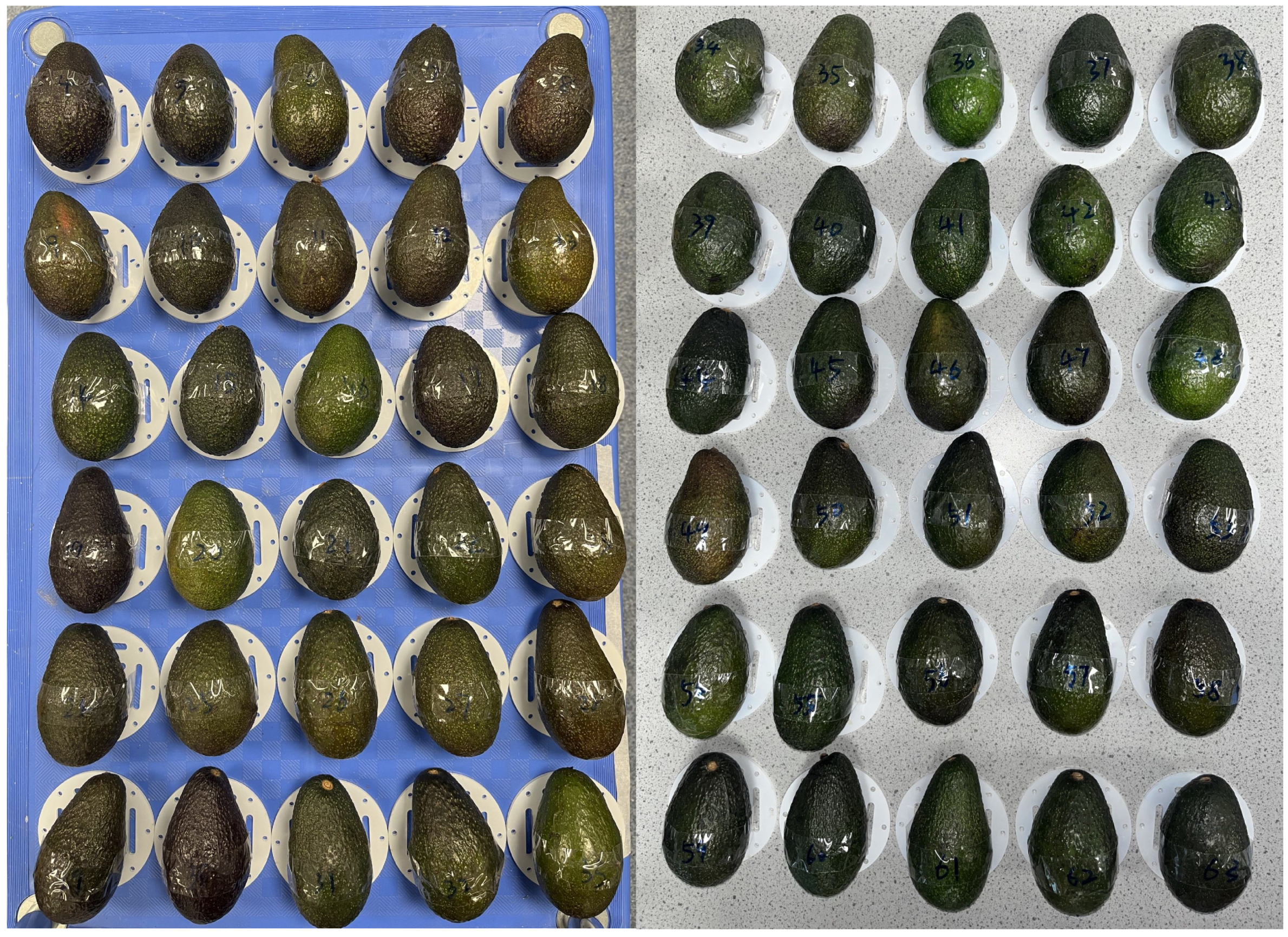
2.3. Sample Preparation
2.4. Measurement Protocol and Data Processing
- Background Scan: Despite the balanced differential design of the receive coil pair, residual voltage signals caused by direct excitation coupling may still persist. To compensate for this, a background scan is conducted during system initialization and between sample batches to account for sensor drift. This baseline measurement is obtained by scanning an empty sensing region.
- Phase Calibration: Phase shifts introduced by the system, including those from amplifiers, shielding effects [26], and coil geometry, distort the response defined in Equation (7). To correct for these phase artifacts, a non-conductive magnetically permeable ferrite rod (4 mm diameter, 16 mm length; 4B1, Ferroxcube) is scanned as a reference object for phase calibration.
- Magnitude Calibration: As indicated in (7), the response magnitude includes a constant scaling factor P, which accounts for the system’s geometric configuration. To determine this factor, a 150 mL saline solution with a conductivity of 9.92 mS/cm—selected to resemble that of the target biological sample—is used for absolute conductivity calibration.
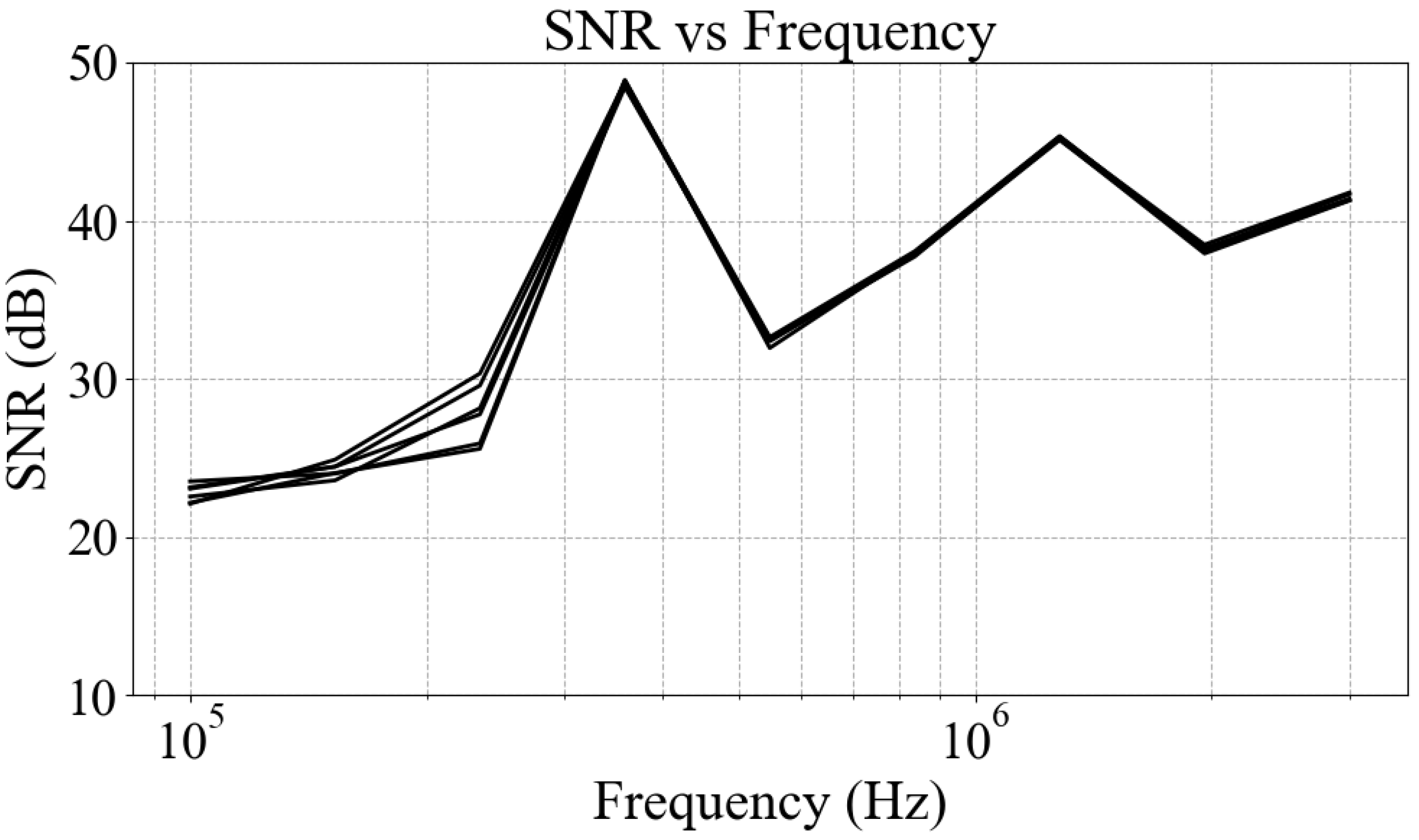
2.5. System Performance
3. Results and Discussion
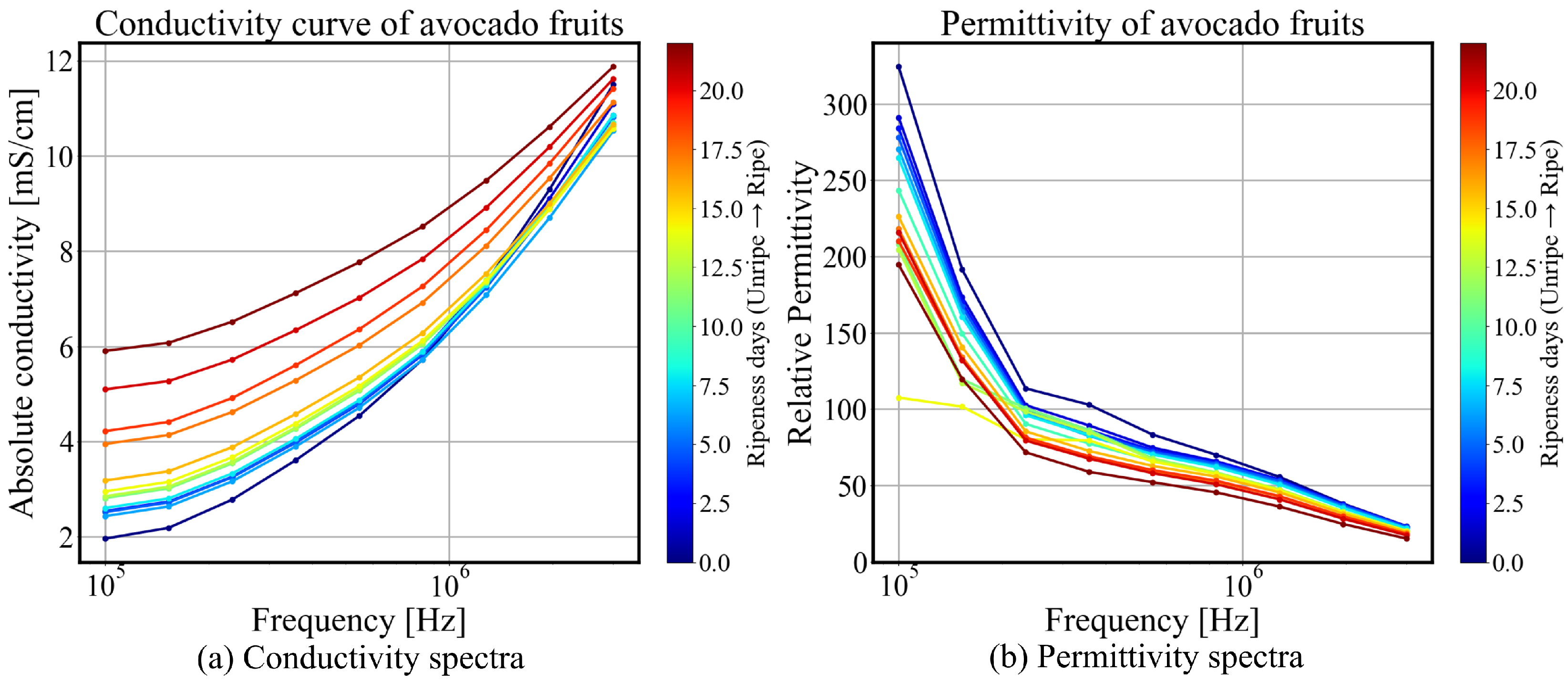
3.1. Bioimpedance and Permittivity Spectroscopy
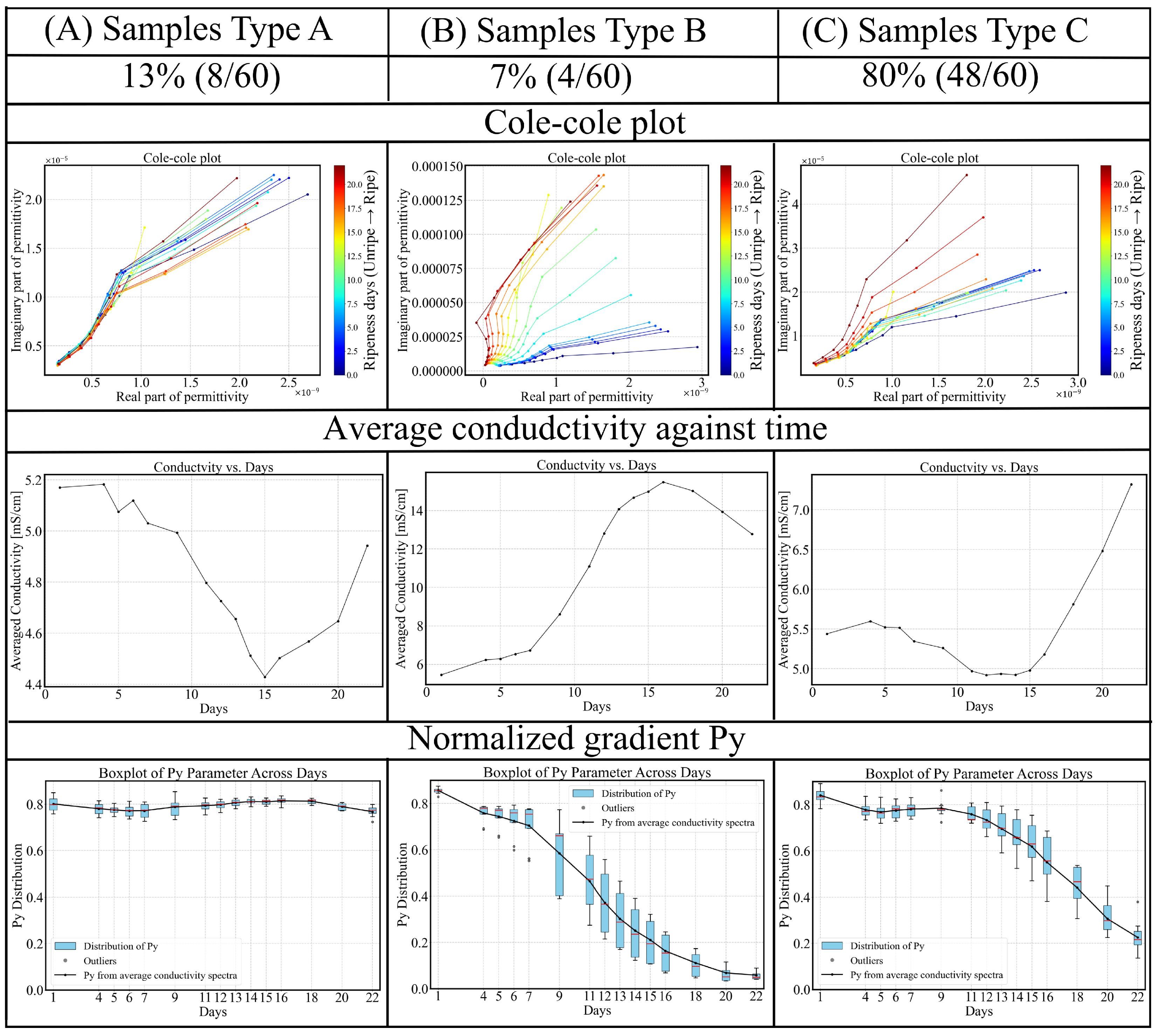
3.2. Quantification and Correlation of Ripening and Statistical Analysis
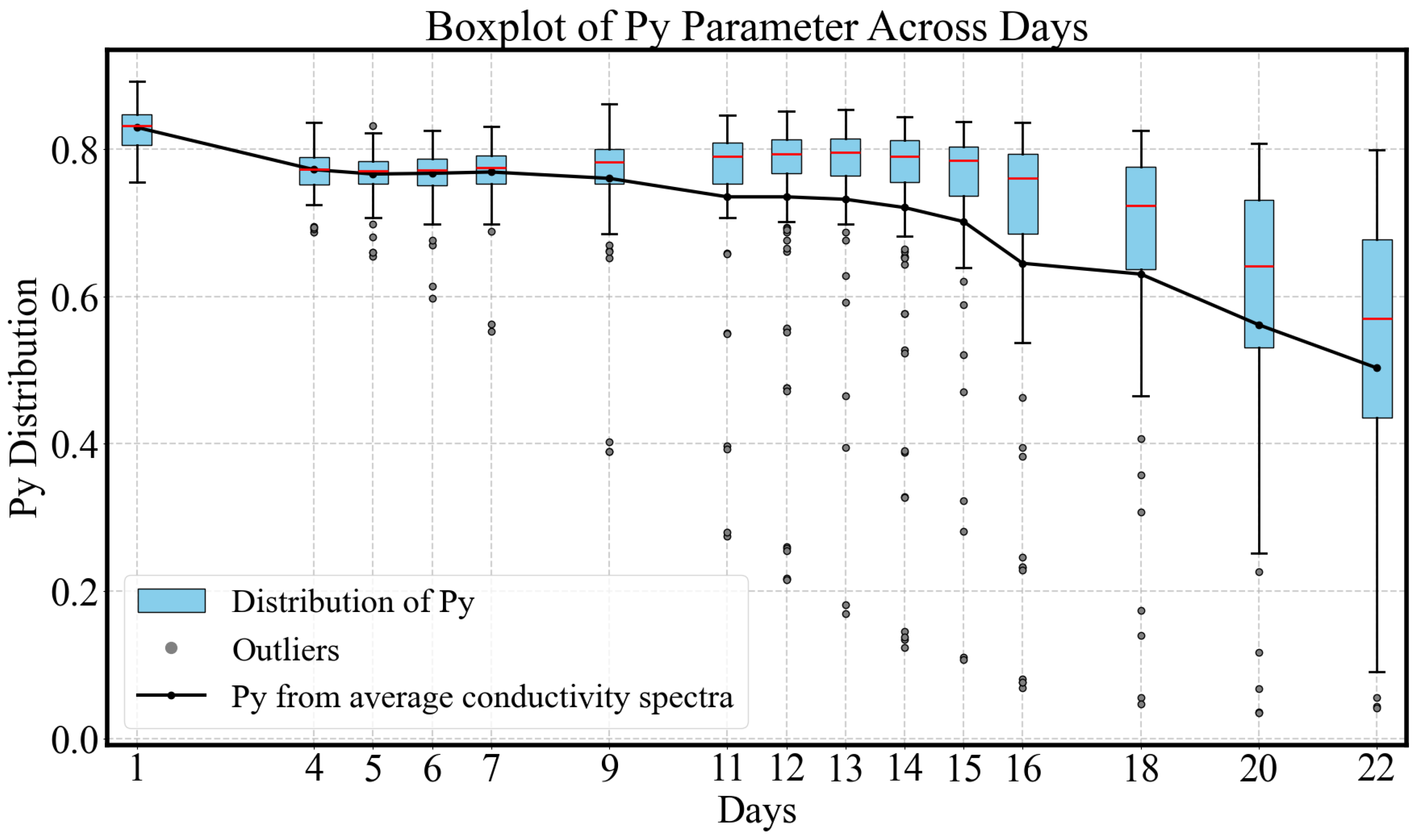
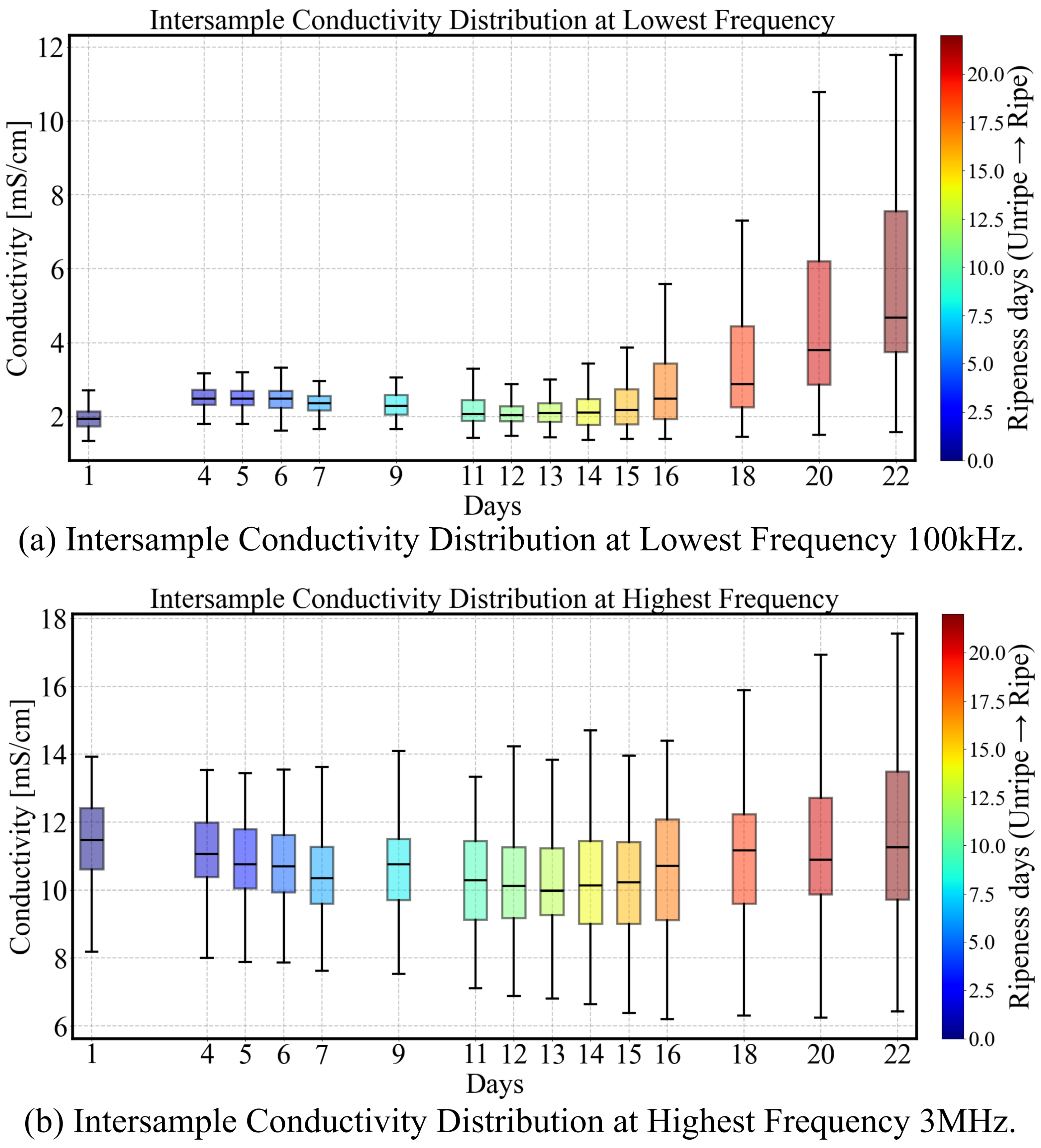
3.2.1. Normalized Gradient of Conductivity Spectrum
- Early Pre-ripe Stage (0–5 days): The initially declines corresponding to the transition from the underripe stage to the ripe stage.
- Stable Ripe Stage (5–15 days): During this period, when the fruit is ready for consumption, the parameter remains relatively stable, with a slight decrease.
- Overripe Stage (15–22 days): The avocado fruit peel softens and darkens to a deep black color, indicating overripeness. This stage is associated with a rapid decline in .
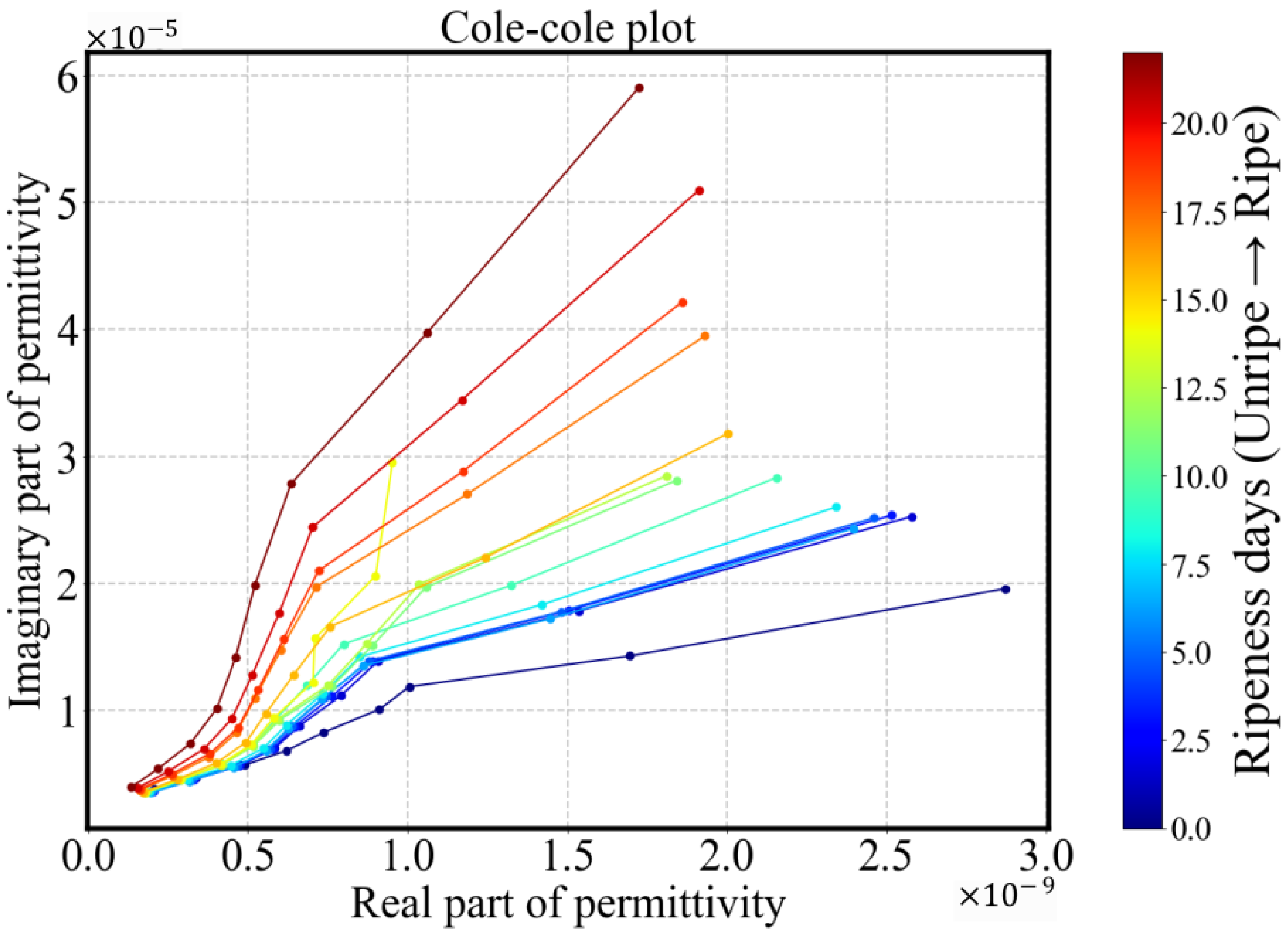
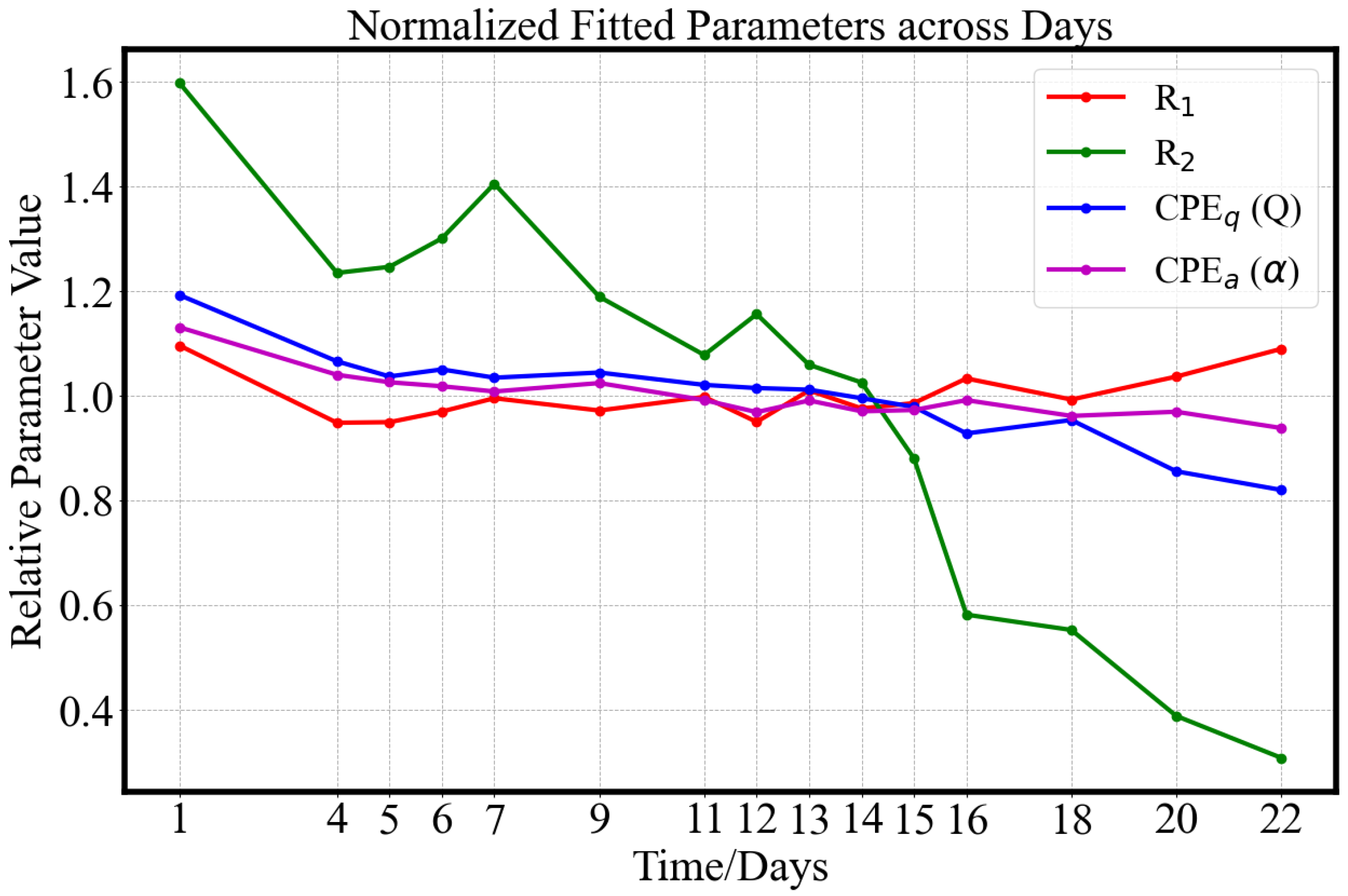
3.2.2. Equivalent Circuits
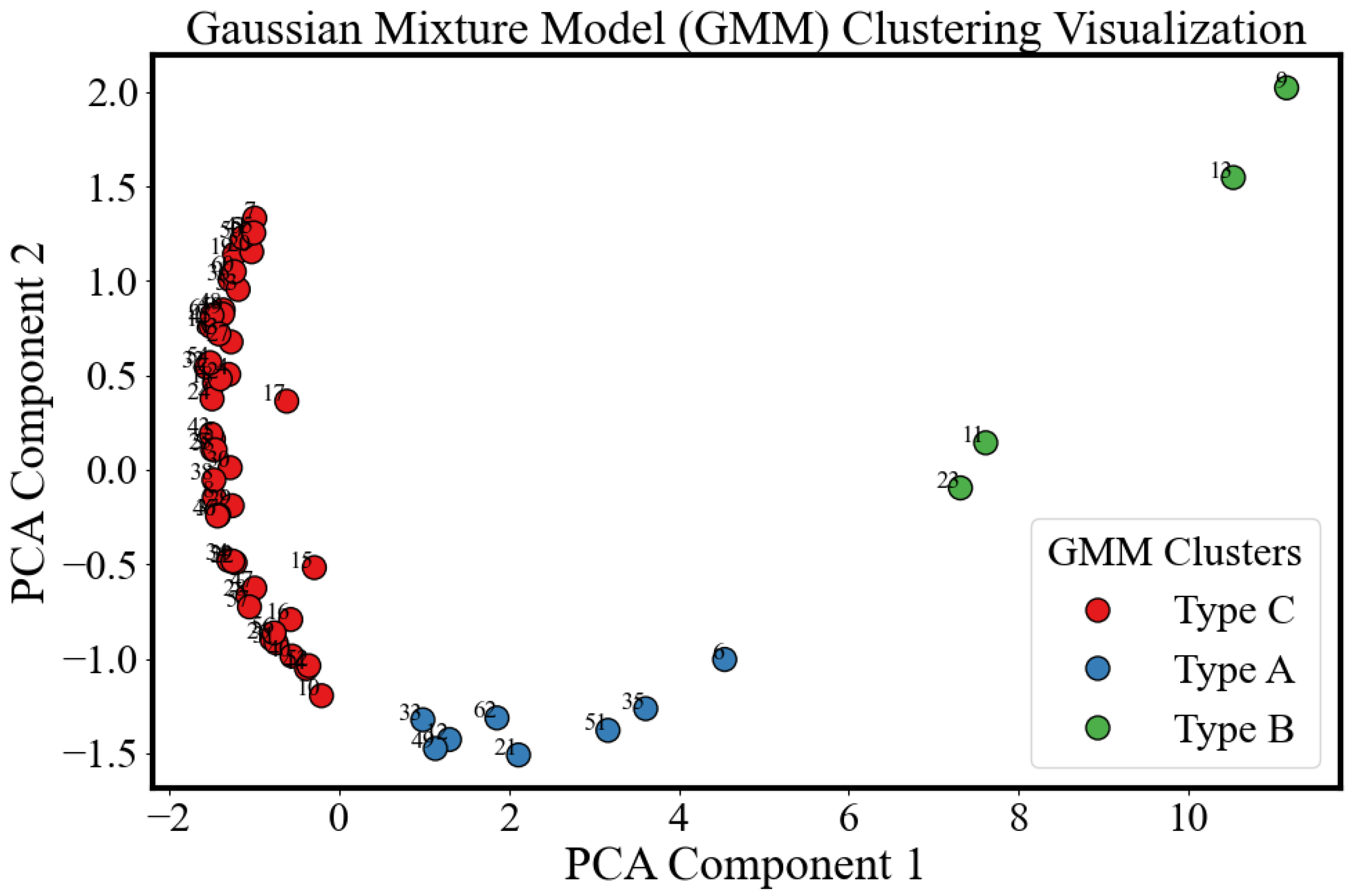
3.3. Inter-Sample Variability and Classification
- Type A (blue): For this group, the parameter remains remarkably stable, around 0.80 throughout the 22-day ripening period, indicating minimal change in dispersion behavior. The average conductivity shows a decreasing trend during the underripe and ripe stages (0–15 days), followed by an increase during the overripe phase (>15 days). Due to the nearly unchanged , dispersion shape remains similar, making conductivity-based ripeness assessment particularly challenging for this sample type. However, this group accounts for only 13% (8/60) of the dataset.
- Type B (green): This is the smallest (4/60) and most dispersed cluster in PCA space, indicating distinct behavior. Samples classified as Type B exhibit rapid ripening and overripening characteristics. The corresponding conductivity spectra displays a sharp increase during the underripe and ripe stages (0–15 days), followed by a significant decline in the overripe stage. This post-peak drop in conductivity could be attributed to both structural degradation and shrinkage-induced volume loss. Type B samples exhibit high temporal sensitivity in conductivity measurements, with a parameter that consistently declines across the 22-day period. In the Cole–Cole plots, the trajectory transitions gradually from alignment along the x-axis to orientation along the y-axis, reflecting a shift from resistive to capacitive dominance as the tissue properties evolve.
- Type C (red): This group represents the largest and most tightly clustered category, comprising 80% (48/60) of the samples and suggesting a high degree of homogeneity in . It dominates the aggregate behavior shown in Figure 6. The cluster is centered near the origin in the PCA space, likely corresponding to the baseline values of . The trajectory closely follows the overall trend previously shown in Figure 8: a decline during the underripe phase, relative stabilization during the ripe stage (days 5–15), and a sharp decrease after day 15 in the overripe stage.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BIS | Bioimpedance Spectroscopy |
| CPE | Constant Phase Element |
| GMM | Gaussian Mixture Model |
| IQR | Interquartile Range |
| MIS | Magnetic Induction Spectroscopy |
| MWS | Maxwell–Wagner–Sillars |
| PCA | Principal Component Analysis |
| RMSE | Root Mean Square Error |
| SSD | Soluble Solid Content |
References
- Eaks, I. Ripening, Respiration, and Ethylene Production of ‘Hass’ Avocado Fruits at 20° to 40 °C. J. Am. Soc. Hortic. Sci. 1978, 103, 576–578. [Google Scholar] [CrossRef]
- Burg, S.; Burg, E. Post-Harvest Ripening of Avocados. Nature 1962, 194, 398–399. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Seeking End to Loss and Waste of Food Along Production Chain; FAO: Rome, Italy, 2025. [Google Scholar]
- Swarts, D. The Firmometer—For Monitoring Ripening of Avocados (and Other Fruit). S. Afr. Avocado Grow. Assoc. 1981, 4, 42–46. [Google Scholar]
- Landahl, S.; Terry, L. Non-Destructive Discrimination of Avocado Fruit Ripeness Using Laser Doppler Vibrometry. Biosyst. Eng. 2020, 194, 106–113. [Google Scholar] [CrossRef]
- Andasuryani, I. Assessment of the Volatile Organic Compound of Avocado during Ripening Process and Mechanical Damage Using Electronic-Nose System. IOP Conf. Ser. Earth Environ. Sci. 2022, 1059, 012023. [Google Scholar] [CrossRef]
- Olivares, D.; García-Rojas, M.; Ulloa, P.; Riveros, A.; Pedreschi, R.; Campos-Vargas, R.; Meneses, C.; Defilippi, B. Response Mechanisms of ‘Hass’ Avocado to Sequential 1-Methylcyclopropene Applications at Different Maturity Stages during Cold Storage. Plants 2022, 11, 1781. [Google Scholar] [CrossRef]
- Solomos, T.; Laties, G. Cellular Organization and Fruit Ripening. Nature 1973, 245, 390–392. [Google Scholar] [CrossRef]
- Yang, S.; Hallett, I.; Oh, H.; Woolf, A.; Wong, M. The Impact of Fruit Softening on Avocado Cell Microstructure Changes Monitored by Electrical Impedance and Conductivity for Cold-Pressed Oil Extraction. J. Food Process Eng. 2019, 42, e13068. [Google Scholar] [CrossRef]
- Islam, M.; Wahid, K.; Dinh, A. Assessment of Ripening Degree of Avocado by Electrical Impedance Spectroscopy and Support Vector Machine. J. Food Qual. 2018, 2018, 4706147. [Google Scholar] [CrossRef]
- O’Toole, M.; Marsh, L.; Davidson, J.; Tan, Y.; Armitage, D.; Peyton, A. Non-Contact Multi-Frequency Magnetic Induction Spectroscopy System for Industrial-Scale Bio-Impedance Measurement. Meas. Sci. Technol. 2015, 26, 035102. [Google Scholar] [CrossRef]
- O’Toole, M.; Colgan, R.; Anvar, A.; Peyton, A. Non-Contact Assessment of Apple Condition Using Magnetic Induction Spectroscopy: Preliminary Results and Indications. In Proceedings of the 2021 IEEE International Workshop on Metrology for Agriculture and Forestry (MetroAgriFor), Trento-Bolzano, Italy, 3–5 November 2021; pp. 135–139. [Google Scholar] [CrossRef]
- Teichmann, D.; Kuhn, A.; Leonhardt, S.; Walter, M. The MAIN Shirt: A Textile-Integrated Magnetic Induction Sensor Array. Sensors 2014, 14, 1039–1056. [Google Scholar] [CrossRef] [PubMed]
- Ojarand, J.; Pille, S.; Min, M.; Land, R.; Oleitšuk, J. Magnetic Induction Sensor for Respiration Monitoring. In Proceedings of the 10th International Conference on Bioelectromagnetism (ICBEM2015), Tallinn, Estonia, 16–18 June 2015; pp. 1–4. [Google Scholar]
- Ding, T.; Chen, X.; Huang, Y. Ultra-Thin Flexible Eddy Current Sensor Array for Gap Measurements. Tsinghua Sci. Technol. 2004, 9, 667–671. [Google Scholar]
- Tsukada, K.; Haga, Y.; Morita, K.; Nannan, S.; Sakai, K.; Kiwa, T.; Cheng, W. Detection of Inner Corrosion of Steel Construction Using Magnetic Resistance Sensor and Magnetic Spectroscopy Analysis. IEEE Trans. Magn. 2016, 52, 6201504. [Google Scholar] [CrossRef]
- Williams, K.; O’Toole, M.; Peyton, A. Classification of Wrought and Cast Aluminium Using Magnetic Induction Spectroscopy and Machine Vision. In Proceedings of the 2024 IEEE Sensors Applications Symposium (SAS), Naples, Italy, 23–25 July 2024. [Google Scholar]
- O’Toole, M.; Karimian, N.; Peyton, A. Classification of Nonferrous Metals Using Magnetic Induction Spectroscopy. IEEE Trans. Ind. Inform. 2018, 14, 3477–3485. [Google Scholar] [CrossRef]
- Muttakin, I.; Soleimani, M. Noninvasive Conductivity and Temperature Sensing Using Magnetic Induction Spectroscopy Imaging. IEEE Trans. Instrum. Meas. 2021, 70, 4500211. [Google Scholar] [CrossRef]
- Schwan, H. Electrical Properties of Tissues and Cell Suspensions: Mechanisms and Models. In Proceedings of the 16th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Baltimore, MD, USA, 3–6 November 1994. [Google Scholar]
- Sacher, J. Relations Between Changes in Membrane Permeability and the Climacteric in Banana and Avocado. Nature 1962, 195, 577–578. [Google Scholar] [CrossRef]
- Jesus, I.; Machado, J.; Cunha, J. Fractional Electrical Impedances in Botanical Elements. J. Vib. Control 2008, 14, 1389–1402. [Google Scholar] [CrossRef]
- Neto, A.; Olivier, N.; Cordeiro, E.; de Oliveira, H. Determination of Mango Ripening Degree by Electrical Impedance Spectroscopy. Comput. Electron. Agric. 2017, 143, 222–226. [Google Scholar] [CrossRef]
- Montoya, M.; Plaza, J.; López-Rodriguez, V. Electrical Conductivity of Avocado Fruits during Cold Storage and Ripening. LWT-Food Sci. Technol. 1994, 27, 34–38. [Google Scholar] [CrossRef]
- Bidinosti, C.; Chapple, E.; Hayden, M. The Sphere in a Uniform RF Field—Revisited. Concepts Magn. Reson. B 2007, 31, 191–202. [Google Scholar] [CrossRef]
- Spikic, D.; Švraka, M.; Vasic, D. Effectiveness of Electrostatic Shielding in High-Frequency Electromagnetic Induction Soil Sensing. Sensors 2022, 22, 3000. [Google Scholar] [CrossRef]
- Cole, K. Electric Impedance of Suspensions of Spheres. J. Gen. Physiol. 1928, 12, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Hallett, I.; Rebstock, R.; Oh, H.; Kam, R.; Woolf, A.; Wong, M. Cellular Changes in ’Hass’ Avocado Mesocarp during Cold-Pressed Oil Extraction. J. Am. Oil Chem. Soc. 2018, 95, 229–238. [Google Scholar] [CrossRef]
- Ibba, P.; Falco, A.; Abera, B.; Cantarella, G.; Petti, L.; Lugli, P. Bio-Impedance and Circuit Parameters: An Analysis for Tracking Fruit Ripening. Postharvest Biol. Technol. 2020, 159, 110978. [Google Scholar] [CrossRef]
- Pliquett, U.; Altmann, M.; Pliquett, F.; Schoberlein, L. Py: A Parameter for Meat Quality. Meat Sci. 2003, 65, 1429–1437. [Google Scholar] [CrossRef]
- O’Toole, M.; Glowacz, M.; Xu, L. Bioimpedance Measurement of Avocado Fruit Using Magnetic Induction Spectroscopy. IEEE Trans. AgriFood Electron. 2023, 1, 99–107. [Google Scholar] [CrossRef]
- Storn, R.; Price, K. Differential Evolution—A Simple and Efficient Heuristic for Global Optimization over Continuous Spaces. J. Glob. Optim. 1997, 11, 341–359. [Google Scholar] [CrossRef]
- Freeborn, T. A Survey of Fractional-Order Circuit Models for Biology and Biomedicine. IEEE J. Emerg. Sel. Top. Circuits Syst. 2013, 3, 416–424. [Google Scholar] [CrossRef]
- Grimnes, S.; Martinsen, Ø.G. Chapter 4—Passive Tissue Electrical Properties. In Bioimpedance and Bioelectricity Basics, 3rd ed.; Grimnes, S., Martinsen, Ø.G., Eds.; Academic Press: Oxford, UK, 2015; pp. 77–118. [Google Scholar] [CrossRef]
- Sakoe, H.; Chiba, S. Dynamic Programming Algorithm Optimization for Spoken Word Recognition. IEEE Trans. Acoust. Speech Signal Process. 1978, 26, 43–49. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, T.; Fletcher, A.D.; Colgan, R.J.; O’Toole, M.D. Magnetic Induction Spectroscopy-Based Non-Contact Assessment of Avocado Fruit Condition. Sensors 2025, 25, 4195. https://doi.org/10.3390/s25134195
Lu T, Fletcher AD, Colgan RJ, O’Toole MD. Magnetic Induction Spectroscopy-Based Non-Contact Assessment of Avocado Fruit Condition. Sensors. 2025; 25(13):4195. https://doi.org/10.3390/s25134195
Chicago/Turabian StyleLu, Tianyang, Adam D. Fletcher, Richard John Colgan, and Michael D. O’Toole. 2025. "Magnetic Induction Spectroscopy-Based Non-Contact Assessment of Avocado Fruit Condition" Sensors 25, no. 13: 4195. https://doi.org/10.3390/s25134195
APA StyleLu, T., Fletcher, A. D., Colgan, R. J., & O’Toole, M. D. (2025). Magnetic Induction Spectroscopy-Based Non-Contact Assessment of Avocado Fruit Condition. Sensors, 25(13), 4195. https://doi.org/10.3390/s25134195





