Machine Learning Algorithms in EEG Analysis of Kleefstra Syndrome: Current Evidence and Future Directions
Abstract
1. Introduction
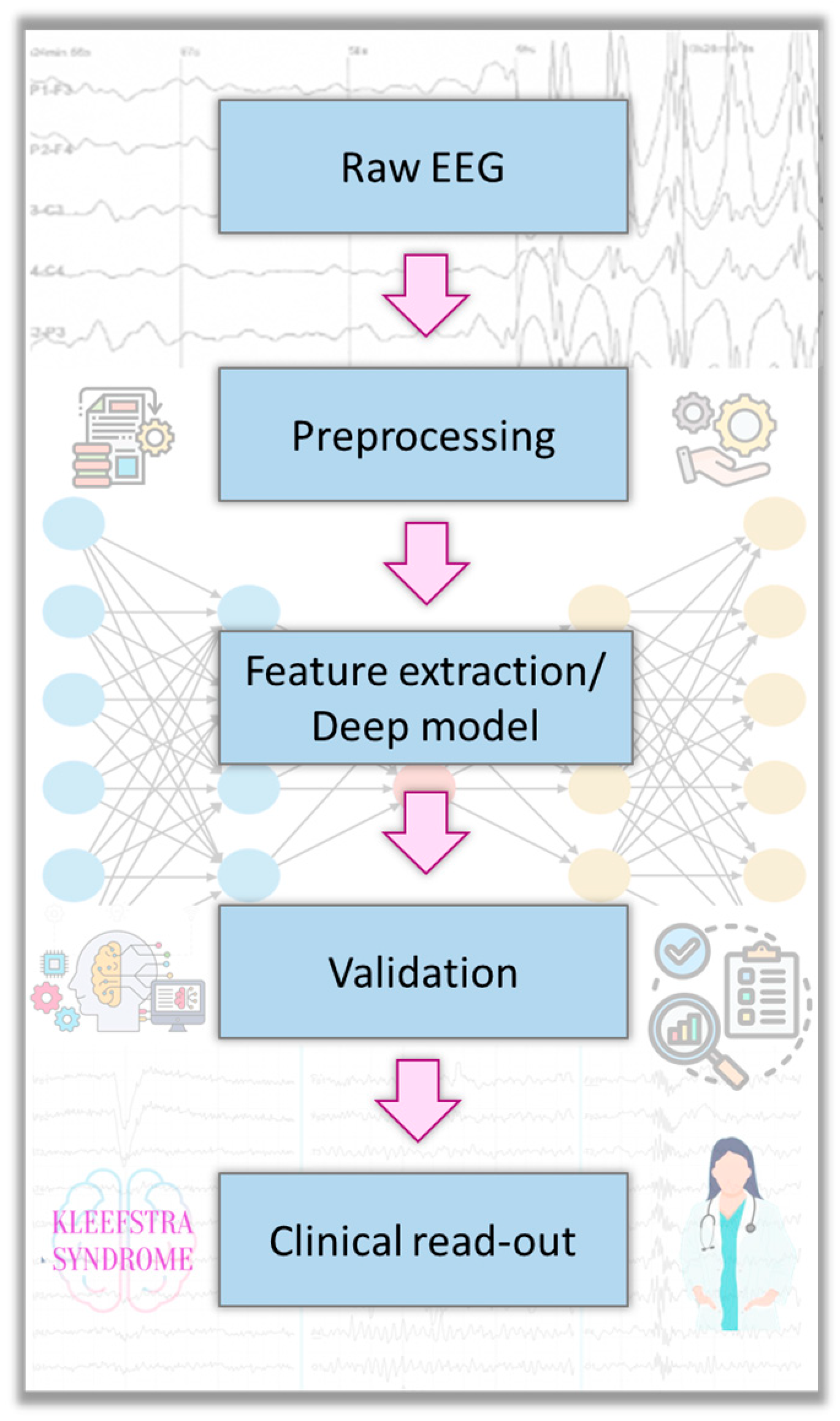
2. Materials and Methods
- (i)
- molecular-genetic or clinical reports with no EEG data (n = 18);
- (ii)
- animal-only studies (n = 7).
3. Results
3.1. EEG Abnormalities in Kleefstra Syndrome
3.2. EEG Data Collection and Processing in Kleefstra Syndrome
3.3. EEG Datasets and Studies in Rare Disorders
3.4. The BRAINMODEL Project
3.5. COMBINEDBrain Consortium
4. Discussion
4.1. Comparative Data and Methodologies from Other Rare Syndromes
4.2. Machine Learning in EEG Analysis for Rare Disorders
4.3. Applications of Machine Learning—Current State
- Diagnostic Classification: Machine learning can classify patient vs control EEGs with high accuracy in certain conditions. In the Bosl et al. autism study [11] for example, researchers computed nonlinear EEG features (e.g., fractal dimensions, entropies) for each infant and then fed these into algorithms like support vector machines (SVM) to predict outcome [11]. They tried k-nearest neighbors, random forests and SVM, ultimately using an SVM with an RBF kernel, which achieved >95% accuracy in distinguishing ASD vs non-ASD based on EEG [11]. While ASD is more common than KS, the principle holds that early brain functional differences can be detected via ML. In rare genetic syndromes, one could envision a tool that analyzes a child’s EEG and outputs a probability of a specific syndrome (e.g., KS vs Angelman vs others) to prompt targeted genetic testing. In fact, patient advocates have suggested using AI on routine EEGs as a screening proxy for genetic testing in undiagnosed children [10]. Such an approach is in its infancy, but the conceptual feasibility is supported by results in more studied conditions (e.g., distinguishing schizophrenia patients by EEG patterns via deep learning [10].
- Biomarker Discovery: Quantitative EEG changes correlated with phenotype have been discovered through ML and advanced analysis. In Rett syndrome clinical trials, network analysis combined with machine learning was able to separate treatment responders from non-responders purely from pretreatment EEG, with 100% accuracy in one small study [41]. For KS, applying similar analyses could be highly informative. For instance, does a KS child with more pronounced EEG slowing have a more severe cognitive impairment? Are there EEG metrics that correlate with the degree of regression in adolescence? These questions remain unanswered. Filling this gap could not only improve clinical monitoring but also provide objective endpoints for any future therapeutic trials in KS (where EEG could serve as a measure of neurological improvement or stabilization).
- Seizure Prediction and Management: Outside of syndrome diagnosis, ML applied to EEG is extensively used in seizure detection/prediction. For KS patients with epilepsy, adapting those tools could improve care. No study specifically addresses KS seizure prediction, but given that most KS seizures are focal, one interesting avenue is the use of ML to detect the precursors of focal seizures (e.g., changes in EEG power or network topology minutes before a seizure). Modern algorithms can sometimes forecast seizures by recognizing subtle pre-ictal patterns. If KS caregivers were equipped with seizure prediction devices (e.g., wearable EEG with AI), it could enhance safety. This is speculative but highlights a potential area for contribution: customizing general epilepsy AI technology to the specific seizure profiles and EEG morphologies seen in KS.
- (1)
- There is no comprehensive description of EEG features in a large KS cohort—we lack knowledge of whether KS has a mild but consistent EEG signature (for example, do all KS patients show a slightly lower dominant frequency compared to peers, as CNV carriers did in one study? [22]).
- (2)
- There are no public or large shared EEG datasets for KS, which slows research progress.
- (3)
- Machine learning techniques, which could uncover hidden patterns in KS EEG, have not yet been applied, leaving a rich potential unexploited.
- (4)
- Because of these, clinicians currently do not use EEG for anything beyond conventional seizure management in KS—unlike some other syndromes where EEG might aid earlier diagnosis (e.g., the notched delta in Angelman can raise clinical suspicion of that diagnosis [12]).
4.4. Potential Areas for Future Contribution
- Feature-Based Machine Learning: This involves manually extracting features from EEG signals and then using classical ML algorithms for classification or regression. In any future KS EEG study, one might extract features such as average slow-wave (delta) power, spike frequency or network synchrony metrics and then train a classifier to distinguish KS patients from other groups. Support vector machines and Random Forests are popular choices for such small-data classification problems because they can work well with limited samples and many features, especially with careful cross-validation to avoid overfitting. Other methods like logistic regression or Gaussian naive Bayes could also be tested as baselines. Feature selection or dimensionality reduction (e.g., principal component analysis) is often incorporated to handle the high-dimensional feature space relative to the number of subjects.
- Deep Learning on EEG: Deep learning models, particularly CNNs, have revolutionized EEG analysis in larger datasets, as they can learn complex spatiotemporal features directly from raw signals or from transformed representations (like time–frequency spectrograms). For rare disorders with small N, deep learning must be used cautiously to avoid overfitting. However, another methodology is transfer learning: using a CNN pre-trained on a big EEG dataset (for example, a seizure detection CNN trained on thousands of hours of EEG from an epilepsy database) and then fine-tuning it on the rare disorder EEG data to detect more subtle patterns. Another approach is data augmentation—creating synthetic EEG segments (perhaps using methods like GANs or by simple slicing/windowing techniques) to effectively increase the training set size. Although we did not find an example of CNN or RNN applied specifically to a rare syndrome EEG classification, these techniques are well documented in the epilepsy literature [29,44]. For instance, CNN-based algorithms have achieved impressive accuracy in identifying epileptic vs normal EEG segments and even differentiating seizure types. An RNN (such as an LSTM network) could capture temporal dynamics in KS EEG, like sequences of microstates or recurrent patterns over minutes. Hybrid models (combining CNN for spatial feature extraction and RNN for temporal modeling) have also been proposed for EEG analysis. The DICE-Net architecture (a convolution-transformer network) for Alzheimer’s EEG classification [23] exemplifies cutting-edge deep learning that could conceivably be adapted to detect subtle encephalopathic patterns in KS.
- Validation and Evaluation: For any ML methodology applied to EEG, rigorous validation is crucial. The literature emphasizes techniques such as k-fold cross-validation or leave-one-subject-out validation when sample sizes are small [45]. Performance metrics commonly reported include accuracy, sensitivity (recall), specificity and the area under the ROC curve (AUC). In an ASD prediction study, for instance, the model’s positive predictive value (PPV) and sensitivity were extremely high (>95%) when evaluated on held-out data at certain time points [11]. In a KS context, one might also consider unsupervised learning methods due to limited labeled data—for example, clustering algorithms to see if EEG feature profiles cluster KS patients separately from others, or anomaly detection algorithms that treat KS as “outliers” against a background of typical EEGs.
- Data Collection and Sharing: A foundational step is to gather a larger dataset of EEG recordings from individuals with Kleefstra syndrome. Multi-center collaborations or family-led registries could enable the collection of standardized resting-state and sleep EEG from dozens of KS patients. Importantly, making such a dataset publicly available (with proper anonymization and consent) would invite machine learning experts to contribute analyses [10]. As seen with other disorders, when data is shared, progress accelerates. The KS community, possibly through organizations like IDefine [15] and IDefine Europe [16] or partnerships with platforms like Rare-X and GestaltMatcher, could spearhead an “EEG data commons” for Kleefstra syndrome. This would directly address the current lack of data.
- Quantitative EEG Characterization: Even prior to complex ML, performing a thorough quantitative analysis of KS EEGs would be valuable. Future studies should compute spectral features, connectivity metrics and sleep microstructure (if overnight EEG or polysomnography is available) for KS cohorts and compare them to neurotypical controls or to individuals with other ID syndromes. This might reveal, for example, that KS brains have a particular profile of oscillatory activity. Any distinctive pattern, once confirmed, could become a biomarker. This is low-hanging fruit that modern EEG analysis software can accomplish, but it requires assembling the recordings first.
- Machine Learning Diagnostic Models: With enough data, researchers can train ML models to classify EEGs as KS or non-KS. Even if perfection is not attainable, a moderately accurate model could be useful as a screening tool. For instance, in an undiagnosed child with developmental delay, an “EEG ML panel” might output probabilities for several genetic syndromes (Kleefstra, Angelman, Tuberous Sclerosis, etc.) based on the EEG pattern. As highlighted by the SLC6A1 initiative, this could guide genetic testing earlier and shorten the diagnostic odyssey [10]. Developing such models for KS would contribute to precision medicine by leveraging an easily obtainable test (EEG) to hint at a genetic diagnosis.
- Prognostic and Monitoring Tools: Another potential contribution is using EEG-based ML to track disease progression or therapeutic response in KS. For example, if a future clinical trial tests a drug targeting EHMT1 pathways, EEG could be used to quantify brain function changes. ML could potentially be explored for KS patients, which could serve as an outcome measure. Additionally, longitudinal EEG data analyzed with ML might predict which children are at risk of neuropsychiatric regression in adolescence—a known concern in KS [5,7]. Early predictions could prompt proactive interventions.
- Cross-Disorder Insights: Finally, a broader contribution would be to integrate KS EEG findings into the larger map of rare neurodevelopmental disorders [46]. By applying uniform ML analyses across multiple syndromes (e.g., KS, KBG, Pitt–Hopkins, Phelan–McDermid, etc.), we can identify shared neural signatures or important differences. The recent CNV EEG study hinted that many disorders share EEG alterations despite different genetics [22]. Confirming this with more syndromes could shift the focus toward treating common circuit-level problems rather than each genetic disorder in isolation. For KS, contributing data to such comparative studies ensures it is included in the development of broadly effective neurotherapeutic strategies.
5. Conclusions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| KS | Kleefstra syndrome |
| ML | Machine learning |
| EEG | Electroencephalogram |
| ICA | Independent component analysis |
| SVM | Support vector machines |
| ASD | Autism spectrum disorder |
| CNNs | Convolutional neural networks |
References
- Giacomini, T.; Cordani, R.; Bagnasco, I.; Vercellino, F.; Giordano, L.; Milito, G.; Ferrero, G.B.; Mandrile, G.; Scala, M.; Meli, M.; et al. Electroclinical Features of Epilepsy in Kleefstra Syndrome. Neuropediatrics 2023, 54, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Morison, L.D.; Kennis, M.G.P.; Rots, D.; Bouman, A.; Kummeling, J.; Palmer, E.; Vogel, A.P.; Liegeois, F.; Brignell, A.; Srivastava, S.; et al. Expanding the Phenotype of Kleefstra Syndrome: Speech, Language and Cognition in 103 Individuals. J. Med. Genet. 2024, 61, 578–585. [Google Scholar] [CrossRef]
- Rots, D.; Bouman, A.; Yamada, A.; Levy, M.; Dingemans, A.J.M.; de Vries, B.B.A.; Ruiterkamp-Versteeg, M.; de Leeuw, N.; Ockeloen, C.W.; Pfundt, R.; et al. Comprehensive EHMT1 Variants Analysis Broadens Genotype-Phenotype Associations and Molecular Mechanisms in Kleefstra Syndrome. Am. J. Hum. Genet. 2024, 111, 1605–1625. [Google Scholar] [CrossRef]
- Zdolšek Draksler, T.; Bouman, A.; Guček, A.; Novak, E.; Burger, P.; Colin, F.; Kleefstra, T. Exploring Kleefstra Syndrome Cohort Phenotype Characteristics: Prevalence Insights from Caregiver-Reported Outcomes. Eur. J. Med. Genet. 2024, 72, 104974. [Google Scholar] [CrossRef] [PubMed]
- Ettore, C. Kleefstra Syndrome and Sleep Disorders: An Italian Case Report. J. Neurol. Neurol. Sci. Disord. 2023, 9, 033–040. [Google Scholar] [CrossRef]
- Kleefstra Syndrome UK. What Is Kleefstra Syndrome? Available online: https://www.kleefstrasyndrome.org/what-is-kleefstra-syndrome/ (accessed on 29 March 2025).
- Kleefstra, T.; de Leeuw, N. Kleefstra Syndrome; University of Washington: Seattle, WA, USA, 2023. [Google Scholar]
- Frazier, Z.J.; Kilic, S.; Osika, H.; Mo, A.; Quinn, M.; Ballal, S.; Katz, T.; Shearer, A.E.; Horlbeck, M.A.; Pais, L.S.; et al. Novel Phenotypes and Genotype–Phenotype Correlations in a Large Clinical Cohort of Patients With Kleefstra Syndrome. Clin. Genet. 2025, 107, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Tzimourta, K.D.; Christou, V.; Tzallas, A.T.; Giannakeas, N.; Astrakas, L.G.; Angelidis, P.; Tsalikakis, D.; Tsipouras, M.G. Machine Learning Algorithms and Statistical Approaches for Alzheimer’s Disease Analysis Based on Resting-State EEG Recordings: A Systematic Review. Int. J. Neural Syst. 2021, 31, 2130002. [Google Scholar] [CrossRef]
- McEntee, K. SLC6A1 Connect Proposes Public EEG Dataset For Diagnostic Research. Available online: https://slc6a1connect.org/2023/11/10/slc6a1-connect-proposes-public-eeg-dataset-for-diagnostic-research/ (accessed on 20 March 2025).
- Bosl, W.J.; Tager-Flusberg, H.; Nelson, C.A. EEG Analytics for Early Detection of Autism Spectrum Disorder: A Data-Driven Approach. Sci. Rep. 2018, 8, 6828. [Google Scholar] [CrossRef]
- Goodspeed, K.; Armstrong, D.; Dolce, A.; Evans, P.; Said, R.; Tsai, P.; Sirsi, D. Electroencephalographic (EEG) Biomarkers in Genetic Neurodevelopmental Disorders. J. Child Neurol. 2023, 38, 466–477. [Google Scholar] [CrossRef]
- Geertjens, L.; van Voorst, T.W.; Bouman, A.; van Boven, M.A.; Kleefstra, T.; Verhage, M.; Linkenkaer-Hansen, K.; Kasri, N.N.; Cornelisse, L.N.; Bruining, H. Following Excitation/Inhibition Ratio Homeostasis from Synapse to EEG in Monogenetic Neurodevelopmental Disorders. Genes 2022, 13, 390. [Google Scholar] [CrossRef]
- Vargiami, E.; Ververi, A.; Al-Mutawa, H.; Gioula, G.; Gerou, S.; Rouvalis, F.; Kambouris, M.; Zafeiriou, D.I. Multiple Coronary Artery Microfistulas in a Girl with Kleefstra Syndrome. Case Rep. Genet. 2016, 2016, 3056053. [Google Scholar] [CrossRef] [PubMed][Green Version]
- IDefine The Kleefstra Syndrome Foundation. What Is Kleefstra Syndrome? 2025. Available online: https://www.idefine.org/about-kleefstra-syndrome/ (accessed on 29 March 2025).
- IDefine Europe. What Are the Symptoms and Causes? Available online: https://idefine-europe.org/kleefstra-syndrome/ (accessed on 29 April 2025).
- Guo, L.; Park, J.; Yi, E.; Marchi, E.; Hsieh, T.-C.; Kibalnyk, Y.; Moreno-Sáez, Y.; Biskup, S.; Puk, O.; Beger, C.; et al. KBG Syndrome: Prospective Videoconferencing and Use of AI-Driven Facial Phenotyping in 25 New Patients 2021. Eur. J. Hum. Genet. 2022, 30, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Paprocka, J.; Coppola, A.; Cuccurullo, C.; Stawicka, E.; Striano, P. Epilepsy, EEG and Chromosomal Rearrangements. Epilepsia Open 2024, 9, 1192–1232. [Google Scholar] [CrossRef]
- Kukla, M.; Karoń, M.; Chrościńska-Krawczyk, M. Kleefstra Syndrome—Case Report. Child Neurol. 2019, 28, 49–52. [Google Scholar] [CrossRef]
- Ciaccio, C.; Scuvera, G.; Tucci, A.; Gentilin, B.; Baccarin, M.; Marchisio, P.; Avignone, S.; Milani, D. New Insights into Kleefstra Syndrome: Report of Two Novel Cases with Previously Unreported Features and Literature Review. Cytogenet. Genome Res. 2019, 156, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Tzimourta, K.D.; Afrantou, T.; Ioannidis, P.; Karatzikou, M.; Tzallas, A.T.; Giannakeas, N.; Astrakas, L.G.; Angelidis, P.; Glavas, E.; Grigoriadis, N.; et al. Analysis of Electroencephalographic Signals Complexity Regarding Alzheimer’s Disease. Comput. Electr. Eng. 2019, 76, 198–212. [Google Scholar] [CrossRef]
- Dubois, A.E.E.; Audet-Duchesne, E.; Knoth, I.S.; Martin, C.-O.; Jizi, K.; Tamer, P.; Younis, N.; Jacquemont, S.; Dumas, G.; Lippé, S. Genetic Modulation of Brain Dynamics in Neurodevelopmental Disorders: The Impact of Copy Number Variations on Resting-State EEG. Transl. Psychiatry 2025, 15, 139. [Google Scholar] [CrossRef]
- Miltiadous, A.; Gionanidis, E.; Tzimourta, K.D.; Giannakeas, N.; Tzallas, A.T. DICE-Net: A Novel Convolution-Transformer Architecture for Alzheimer Detection in EEG Signals. IEEE Access 2023, 11, 71840–71858. [Google Scholar] [CrossRef]
- Simons Searchlight Registry Update: EHMT1; Simons Searchlight: New York, NY, USA, 2025.
- Laan, L.A.E.M.; Vein, A.A. Angelman Syndrome: Is There a Characteristic EEG? Brain Dev. 2005, 27, 80–87. [Google Scholar] [CrossRef]
- Pintaudi, M.; Calevo, M.G.; Vignoli, A.; Parodi, E.; Aiello, F.; Baglietto, M.G.; Hayek, Y.; Buoni, S.; Renieri, A.; Russo, S.; et al. Epilepsy in Rett Syndrome: Clinical and Genetic Features. Epilepsy Behav. 2010, 19, 296–300. [Google Scholar] [CrossRef]
- Portnova, G.; Neklyudova, A.; Voinova, V.; Sysoeva, O. Clinical EEG of Rett Syndrome: Group Analysis Supplemented with Longitudinal Case Report. J. Pers. Med. 2022, 12, 1973. [Google Scholar] [CrossRef] [PubMed]
- Giertuga, K.; Zakrzewska, M.Z.; Bielecki, M.; Racicka-Pawlukiewicz, E.; Kossut, M.; Cybulska-Klosowicz, A. Age-Related Changes in Resting-State Eeg Activity in Attention Deficit/Hyperactivity Disorder: A Cross-Sectional Study. Front. Hum. Neurosci. 2017, 11, 285. [Google Scholar] [CrossRef]
- Misirlis, Y.; Tzimourta, K.D.; Angelidis, P.; Giannakeas, N.; Tzallas, A.T.; Tsipouras, M.G. Pediatric Epilepsy Assessment Based on EEG Analysis. In Proceedings of the 2022 45th International Conference on Telecommunications and Signal Processing (TSP), Prague, Czech Republic, 13–15 July 2022; pp. 377–380. [Google Scholar]
- BRAINMODEL: Precision Medicine for Brain Disorders. Available online: https://brainmodel.nl/ (accessed on 13 April 2025).
- Frega, M.; Linda, K.; Keller, J.M.; Gümüş-Akay, G.; Mossink, B.; van Rhijn, J.R.; Negwer, M.; Klein Gunnewiek, T.; Foreman, K.; Kompier, N.; et al. Neuronal Network Dysfunction in a Model for Kleefstra Syndrome Mediated by Enhanced NMDAR Signaling. Nat. Commun. 2019, 10, 4928. [Google Scholar] [CrossRef]
- COMBINEDBrain—Consortium for Outcome Measures and Biomarkers for Neurodevelopmental Disorders. Available online: https://rarediseases.org/organizations/combinedbrain-consortium-for-outcome-measures-and-biomarkers-for-neurodevelopmental-disorders/ (accessed on 11 April 2025).
- Hatcher, K. COMBINEDBrain Received the Global Genes 2022 Health Equity RARE Patient Impact Grant. Available online: https://combinedbrain.org (accessed on 29 March 2025).
- Prince, S.; Bonkowski, E.; McGraw, C.; SanInocencio, C.; Mefford, H.C.; Carvill, G.; Broadbent, B. A Roadmap to Cure CHD2-Related Disorders. Ther. Adv. Rare Dis. 2024, 5, 26330040241283749. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.C.; Bar-Haim, A.; Moosa, S.; Ehmke, N.; Gripp, K.W.; Pantel, J.T.; Danyel, M.; Mensah, M.A.; Horn, D.; Rosnev, S.; et al. GestaltMatcher Facilitates Rare Disease Matching Using Facial Phenotype Descriptors. Nat. Genet. 2022, 54, 349–357. [Google Scholar] [CrossRef]
- Miltiadous, A.; Tzimourta, K.D.; Giannakeas, N.; Tsipouras, M.G.; Glavas, E.; Kalafatakis, K.; Tzallas, A.T. Machine Learning Algorithms for Epilepsy Detection Based on Published EEG Databases: A Systematic Review. IEEE Access 2023, 11, 564–594. [Google Scholar] [CrossRef]
- Obeid, I.; Picone, J. The Temple University Hospital EEG Data Corpus. Front. Neurosci. 2016, 10, 196. [Google Scholar] [CrossRef]
- Guttag, J. CHB-MIT Scalp EEG Database (Version 1.0.0); PhysioNet—MIT Laboratory for Computational Physiology: Cambridge, MA, USA, 2010. [Google Scholar]
- Islah, N.; Koerner, J.; Genov, R.; Valiante, T.A.; O’Leary, G. Machine Learning with Imbalanced EEG Datasets Using Outlier-Based Sampling. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 112–115. [Google Scholar]
- Han, D.; Qi, H.; Wang, S.X.; Hou, D.; Wang, C. Adaptive Stepsize Forward–Backward Pursuit and Acoustic Emission-Based Health State Assessment of High-Speed Train Bearings. Struct. Health Monit. 2024, 1–20. [Google Scholar] [CrossRef]
- Keogh, C.; Pini, G.; Gemo, I.; Kaufmann, W.E.; Tropea, D. Functional Network Mapping Reveals State-Dependent Response to IGF1 Treatment in Rett Syndrome. Brain Sci. 2020, 10, 515. [Google Scholar] [CrossRef]
- Hipp, J.F.; Frohlich, J.; Keute, M.; Tan, W.H.; Bird, L.M. Electrophysiological Abnormalities in Angelman Syndrome Correlate With Symptom Severity. Biol. Psychiatry Glob. Open Sci. 2021, 1, 201–209. [Google Scholar] [CrossRef]
- Ethridge, L.E.; Pedapati, E.V.; Schmitt, L.M.; Norris, J.E.; Auger, E.; De Stefano, L.A.; Sweeney, J.A.; Erickson, C.A. Validating Brain Activity Measures as Reliable Indicators of Individual Diagnostic Group and Genetically Mediated Sub-Group Membership in Fragile X Syndrome. Sci. Rep. 2024, 14, 22982. [Google Scholar] [CrossRef] [PubMed]
- Tzimourta, K.D.; Giannakeas, N.; Tzallas, A.T.; Astrakas, L.G.; Afrantou, T.; Ioannidis, P.; Grigoriadis, N.; Angelidis, P.; Tsalikakis, D.G.; Tsipouras, M.G. EEG Window Length Evaluation for the Detection of Alzheimer’s Disease over Different Brain Regions. Brain Sci. 2019, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Miltiadous, A.; Tzimourta, K.D.; Giannakeas, N.; Tsipouras, M.G.; Afrantou, T.; Ioannidis, P.; Tzallas, A.T. Alzheimer’s Disease and Frontotemporal Dementia: A Robust Classification Method of EEG Signals and a Comparison of Validation Methods. Diagnostics 2021, 11, 1437. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, J.; Lehne, M.; Schepers, J.; Prasser, F.; Thun, S. The Use of Machine Learning in Rare Diseases: A Scoping Review. Orphanet J. Rare Dis. 2020, 15, 145. [Google Scholar] [CrossRef]
| EEG Phenotype | EEG Description | Clinical Context/Notes | Reported Incidence | Ref. |
|---|---|---|---|---|
| Focal epileptiform discharges | Spikes/sharp waves confined to one region (often centro- temporal, temporal, or frontal) | Interictal correlate of the predominantly focal seizures described in KS; occasionally seen in patients without overt epilepsy | Common in epileptic KS cohorts | [1,4,5,7] |
| Diffuse/ generalized background slowing | Globally reduced posterior dominant rhythm; disorganized or low-frequency background | Mirrors moderate-severe intellectual disability and hypotonia typical of KS | Recurrent finding across small series and case reports but not systematically quantified | [5,17] |
| Generalized epileptiform activity | Generalized spike–wave or polyspike–wave bursts | Generalized tonic–clonic or absence seizures | Occasional (isolated case descriptions) | [1,4,5,7] |
| Refractory epilepsy (Infantile Spasms/ Lennox–Gastaut pattern) | Generalized slow spike-and-wave with paroxysmal fast activity | Not syndrome-specific but noted in a minority of KS children with seizures | Rare (individual cases) | [4,8] |
| Normal EEG | No epileptiform discharges; age-appropriate background | More often in younger children or KS individuals without seizures | Subset of patients (exact proportion unknown) | [3,19,20] |
| Syndrome | EEG Abnormalities | EEG Signature | Study (Year) | No. of Cases | ML Method | Results |
|---|---|---|---|---|---|---|
| Angelman | Rhythmic delta, frontal notch | Yes | Hip et al. (2021) [42] | 45 patients | Delta Spectral Power, Linear Support Vector Regression | Delta power correlates with severity |
| Rett | Stage-dependent, slowing | Yes | Keogh et al. (2020) [41] | 18 patients (9 treated and 9 non-treated) | Spectral Power, SVM | Accuracy: 100% |
| Fragile X | Increased resting gamma power | Yes | Ethridge et al. (2024) [43] | 141 cases (70 patients 71 controls) | Spectral power, Alpha peak frequency, Theta/beta ratio, multi-scale entropy, naive Bayes | AUC = 0.9610 |
| KGB | Nonspecific, frequent | No | Not reported | Not reported | Not reported | Not reported |
| Kleefstra | Focal sharp waves, slowing | No | Not reported | Not reported | Not reported | Not reported |
| Domain | Current Gap | Proposed Action | Expected Impact |
|---|---|---|---|
| Data availability | No open, large-scale EEG repositories for KS; limited published studies |
| Enables reproducible research; provides training data for ML models; accelerates biomarker discovery |
| Acquisition standards | Heterogeneous clinical recordings (different montages, durations, states) hamper pooling | Agree on core protocol: 10-min resting-state (eyes-open/closed) + overnight PSG where feasible; record sampling rate, state, meds | comparable quantitative features; reduces confounds in cross-site ML |
| Machine-learning use | No ML model trained on KS EEG; small and imbalanced data deter DL |
| Proof-of-concept classifiers; early screening tool in clinics where genetics is delayed |
| Longitudinal insight | Lack of serial EEG to track natural history/ treatment effect | Embed annual EEG in registries; couple with developmental scales | Allows modelling of progression, seizure remission trends; supplies endpoints for clinical trials |
| Cross- disorder context | Unknown if KS shares EEG signature with other chromatinopathies | Joint analyses across KS, KBG, PMS, CHD2, etc.; multivariate ML to find convergent biomarkers | Identifies common circuit broader therapeutic targets; positions KS within pan-syndromic frameworks |
| Multimodal integration | EEG studied in isolation; omits facial AI, genetics, iPSC networks | Fuse EEG features with facial-phenotype scores and cell-level E/I metrics via multimodal ML | behavior |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzimourta, K.D. Machine Learning Algorithms in EEG Analysis of Kleefstra Syndrome: Current Evidence and Future Directions. Sensors 2025, 25, 3420. https://doi.org/10.3390/s25113420
Tzimourta KD. Machine Learning Algorithms in EEG Analysis of Kleefstra Syndrome: Current Evidence and Future Directions. Sensors. 2025; 25(11):3420. https://doi.org/10.3390/s25113420
Chicago/Turabian StyleTzimourta, Katerina D. 2025. "Machine Learning Algorithms in EEG Analysis of Kleefstra Syndrome: Current Evidence and Future Directions" Sensors 25, no. 11: 3420. https://doi.org/10.3390/s25113420
APA StyleTzimourta, K. D. (2025). Machine Learning Algorithms in EEG Analysis of Kleefstra Syndrome: Current Evidence and Future Directions. Sensors, 25(11), 3420. https://doi.org/10.3390/s25113420






