A Sensitive and Selective Sensor Based on Orthorhombic Copper Molybdate Decorated on Reduced Graphene Oxide for the Detection of Promethazine Hydrochloride
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
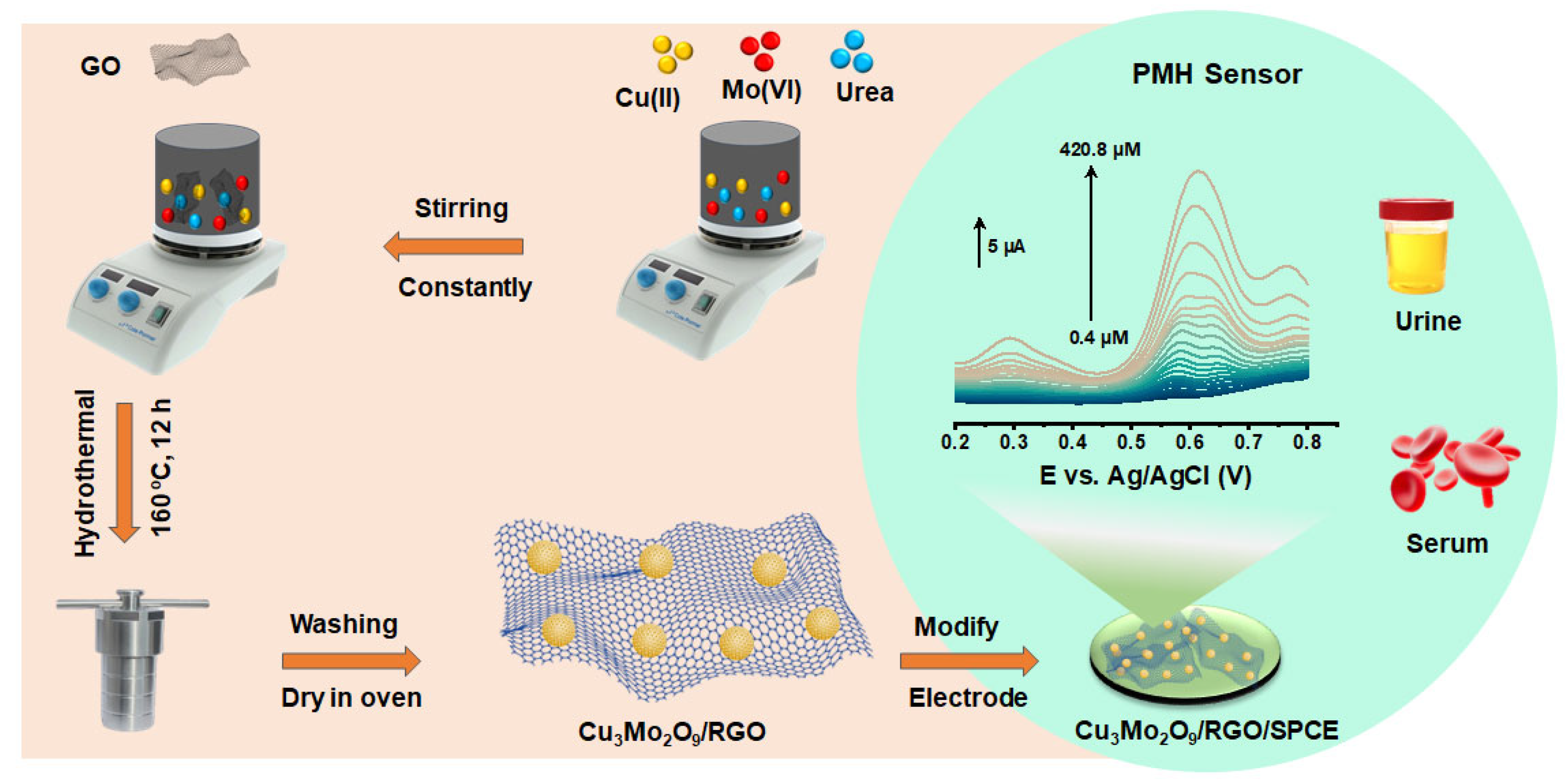
2.2. Synthesis of Cu3Mo2O9/RGO Hybrid
2.3. Instrumentation
2.4. Fabrication of Cu3Mo2O9/RGO/SPCE
2.5. Preparation of Real Samples
3. Results and Discussion
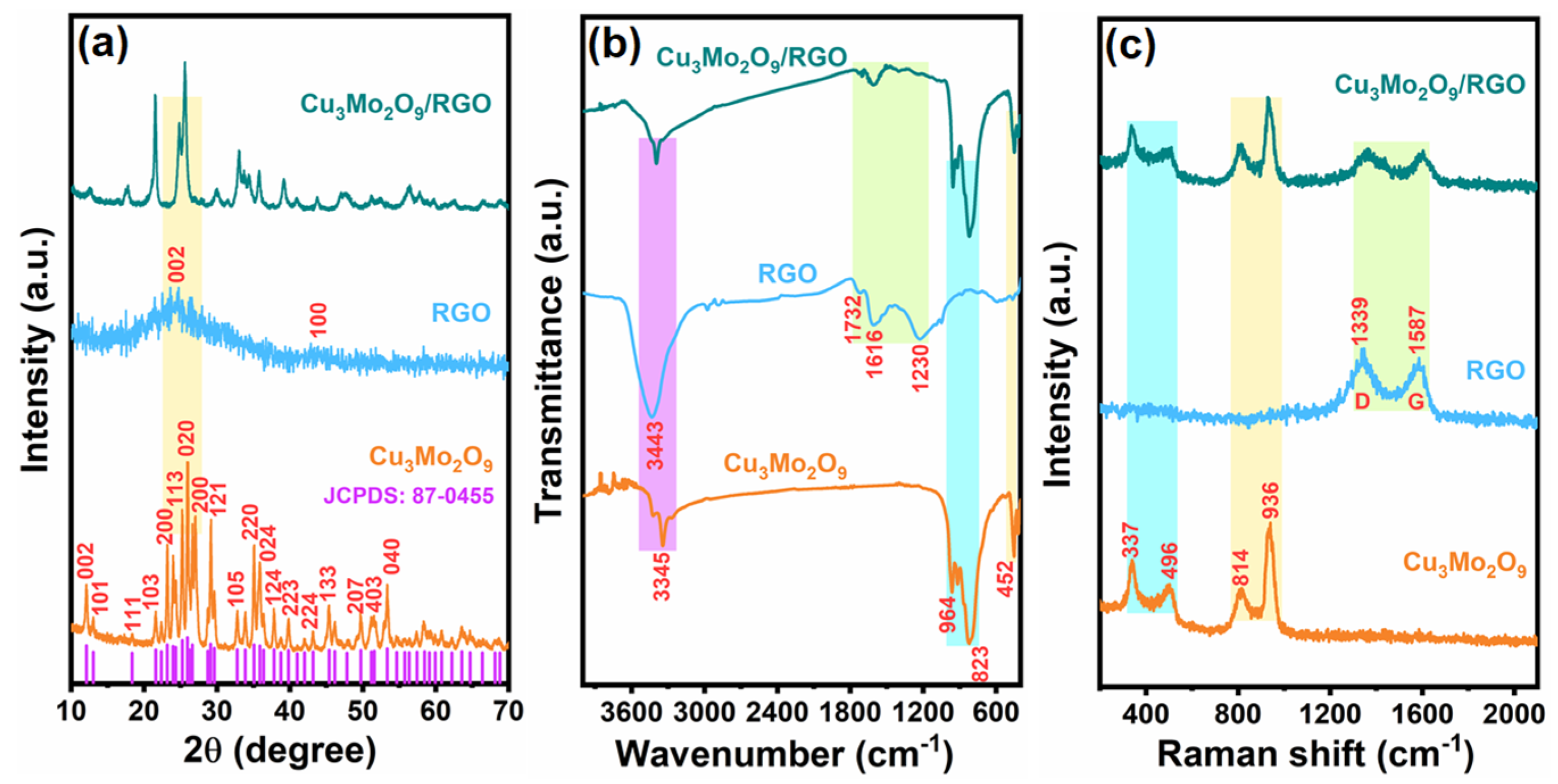
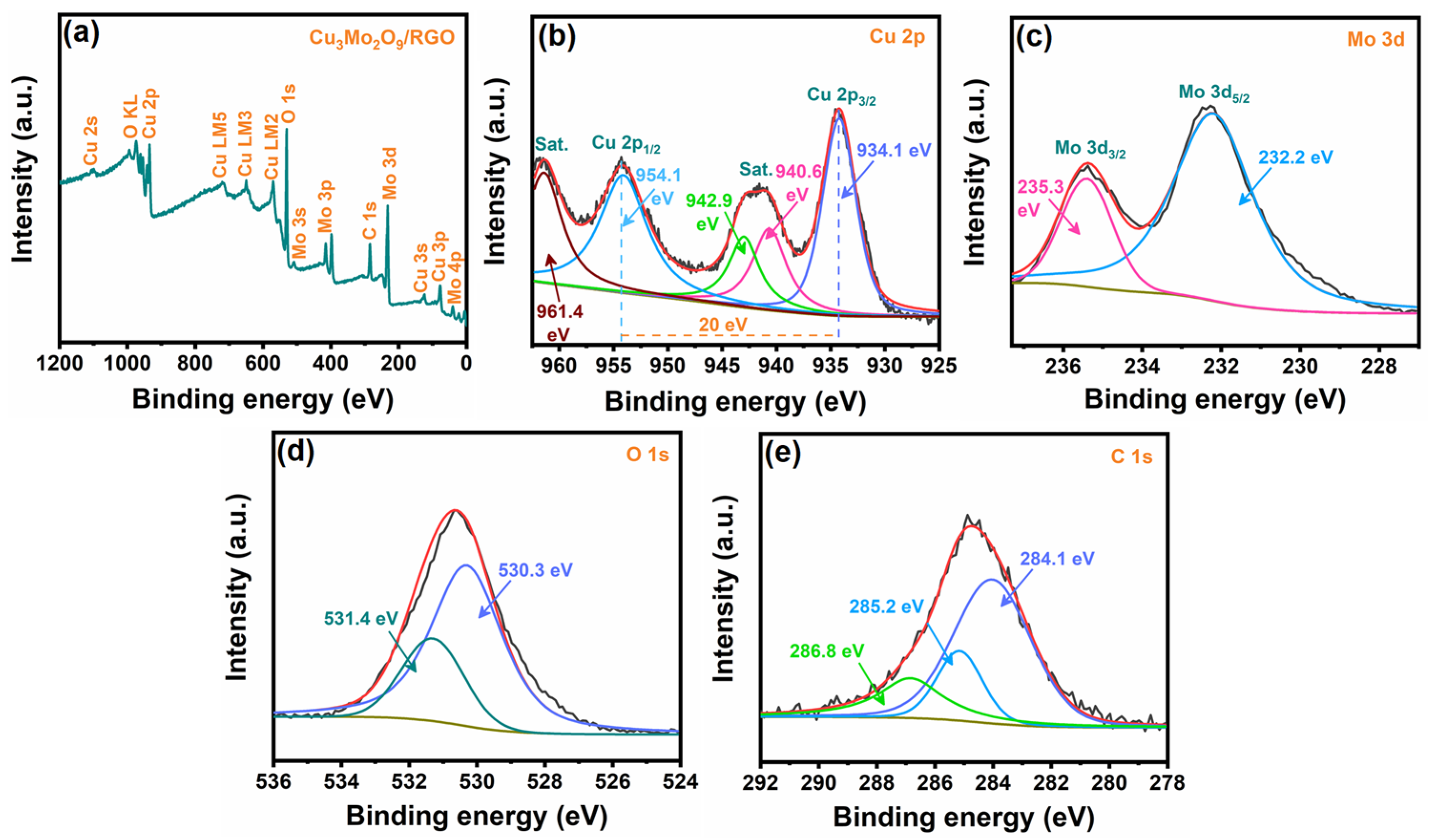
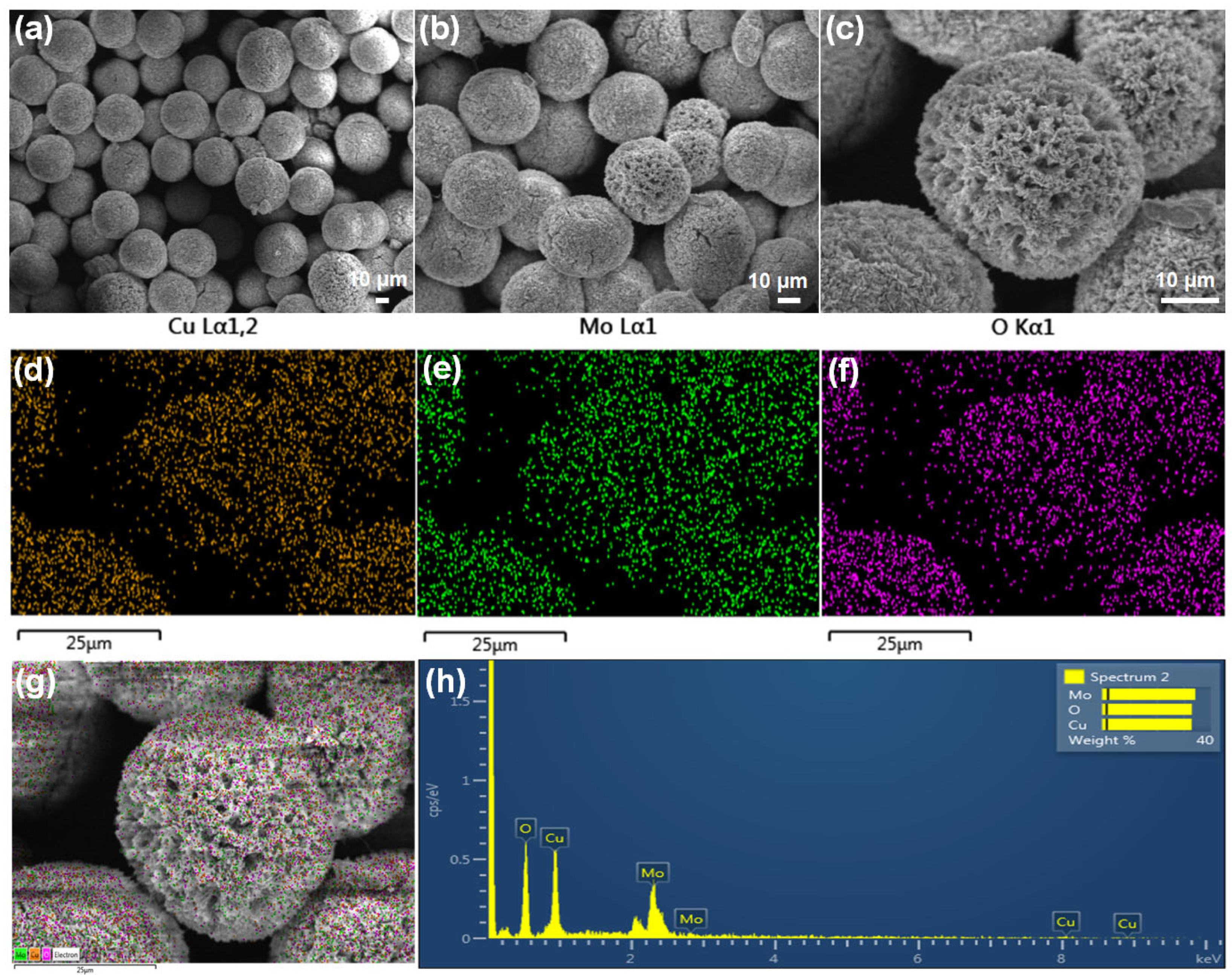
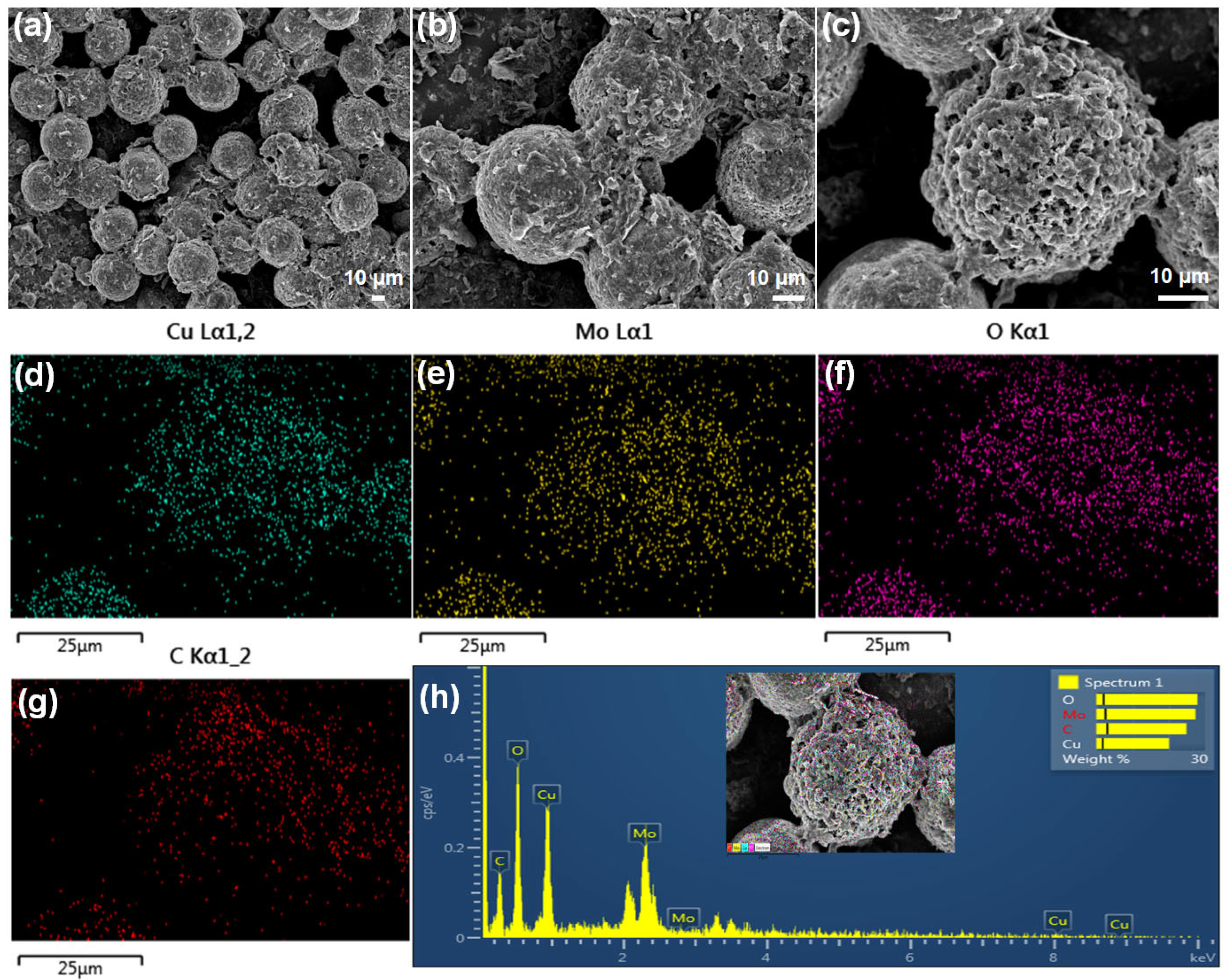
3.1. Physical Characterization of Cu3Mo2O9/RGO Hybrid
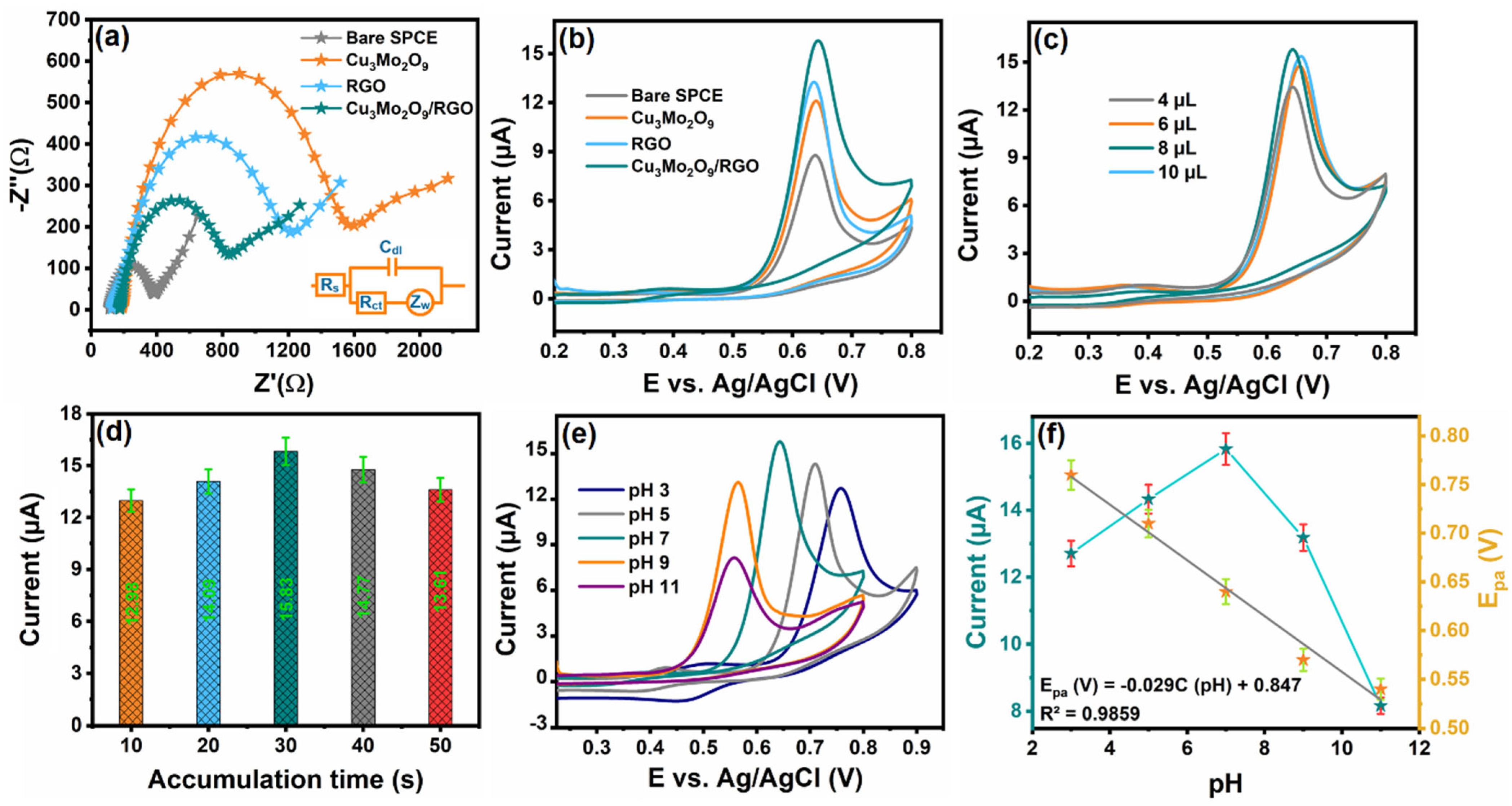
3.2. Electrochemical Impedance Analysis at Different Modified Electrodes
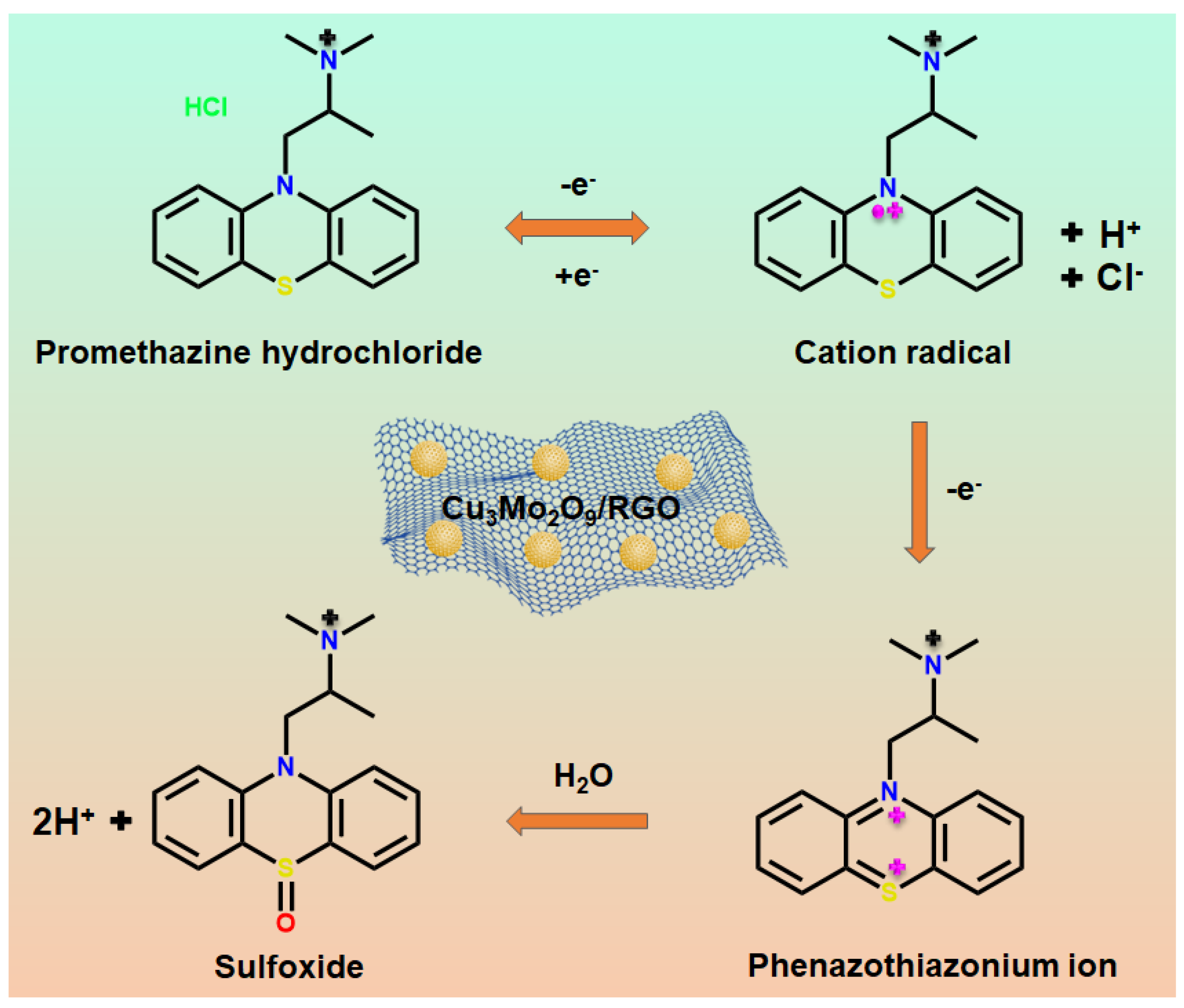
3.3. Electrochemical Behavior of PMH on Various Interfaces
3.4. Experimental Condition Optimization
3.4.1. Influence of Loading Catalyst
3.4.2. Influence of Accumulation Time
3.4.3. Influence of pH
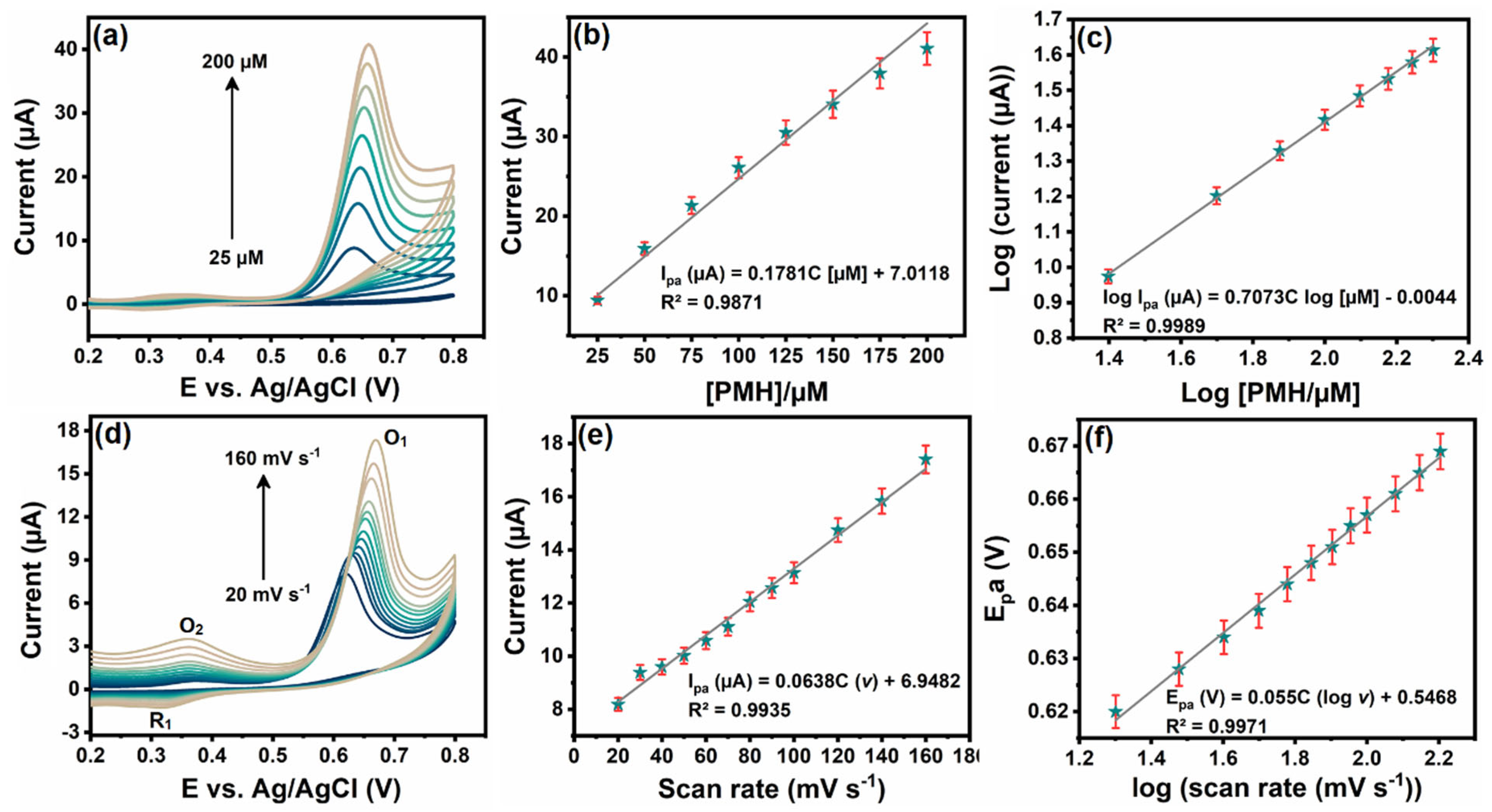
3.5. Influence of Increasing Concentration and Scan Rate
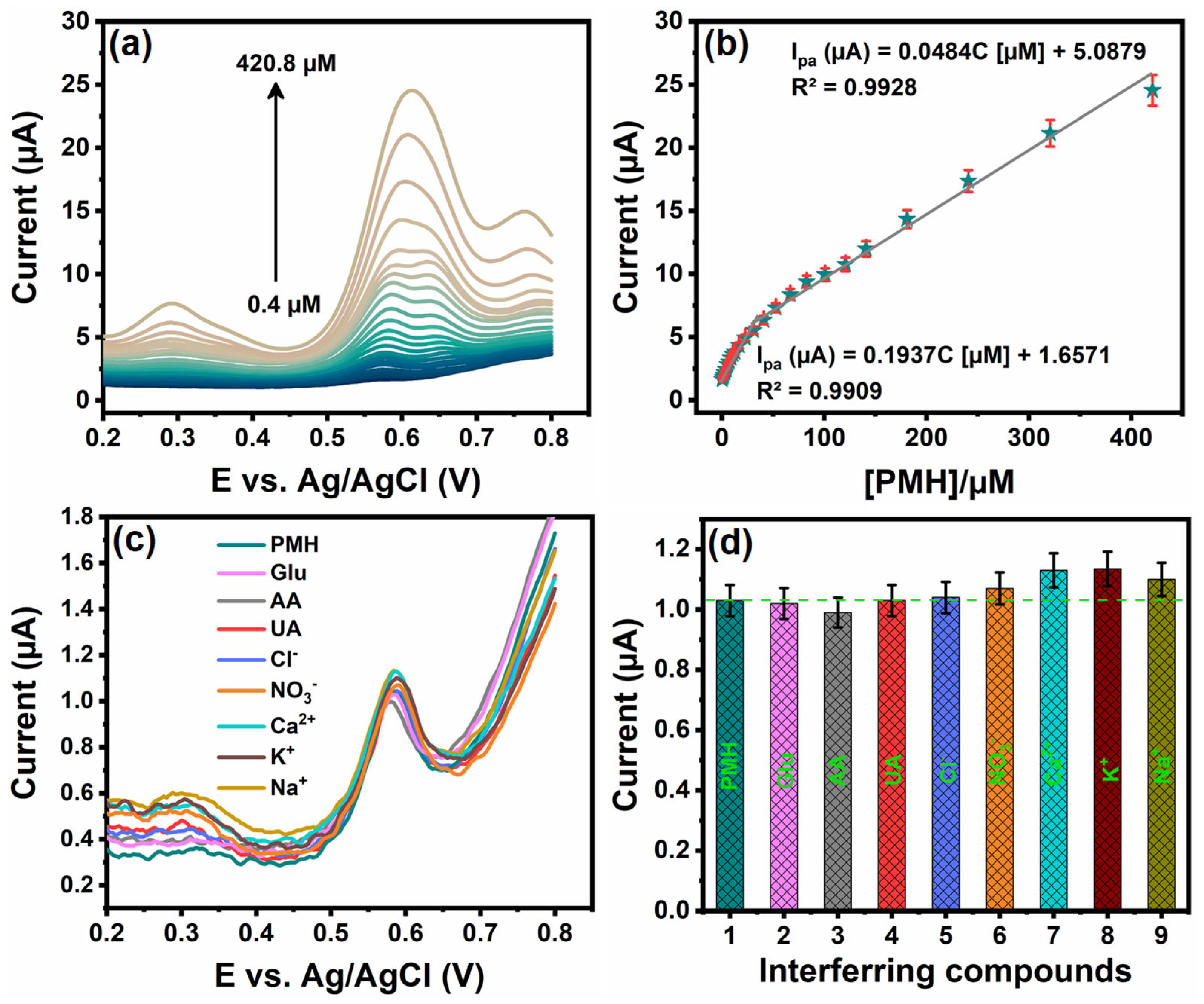
3.6. Determination of PMH with Cu3Mo2O9/RGO/SPCE Using DPV Technique
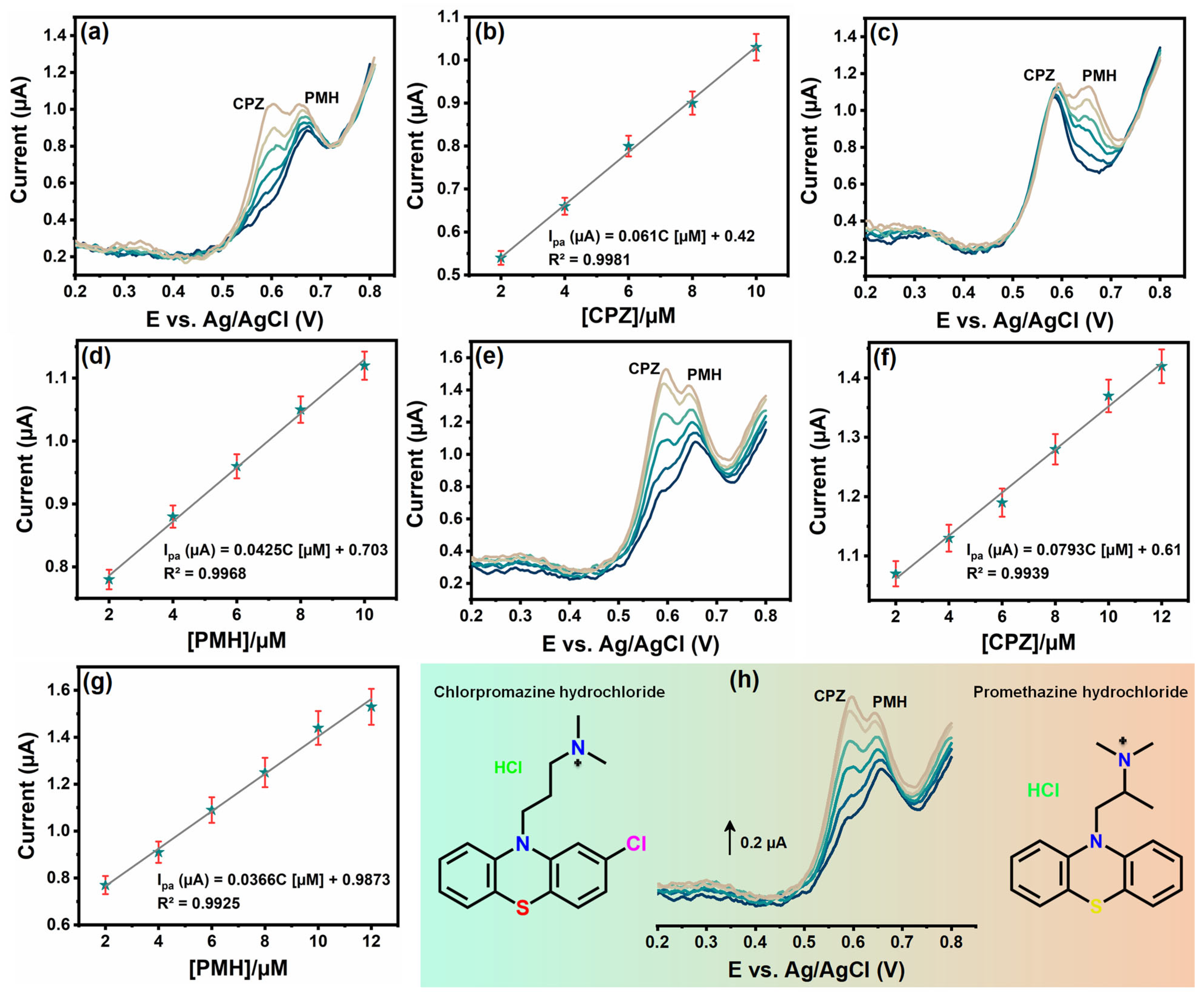
3.7. Interference Studies of Cu3Mo2O9/RGO/SPCE
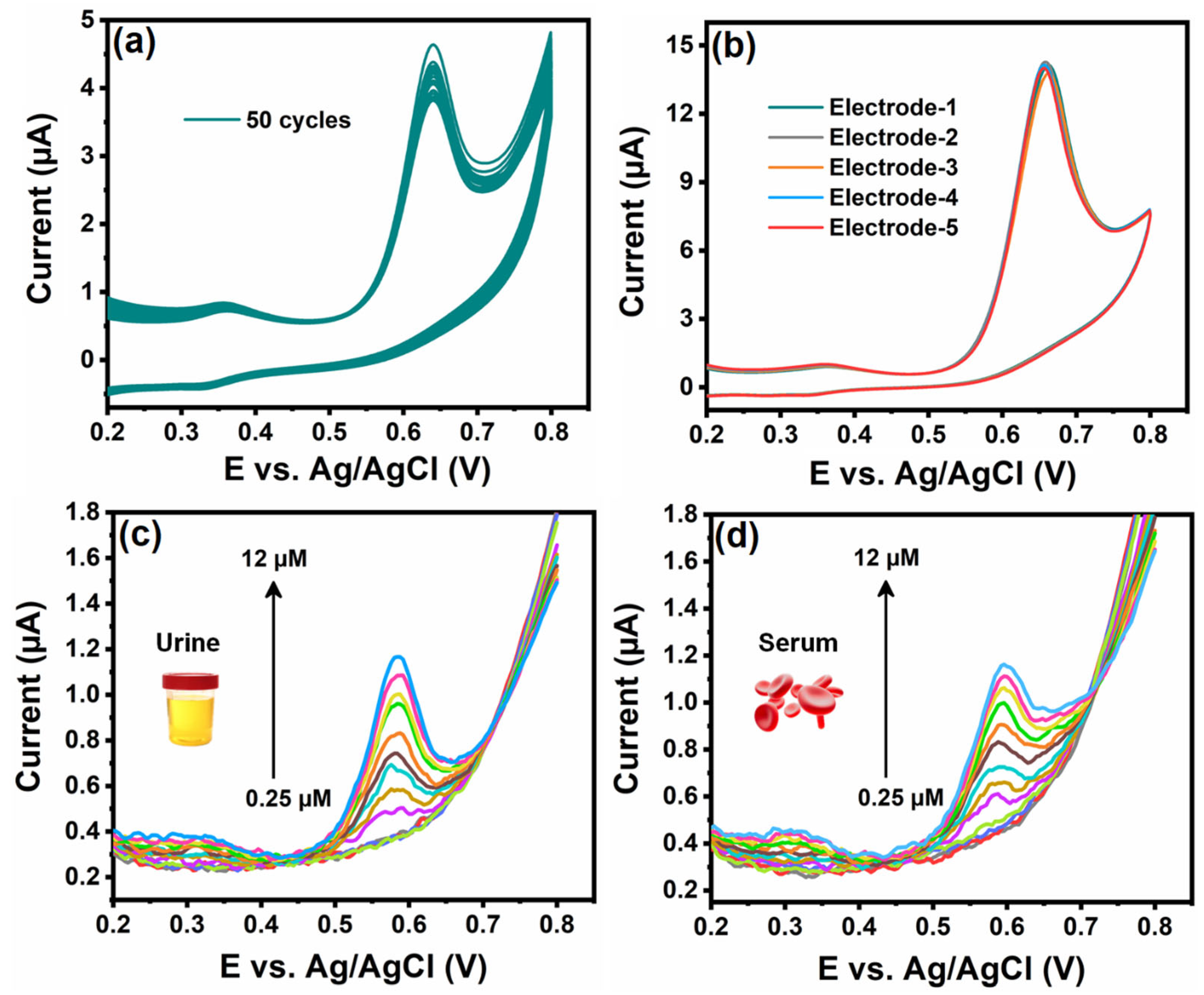
3.8. Cyclic Stability and Reproducibility of the Cu3Mo2O9/RGO/SPCE Sensor
3.9. Real-Time Monitoring of PMH in Human Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, H.; Zhang, C.; Wang, C.; Fan, K.; Zhang, Y.; Fang, L.; Li, L.; Ren, C.; Yin, Z.-Z.; Lü, Z. A highly selective and sensitive sensor for promethazine based on molecularly imprinted interface coated Au/Sn bimetal nanoclusters functionalized acupuncture needle microelectrode. Anal. Chim. Acta 2023, 1269, 341395. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, N.; Yu, W.-C.; Hu, Y.-C.; Balram, D.; Yu, Y.-H. Morphological evolution of nanosheets-stacked spherical ZnO for preparation of GO-Zn/ZnO ternary nanocomposite: A novel electrochemical platform for nanomolar detection of antihistamine promethazine hydrochloride. J. Alloys Compd. 2022, 890, 161768. [Google Scholar] [CrossRef]
- Purushothama, S.; Subbareddy, S.; Shivamurthy, S.A.; Shadakshari, S.; Dwarakanath, S.C.; Palakollu, V.N. Ternary Metal (W–Ni–Sr) Oxide@Polypyrrole Nanotubes: A New Frontier in the Electrochemical Detection of Promethazine Hydrochloride (PMHC). Langmuir 2025, 41, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Phonchai, A.; Pon-in, S.; Resan, S.; Chuayyok, P.; Nacapricha, D.; Wilairat, P.; Chaisiwamongkhol, K. Simple and rapid paper-based colorimetric spot test for promethazine screening in recreational drug beverages and pharmaceuticals. Microchem. J. 2025, 208, 112353. [Google Scholar] [CrossRef]
- Lara, F.J.; García-Campaña, A.M.; Alés-Barrero, F.; Bosque-Sendra, J.M. Determination of thiazinamium, promazine and promethazine in pharmaceutical formulations using a CZE method. Anal. Chim. Acta 2005, 535, 101–108. [Google Scholar] [CrossRef]
- Sultan, S.M.; Hassan, Y.A.; Abulkibash, A.M. Chemiluminescence assay of promethazine hydrochloride using acidic permanganate employing flow injection mode operated with syringe and peristaltic pumps. Talanta 2003, 59, 1073–1080. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Nasr-Esfahani, P.; Rezaei, B. Synthesis of molecularly imprinted polymer on carbon quantum dots as an optical sensor for selective fluorescent determination of promethazine hydrochloride. Sens. Actuators B Chem. 2018, 257, 889–896. [Google Scholar] [CrossRef]
- Lantam, A.; Limbut, W.; Thiagchanya, A.; Phonchai, A. A portable optical colorimetric sensor for the determination of promethazine in lean cocktail and pharmaceutical doses. Microchem. J. 2020, 159, 105519. [Google Scholar] [CrossRef]
- Calatayud, J.M.; Sarrion, S.N.; Sampedro, A.S.; Benito, C.G. Determination of promethazine hydrochloride with bromophenol blue by a turbidimetric method and flow injection analysis. Microchem. J. 1992, 45, 129–136. [Google Scholar] [CrossRef]
- Borkar, D.D.; Godse, V.P.; Bafana, Y.S.; Bhosale, A.V.; Tal-Purandar, D.-P. Simultaneous estimation of paracetamol and promethazine hydrochloride in pharmaceutical formulations by a RP-HPLC method. Int. J. ChemTech Res. 2009, 2, 667–670. [Google Scholar]
- Vinothkumar, V.; Sakthivel, R.; Chen, S.M.; Arumugam, S.; Kim, T.H. Construction of Sr@Mn3O4/GO nanocomposite: A synergistic electrocatalyst for nitrofurantoin detection in biological and environmental samples. Environ. Sci. Nano 2023, 10, 503–518. [Google Scholar] [CrossRef]
- Kamalasekaran, K.; Sundramoorthy, A.K. Applications of chemically modified screen-printed electrodes in food analysis and quality monitoring: A review. RSC Adv. 2024, 14, 27957–27971. [Google Scholar] [CrossRef] [PubMed]
- Juang, R.S.; Hsieh, C.-T.; Lin, T.A. Highly sensitive detection of promethazine hydrochloride in water and human urine using flower-like ZnO nanorods supported by GO sheets and Pt nanoparticles. J. Mol. Liq. 2024, 399, 124460. [Google Scholar] [CrossRef]
- Tajik, S.; Zaimbashi, R.; Nejad, F.G.; Moghadam, M.T.T.; Askari, M.B.; Beitollahi, H. MnWO4/reduced graphene oxide-based electrochemical sensing platform for simultaneous detection of catechol and resorcinol. Diam. Relat. Mater. 2024, 149, 111579. [Google Scholar] [CrossRef]
- Meng, T.; Jia, H.; Ye, H.; Zeng, T.; Yang, X.; Wang, H.; Zhang, Y. Facile preparation of CoMoO4 nanorods at macroporous carbon hybrid electrocatalyst for non-enzymatic glucose detection. J. Colloid Interface Sci. 2020, 560, 1–10. [Google Scholar] [CrossRef]
- Guo, J.; Wu, J.; Xu, L.; Yuan, X.; Tan, C.; Wang, Q.; Xiong, X. Microplasma-assisted construction of cross-linked network hierarchical structure of NiMoO4 nanorods@NiCo-LDH nanosheets for electrochemical sensing of non-enzymatic H2O2 in food. Food Chem. 2024, 461, 140940. [Google Scholar] [CrossRef]
- Gajraj, V.; Mariappan, C.R. Electrochemical performances of asymmetric aqueous supercapacitor based on porous Cu3Mo2O9 petals and La2Mo3O12 nanoparticles fabricated through a simple co-precipitation method. Appl. Surf. Sci. 2020, 512, 145648. [Google Scholar] [CrossRef]
- Li, J.C.; Feng, F.; Yang, S.H.; Gu, Y.R.; Xue, H.G.; Guo, S.P. Promising electrochemical performance of Cu3Mo2O9 nanorods for lithium-ion batteries. J. Mater. Sci. 2017, 52, 12380–12389. [Google Scholar] [CrossRef]
- Huang, W.; Fu, Z.; Hu, X.; Wang, Q.; Fan, J.; Liu, E. Efficient photocatalytic hydrogen evolution over Cu3Mo2O9/TiO2 pn heterojunction. J. Alloys Compd. 2022, 904, 164089. [Google Scholar] [CrossRef]
- Ghoreishian, S.M.; Raju, G.S.R.; Ranjith, K.S.; Lee, H.; Kwak, C.H.; Park, B.; Nikoo, S.Z.; Han, Y.K.; Huh, Y.S. Construction of 2D/2D/2D rGO/p-C3N4/Cu3Mo2O9 heterostructure as an efficient catalytic platform for cascade photo-degradation and photoelectrochemical activity. Appl. Surf. Sci. 2020, 511, 145469. [Google Scholar] [CrossRef]
- Vinothkumar, V.; Sekhar, Y.C.; Chen, S.M.; Kim, T.H. Development of sensitive Mn@TiO2/RGO nanocomposite-based sensor for the detection of sunset yellow in food samples. FlatChem 2025, 51, 100861. [Google Scholar] [CrossRef]
- Mokoba, T.; Liu, Y.; Wu, Y.; Zhang, T.C.; Yuan, S. Agave-angustifolia-like Cu3Mo2O9 nanoplate-coated copper mesh for effective emulsion separation and photocatalytic degradation of soluble dyes. Ind. Eng. Chem. Res. 2022, 61, 13635–13649. [Google Scholar] [CrossRef]
- Christopher, J.J.; Lydia, I.S.; Sathiyan, A.; Merlin, J.P. Fabrication of Cu3Mo2O9 doped MWCNT nanocomposites as efficient photocatalyst for malachite green dye degradation. Opt. Mater. 2024, 156, 115935. [Google Scholar] [CrossRef]
- Ren, X.; Yang, H.; Gen, S.; Zhou, J.; Yang, T.; Zhang, X.; Cheng, Z.; Sun, S. Controlled growth of LaFeO3 nanoparticles on reduced graphene oxide for highly efficient photocatalysis. Nanoscale 2016, 8, 752–756. [Google Scholar] [CrossRef]
- Mehta, S.; Kumaravel, S.; Jha, S.; Yen, M.; Kundu, S.; Liang, H. Impacts of structure-directing agents on the synthesis of Cu3Mo2O9 for flexible lignin-based supercapacitor electrodes. J. Compos. Sci. 2023, 7, 155. [Google Scholar] [CrossRef]
- Adamu, B.I.; Falak, A.; Tian, Y.; Tan, X.; Meng, X.; Chen, P.; Wang, H.; Chu, W. p–p heterojunction sensors of p-Cu3Mo2O9 micro/nanorods vertically grown on p-CuO layers for room-temperature ultrasensitive and fast recoverable detection of NO2. ACS Appl. Mater. Interfaces 2020, 12, 8411–8421. [Google Scholar] [CrossRef]
- Chen, X.; Abdullah, H.; Kuo, D.H. CuMnOS nanoflowers with different Cu+/Cu2+ ratios for the CO2-to-CH3OH and the CH3OH-to-H2 redox reactions. Sci. Rep. 2017, 7, 41194. [Google Scholar] [CrossRef]
- Bahmani, F.; Kazemi, S.H.; Kazemi, H.; Kiani, M.A.; Feizabadi, S.Y. Nanocomposite of copper–molybdenum–oxide nanosheets with graphene as high-performance materials for supercapacitors. J. Alloys Compd. 2019, 784, 500–512. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Fan, L.; Ma, R.; Zhang, E.; Liu, Q.; Lu, B. An ultrafast rechargeable hybrid sodium-based dual-ion capacitor based on hard carbon cathodes. Adv. Energy Mater. 2018, 8, 1800140. [Google Scholar] [CrossRef]
- Vinothkumar, V.; Koventhan, C.; Chen, S.M.; Huang, Y.F. A facile development of rare earth neodymium nickelate nanoparticles for selective electrochemical determination of antipsychotic drug prochlorperazine. J. Ind. Eng. Chem. 2022, 109, 253–266. [Google Scholar] [CrossRef]
- Saraban, A.; Promsuwan, K.; Saichanapan, J.; Soleh, A.; Saisahas, K.; Samoson, K.; Wangchuk, S.; Sanjailuk, T.; Hasin, P.; Limbut, W. A Ternary nanocomposite based on nano-bimetallic platinum/nickel decorated on multi-walled carbon nanotubes for flow injection amperometric detection of promethazine. J. Electrochem. Soc. 2023, 170, 67504. [Google Scholar] [CrossRef]
- Honarmand, E.; Motaghedifard, M.H.; Ghamari, M. Electroanalytical approach for determination of promethazine hydrochloride on gold nanoparticles-incorporated carbon paste electrode as a nanosensor. RSC Adv. 2014, 4, 35511–35521. [Google Scholar] [CrossRef]
- Promsuwan, K.; Kanatharana, P.; Thavarungkul, P.; Limbut, W. Subnanomolar detection of promethazine abuse using a gold nanoparticle-graphene nanoplatelet-modified electrode. Microchim. Acta 2020, 187, 646. [Google Scholar] [CrossRef] [PubMed]
- Honarmand, E.; Motaghedifard, M.H.; Hadi, M.; Mostaanzadeh, H. Electro-oxidation study of promethazine hydrochloride at the surface of modified gold electrode using molecular self assembly of a novel bis-thio Schiff base from ethanol media. J. Mol. Liq. 2016, 216, 429–439. [Google Scholar] [CrossRef]
- de Oliveira, R.C.; Sousa, C.P.; Morais, S.; de Lima-Neto, P.; Correia, A.N. Polyethylenimine-multi-walled carbon nanotubes/glassy carbon electrode as an efficient sensing platform for promethazine. J. Electrochem. Soc. 2020, 167, 107506. [Google Scholar] [CrossRef]
- Alagumalai, K.; Sivakumar, M.; Kim, S.C.; Lee, D.; Muthukutty, B.; Prakash, K.; Abdelghani, H.T.M. Nonenzymatic electrochemical approaches for promethazine monitoring in aquatic media. Colloids Surf. A Physicochem. Eng. Asp. 2024, 694, 134107. [Google Scholar] [CrossRef]
- Marco, J.P.; Borges, K.B.; Tarley, C.R.T.; Ribeiro, E.S.; Pereira, A.C. Development of a simple, rapid and validated square wave voltametric method for determination of promethazine in raw material and pharmaceutical formulation using DNA modified multiwall carbon nanotube paste electrode. Sens. Actuators B Chem. 2013, 177, 251–259. [Google Scholar] [CrossRef]
- Felix, F.S.; Ferreira, L.M.C.; Vieira, F.; Trindade, G.M.; Ferreira, V.S.S.A.; Angnes, L. Amperometric determination of promethazine in tablets using an electrochemically reduced graphene oxide modified electrode. New J. Chem. 2015, 39, 696–702. [Google Scholar] [CrossRef]
- Pereira, A.C.; Santos, A.P.; de Oliveira, A.E.F.; Ferreira, L.F. Electrochemical Determination of Promethazine Hydrochloride Using Carbon Paste Electrode Modified with Activated Carbon and Silver Nanoparticles. In Handbook of Nanobioelectrochemistry: Application in Devices and Biomolecular Sensing; Springer: Singapore, 2023; pp. 199–218. [Google Scholar]
- Primo, E.N.; Oviedo, M.B.; Sánchez, C.G.; Rubianes, M.D.; Rivas, G.A. Bioelectrochemical sensing of promethazine with bamboo-type multiwalled carbon nanotubes dispersed in calf-thymus double stranded DNA. Bioelectrochemistry 2014, 99, 8–16. [Google Scholar] [CrossRef]
- Viji, S.; Dinesh, A.; Radhakrishnan, K.; Priya, L.S.; Sivasankari, C.; Santhamoorthy, M.; Ayyar, M.; Mohanavel, V.; Hashem, M.; Fouad, H.; et al. One-pot green synthesis of BSA-capped O-CQDs as an effective fluorescent sensing platform for sensitive and selective detection of promethazine drug. Sens. Bio-Sens. Res. 2025, 47, 100756. [Google Scholar] [CrossRef]
| Modified Materials | Techniques | Electrolyte/pH | Linear Range (µM) | Detection Limit (µM) | Real Samples | References |
|---|---|---|---|---|---|---|
| MWCNT/SiO2/Al2O3/Nb2O5/DNA | DPV | PBS/pH 7 | 20–100 | 5.9 | Tablet | [37] |
| RGO/GCE | i–t | ABS/pH 5 | 1.99–1030 | 0.199 | Tablet | [38] |
| Au/CPE | DPV | PBS/pH 6 | 2–225 | 0.065 | Tablet and Urine | [32] |
| Pt/Ni@MWCNT/GCE | FI-i–t | PBS/pH 6 | 0.1–1000 | 0.033 | Saliva, Beverage, and Urine | [31] |
| AC/Ag/CPE | DPV | PBS/pH 7 | 5–40 | 1.30 | Tablet | [39] |
| bCNT-dsDNA/GCE | DPV | ABS/pH 5 | 0.1–6 | 0.023 | Tablet | [40] |
| BSA-O-CQDs | FQ | PBS/pH 8 | 10–90 | 0.2 | Water | [41] |
| fMWCNT-PEI/GCE | SWV | BR/pH 2 | 0.49–5.03 | 0.231 | Tablet | [35] |
| Pt/ZnO@SPCE | DPV | PBS/pH 7 | 0.2–116.2 | 0.0185 | Urine | [13] |
| Cu3Mo2O9/RGO/SPCE | DPV | PBS/pH 7 | 0.4–420.8 | 0.015 | Urine and Serum | This work |
| Samples | Added (µM) | Found (µM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Human urine | H.U-15 | Not detected | – | – |
| H.U-30 | – | – | – | |
| H.U-50 | – | – | – | |
| 0.25 | 0.24 | 96.0 | 2.51 | |
| 0.50 | 0.49 | 98.0 | 2.36 | |
| 1.0 | 0.99 | 99.0 | 2.18 | |
| 2.0 | 1.97 | 98.5 | 2.24 | |
| 4.0 | 3.96 | 99.0 | 2.03 | |
| 6.0 | 5.95 | 99.16 | 1.87 | |
| 8.0 | 7.98 | 99.78 | 1.75 | |
| 10.0 | 9.95 | 99.5 | 1.92 | |
| 12.0 | 11.93 | 99.41 | 1.50 | |
| Human serum | H.S-15 | Not detected | – | – |
| H.S-30 | – | – | – | |
| H.S-50 | – | – | – | |
| 0.25 | 0.235 | 94.0 | 2.78 | |
| 0.50 | 0.48 | 96.0 | 2.61 | |
| 1.0 | 0.98 | 98.0 | 2.45 | |
| 2.0 | 1.99 | 99.5 | 2.37 | |
| 4.0 | 3.94 | 98.5 | 2.13 | |
| 6.0 | 5.99 | 99.83 | 2.06 | |
| 8.0 | 7.97 | 99.62 | 1.94 | |
| 10.0 | 9.93 | 99.3 | 1.72 | |
| 12.0 | 11.91 | 99.25 | 1.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinothkumar, V.; Sekhar, Y.C.; Chen, S.-M.; Manjula, N.; Kim, T.H. A Sensitive and Selective Sensor Based on Orthorhombic Copper Molybdate Decorated on Reduced Graphene Oxide for the Detection of Promethazine Hydrochloride. Sensors 2025, 25, 3569. https://doi.org/10.3390/s25113569
Vinothkumar V, Sekhar YC, Chen S-M, Manjula N, Kim TH. A Sensitive and Selective Sensor Based on Orthorhombic Copper Molybdate Decorated on Reduced Graphene Oxide for the Detection of Promethazine Hydrochloride. Sensors. 2025; 25(11):3569. https://doi.org/10.3390/s25113569
Chicago/Turabian StyleVinothkumar, Venkatachalam, Yellatur Chandra Sekhar, Shen-Ming Chen, Natesan Manjula, and Tae Hyun Kim. 2025. "A Sensitive and Selective Sensor Based on Orthorhombic Copper Molybdate Decorated on Reduced Graphene Oxide for the Detection of Promethazine Hydrochloride" Sensors 25, no. 11: 3569. https://doi.org/10.3390/s25113569
APA StyleVinothkumar, V., Sekhar, Y. C., Chen, S.-M., Manjula, N., & Kim, T. H. (2025). A Sensitive and Selective Sensor Based on Orthorhombic Copper Molybdate Decorated on Reduced Graphene Oxide for the Detection of Promethazine Hydrochloride. Sensors, 25(11), 3569. https://doi.org/10.3390/s25113569










