Electrochemical Determination of Creatinine Based on Multienzyme Cascade-Modified Nafion/Gold Nanoparticles/Screen-Printed Carbon Composite Biosensors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Modification of SPCEs
2.3. Multienzyme Cascade Modification
3. Results
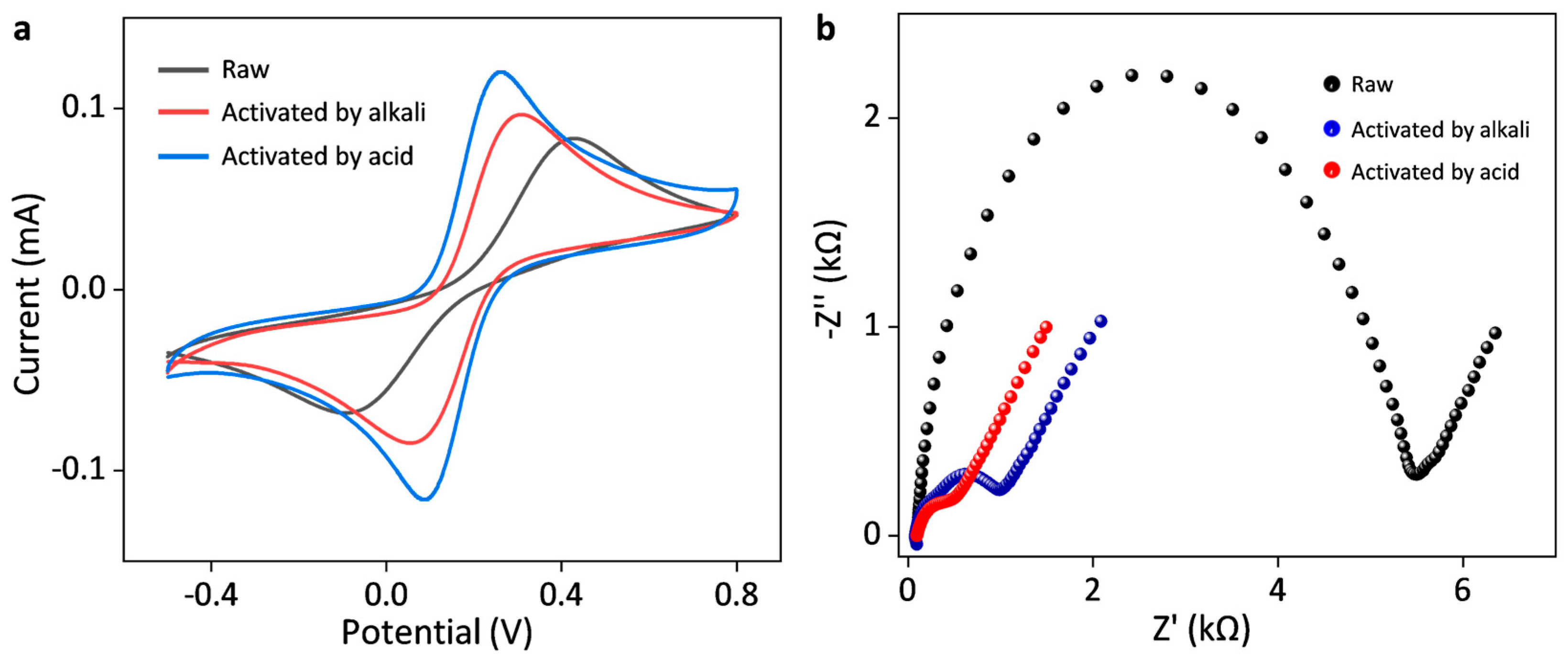
3.1. Effects of Electrochemical Activation on the Electrochemical Performance of SPCE
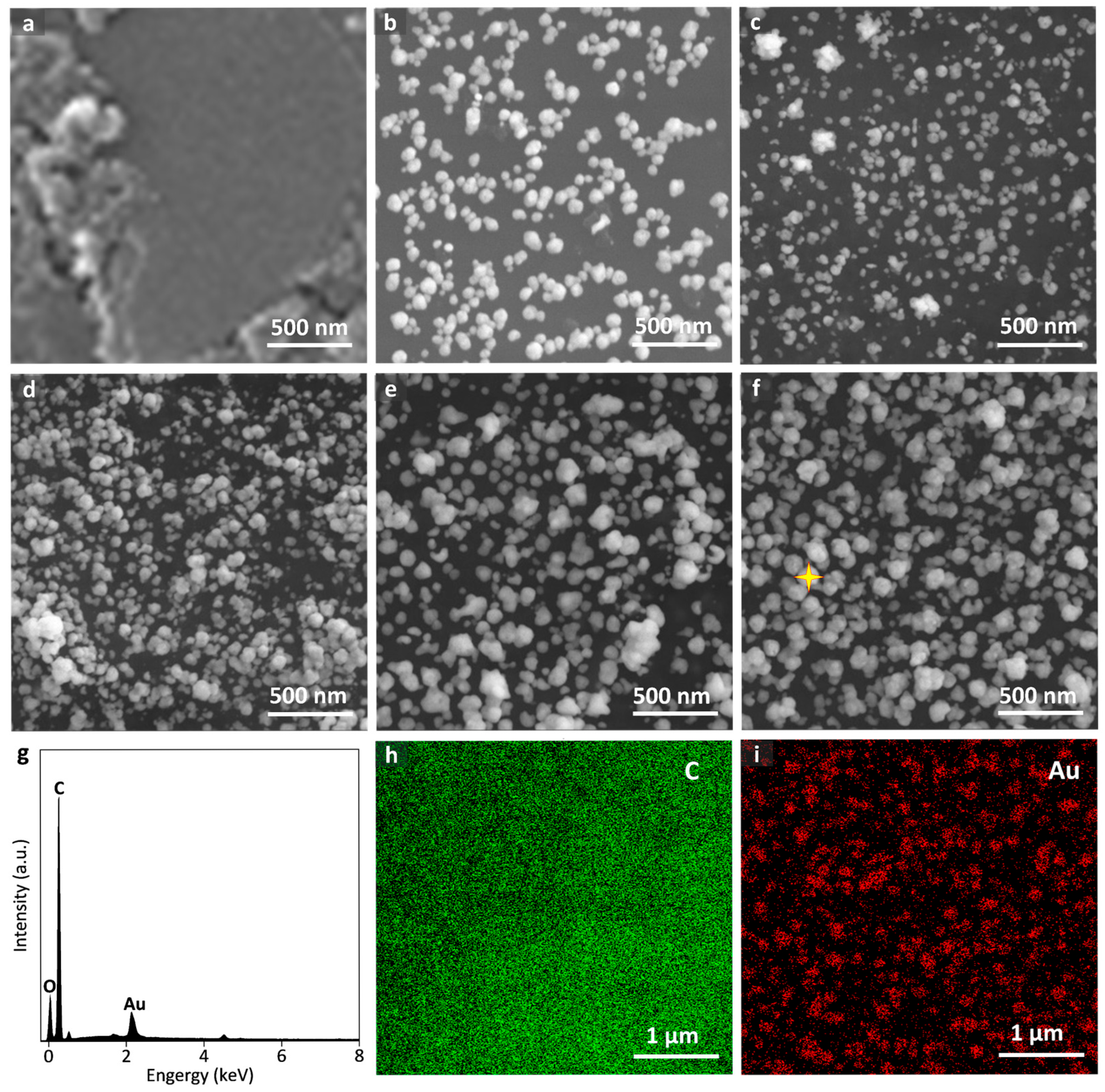
3.2. Surface Modification of SPCEs by AuNPs and Their Resulting Electrochemical Performance
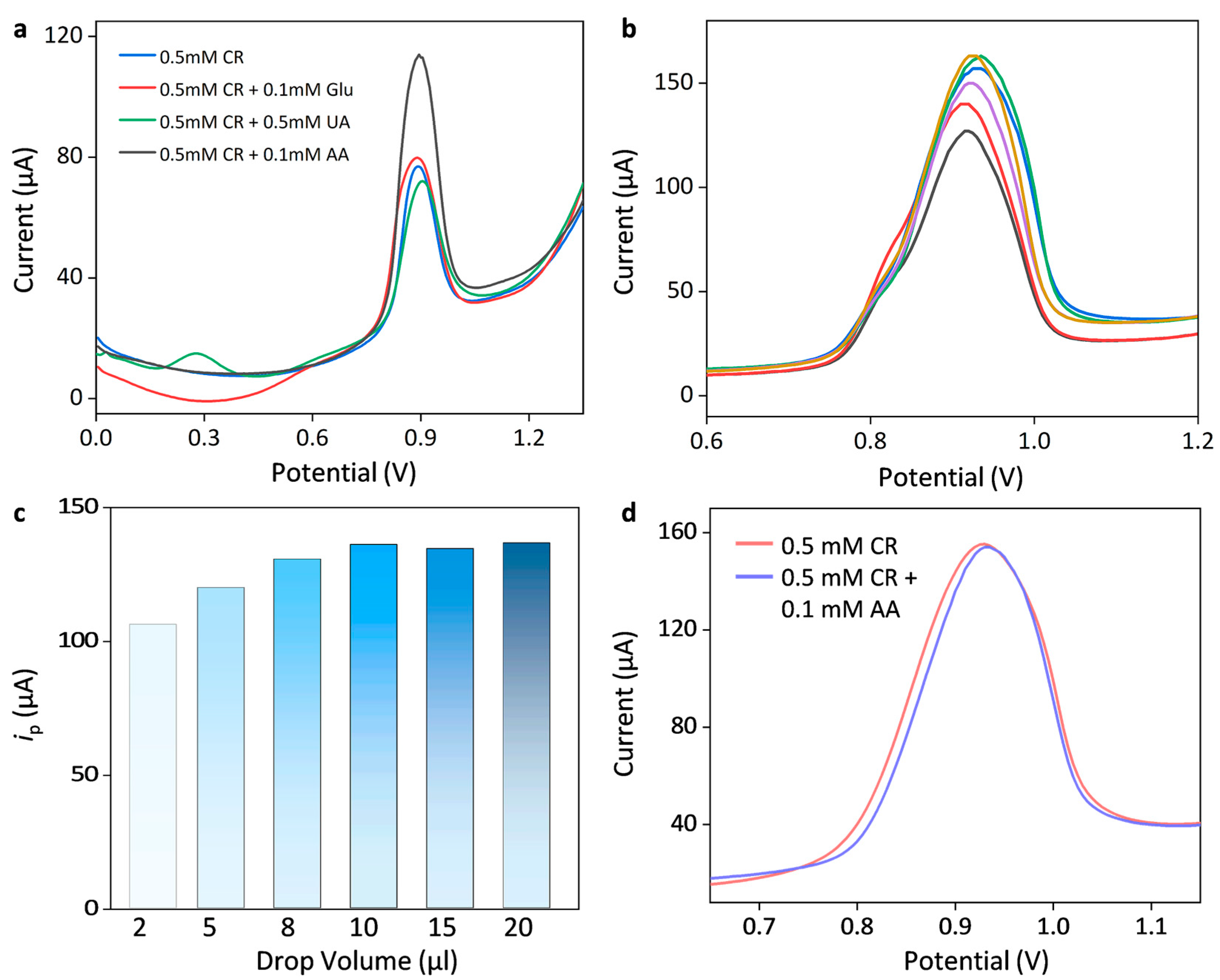
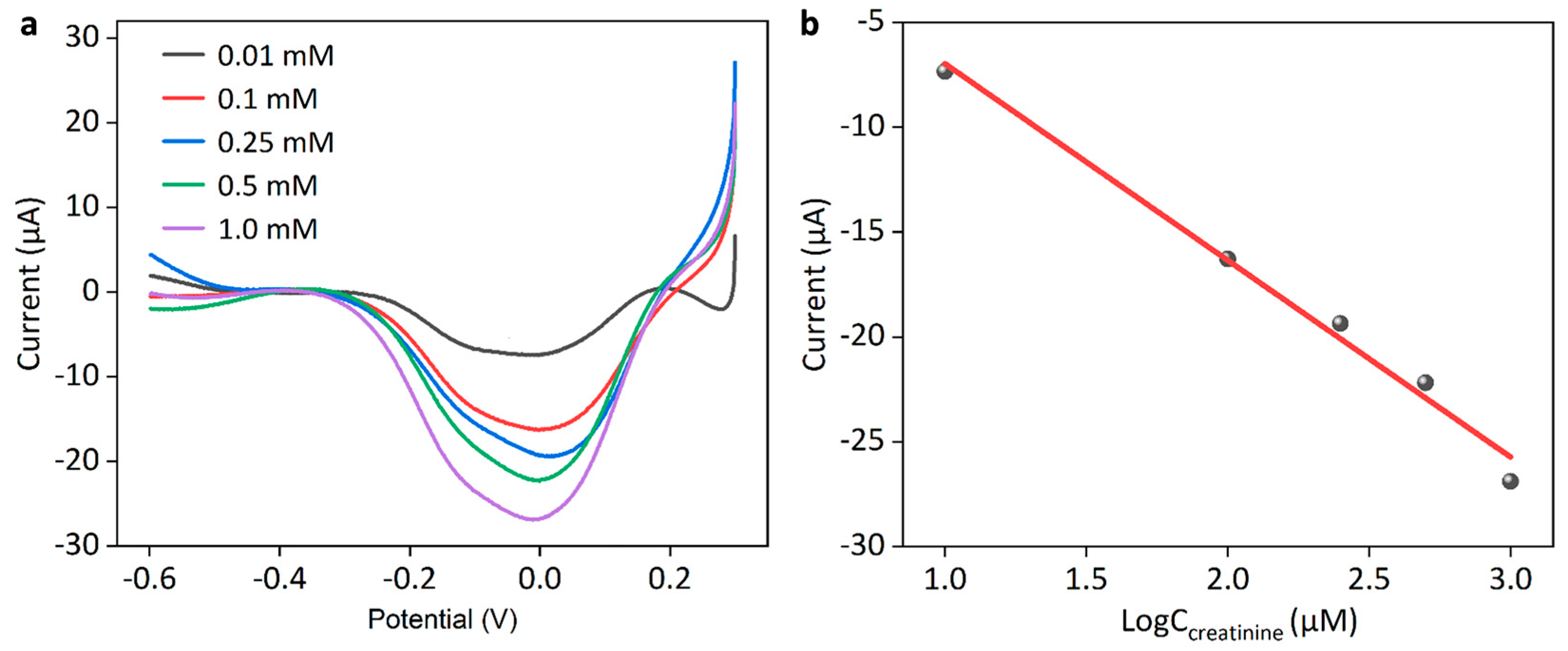
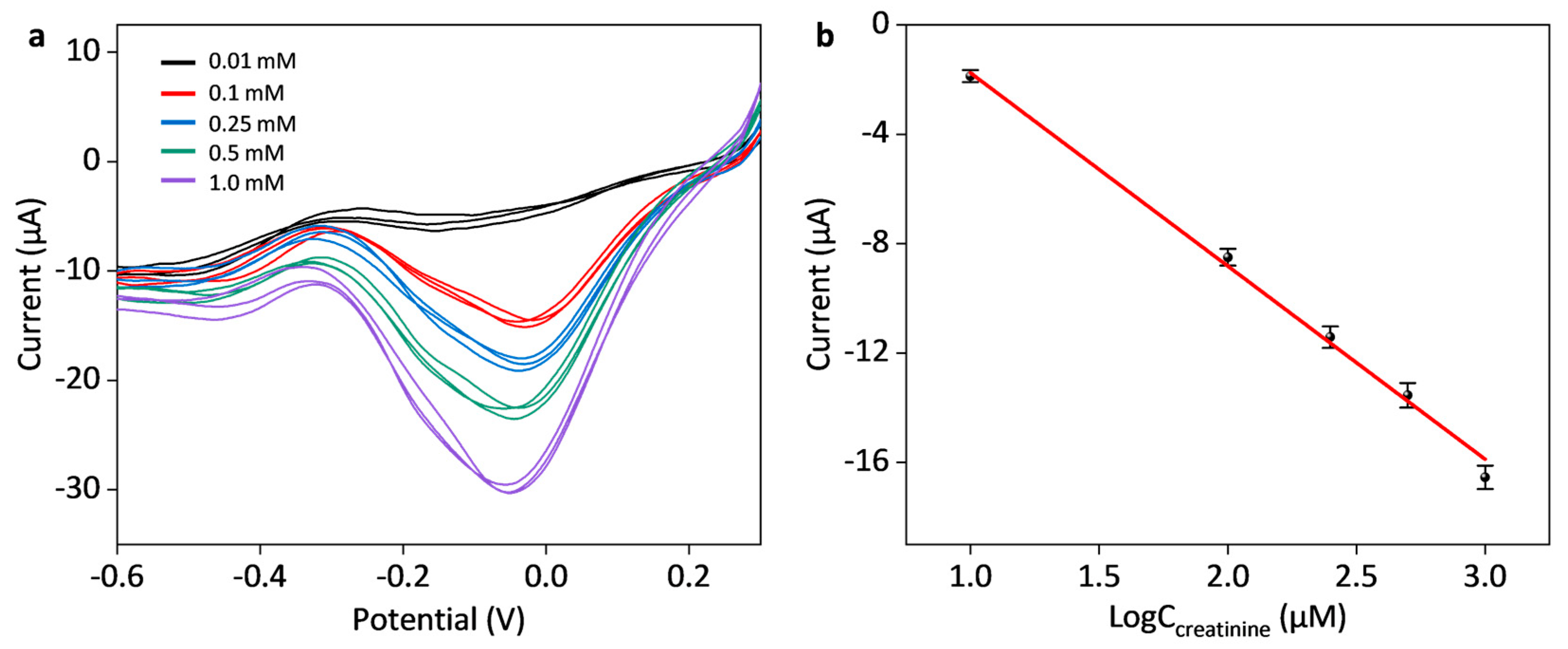
3.3. Construction of Multienzyme Cascade Mmodified Biosensor and Its Sensing Performance
4. Discussions and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.-J. Acute kidney injury. Nat. Rev. Dis. Primers 2021, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Kellum, J.A.; Ronco, C. Acute kidney injury. Lancet 2012, 380, 756–766. [Google Scholar] [CrossRef]
- Narimani, R.; Esmaeili, M.; Rasta, S.H.; Khosroshahi, H.T.; Mobed, A. Trend in creatinine determining methods: Conventional methods to molecular-based methods. Anal. Sci. Adv. 2021, 2, 308–325. [Google Scholar] [CrossRef] [PubMed]
- den Elzen, W.P.; Cobbaert, C.M.; Gunnewiek, J.M.K.; Bakkeren, D.L.; Van Berkel, M.; Frasa, M.A.; Herpers, R.L.; Kuypers, A.W.; Ramakers, C.; Roelofsen-de Beer, R.J. Glucose and total protein: Unacceptable interference on Jaffe creatinine assays in patients. Clin. Chem. Lab. Med. 2018, 56, e185–e187. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Yamamoto, S.; Jung, C.-Y.; Jin, D.Y.; Lee, T.; Kim, J.-S. Wearable sensors for monitoring chronic kidney disease. Commun. Mater. 2024, 5, 153. [Google Scholar] [CrossRef]
- Adelaars, S.; Lapré, C.S.; Raaijmakers, P.; Konings, C.J.; Mischi, M.; Bouwman, R.A.; van de Kerkhof, D. A novel LC-MS/MS assay for low concentrations of creatinine in sweat and saliva to validate biosensors for continuous monitoring of renal function. J. Chromatogr. B 2025, 1252, 124444. [Google Scholar] [CrossRef]
- Li, Y.; Luo, L.; Kong, Y.; George, S.; Li, Y.; Guo, X.; Li, X.; Yeatman, E.; Davenport, A.; Li, Y. A Point-of-Care Sensing Platform for Multiplexed Detection of Chronic Kidney Disease Biomarkers Using Molecularly Imprinted Polymers. Adv. Funct. Mater. 2024, 34, 2316865. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Hsu, Y.-P.; Tain, Y.-L.; Li, N.-S.; Pang, H.-H.; Kuo, S.-W.; Yang, H.-W. Microneedle patches integrated with lateral flow cassettes for blood-free chronic kidney disease point-of-care testing during a pandemic. Biosens. Bioelectron. 2022, 208, 114234. [Google Scholar] [CrossRef]
- Pundir, C.; Kumar, P.; Jaiwal, R. Biosensing methods for determination of creatinine: A review. Biosens. Bioelectron. 2019, 126, 707–724. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Min, J.; Song, Y.; Tu, J.; Mukasa, D.; Ye, C.; Xu, C.; Heflin, N.; McCune, J.S. A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 2022, 6, 1225–1235. [Google Scholar] [CrossRef]
- Saeed, M.; Saddique, Z.; Mujahid, A.; Afzal, A. Discerning biomimetic nanozyme electrodes based on g-C3N4 nanosheets and molecularly imprinted polythiophene nanofibers for detecting creatinine in microliter droplets of human saliva. Biosens. Bioelectron. 2024, 247, 115899. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, H.; Chen, W.; Ma, B.; Ju, H. Device integration of electrochemical biosensors. Nat. Rev. Bioeng. 2023, 1, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cánovas, R.; Crespo, G.A.; Cuartero, M. Thin-layer potentiometry for creatinine detection in undiluted human urine using ion-exchange membranes as barriers for charged interferences. Anal. Chem. 2020, 92, 3315–3323. [Google Scholar] [CrossRef]
- Dong, Y.; Luo, X.; Liu, Y.; Yan, C.; Li, H.; Lv, J.; Yang, L.; Cui, Y. A disposable printed amperometric biosensor for clinical evaluation of creatinine in renal function detection. Talanta 2022, 248, 123592. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, G.; Xu, G.; Li, X.; Shi, Z.; Cheng, C.; Xu, D.; Lu, Y.; Liu, Q. Battery-free and wireless tag for in situ sensing of urinary albumin/creatinine ratio (ACR) for the assessment of albuminuria. Sens. Actuators B 2022, 367, 132050. [Google Scholar] [CrossRef]
- Nontawong, N.; Amatatongchai, M.; Thimoonnee, S.; Laosing, S.; Jarujamrus, P.; Karuwan, C.; Chairam, S. Novel amperometric flow-injection analysis of creatinine using a molecularly-imprinted polymer coated copper oxide nanoparticle-modified carbon-paste-electrode. J. Pharm. Biomed. Anal. 2019, 175, 112770. [Google Scholar] [CrossRef]
- Kalasin, S.; Sangnuang, P.; Khownarumit, P.; Tang, I.M.; Surareungchai, W. Salivary creatinine detection using a Cu (I)/Cu (II) catalyst layer of a supercapacitive hybrid sensor: A wireless IoT device to monitor kidney diseases for remote medical mobility. ACS Biomater. Sci. Eng. 2020, 6, 5895–5910. [Google Scholar] [CrossRef] [PubMed]
- Rakesh Kumar, R.K.; Shaikh, M.O.; Kumar, A.; Liu, C.-H.; Chuang, C.-H. Zwitterion-functionalized cuprous oxide nanoparticles for highly specific and enzymeless electrochemical creatinine biosensing in human serum. ACS Appl. Nano Mater. 2023, 6, 2083–2094. [Google Scholar] [CrossRef]
- Dasgupta, P.; Kumar, V.; Krishnaswamy, P.R.; Bhat, N. Serum creatinine electrochemical biosensor on printed electrodes using monoenzymatic pathway to 1-methylhydantoin detection. ACS Omega 2020, 5, 22459–22464. [Google Scholar] [CrossRef]
- Jadhav, R.B.; Patil, T.; Tiwari, A.P. Trends in sensing of creatinine by electrochemical and optical biosensors. Appl. Surf. Sci. Adv. 2024, 19, 100567. [Google Scholar] [CrossRef]
- Jampasa, S.; Khamcharoen, W.; Traipop, S.; Jesadabundit, W.; Ozer, T.; Chailapakul, O. Recent advances on nanomaterial-modified film-electrode-based sensors: Approach to clinical purpose. Curr. Opin. Electrochem. 2023, 42, 101420. [Google Scholar] [CrossRef]
- Kumar, R.R.; Shaikh, M.O.; Chuang, C.-H. A review of recent advances in non-enzymatic electrochemical creatinine biosensing. Anal. Chim. Acta 2021, 1183, 338748. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.; Abbas, M.N. Non-enzymatic disposable electrochemical sensors based on CuO/Co3O4@ MWCNTs nanocomposite modified screen-printed electrode for the direct determination of urea. Sci. Rep. 2023, 13, 2034. [Google Scholar] [CrossRef]
- Vrabelj, T.; Finšgar, M. Recent progress in non-enzymatic electroanalytical detection of pesticides based on the use of functional nanomaterials as electrode modifiers. Biosensors 2022, 12, 263. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Deng, Z.; Wang, Y.; Zhou, K.; Yu, Z.; Ma, L.; Wei, Q. A nanoporous diamond particle microelectrode and its surface modification. Electrochim. Acta 2022, 430, 141015. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Jiao, Z.; Zhu, R.; Ma, L.; Zhou, K.; Yu, Z.; Wei, Q. Engineering a Au-NPs/Nafion modified nanoporous diamond sensing interface for reliable voltammetric quantification of dopamine in human serum. J. Chem. Eng. 2022, 446, 136927. [Google Scholar] [CrossRef]
- Wang, H.; Sayed, S.Y.; Luber, E.J.; Olsen, B.C.; Shirurkar, S.M.; Venkatakrishnan, S.; Tefashe, U.M.; Farquhar, A.K.; Smotkin, E.S.; McCreery, R.L. Redox flow batteries: How to determine electrochemical kinetic parameters. ACS Nano 2020, 14, 2575–2584. [Google Scholar] [CrossRef]
- Lavagnini, I.; Antiochia, R.; Magno, F. An extended method for the practical evaluation of the standard rate constant from cyclic voltammetric data. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2004, 16, 505–506. [Google Scholar] [CrossRef]
- Kashani, K.; Rosner, M.H.; Ostermann, M. Creatinine: From physiology to clinical application. Eur. J. Intern. Med. 2020, 72, 9–14. [Google Scholar] [CrossRef]
- Bardyn, M.; Chen, J.; Dussiot, M.; Crettaz, D.; Schmid, L.; Längst, E.; Amireault, P.; Tissot, J.-D.; Jolicoeur, M.; Prudent, M. Restoration of physiological levels of uric acid and ascorbic acid reroutes the metabolism of stored red blood cells. Metabolites 2020, 10, 226. [Google Scholar] [CrossRef]
- Lousada, C.M.; Johansson, A.J.; Brinck, T.; Jonsson, M. Mechanism of H2O2 decomposition on transition metal oxide surfaces. J. Phys. Chem. C 2012, 116, 9533–9543. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Yu, R.; Zhang, W.; Wang, Y.; Deng, Z. Electrochemical Determination of Creatinine Based on Multienzyme Cascade-Modified Nafion/Gold Nanoparticles/Screen-Printed Carbon Composite Biosensors. Sensors 2025, 25, 4132. https://doi.org/10.3390/s25134132
Yang J, Yu R, Zhang W, Wang Y, Deng Z. Electrochemical Determination of Creatinine Based on Multienzyme Cascade-Modified Nafion/Gold Nanoparticles/Screen-Printed Carbon Composite Biosensors. Sensors. 2025; 25(13):4132. https://doi.org/10.3390/s25134132
Chicago/Turabian StyleYang, Jialin, Ruizhi Yu, Wanxin Zhang, Yijia Wang, and Zejun Deng. 2025. "Electrochemical Determination of Creatinine Based on Multienzyme Cascade-Modified Nafion/Gold Nanoparticles/Screen-Printed Carbon Composite Biosensors" Sensors 25, no. 13: 4132. https://doi.org/10.3390/s25134132
APA StyleYang, J., Yu, R., Zhang, W., Wang, Y., & Deng, Z. (2025). Electrochemical Determination of Creatinine Based on Multienzyme Cascade-Modified Nafion/Gold Nanoparticles/Screen-Printed Carbon Composite Biosensors. Sensors, 25(13), 4132. https://doi.org/10.3390/s25134132







