A Novel Microelectrode Based on Joule Heating and Impedance Spectroscopy for Inducing and Monitoring the Aggregation of HCV-Specific Probes
Abstract
Highlights
- A new on-chip microelectrode was designed to induce and monitor the aggregation of HCV RNA-attached gold nanoparticles (AuNPs).
- The microelectrode effectively induces the aggregation of RNA-attached AuNPs through controlled Joule heating.
- Impedance spectroscopy was utilized to monitor the aggregation process in real time.
- Integrating Joule heating and impedance spectroscopy enables on-chip system integration and reduces the detection time for nanoparticle aggregation, enhancing its bioanalytical utility.
- The developed microelectrode can heat a droplet to a specific temperature.
- It successfully induces color change based on the presence of hepatitis C virus (HCV) RNA.
- Impedance readings are used to monitor the aggregation process.
Abstract
1. Introduction
2. Materials and Methods
2.1. The Joule Heating Microelectrode (JHM)
- The higher the resistance is, the higher the temperature of the microelectrode is.
- The higher the applied current is, the higher the temperature of the microelectrode is.
- The longer the current is applied for, the higher the temperature of the microelectrode is.
- The lower the volume of the microelectrode is, the higher the temperature of the microelectrode is.
2.2. Impedance Spectroscopy
2.3. Finite Element Method (FEM)
2.3.1. Geometry
- The mathematical method using the following equation, where R is the resistance of the microelectrode, is the material resistivity ( of copper equal [33]), l is the length of the microelectrode, and A is the area of the microelectrode (equal to the width in case of ignoring the thickness of the microelectrode):
- The finite element method: The resistance is estimated by applying a voltage difference to a conductor to create a current flow. The intensity of the current is usually a function of the applied voltage difference. In the most straightforward (linear) case, the current flow and the voltage difference are proportional; the proportionality constant is the device’s resistance. The resistance of each design geometry is estimated by solving the electric current module (ec) based on a stationary study.
2.3.2. Material Properties
2.3.3. Loads and Boundary Conditions
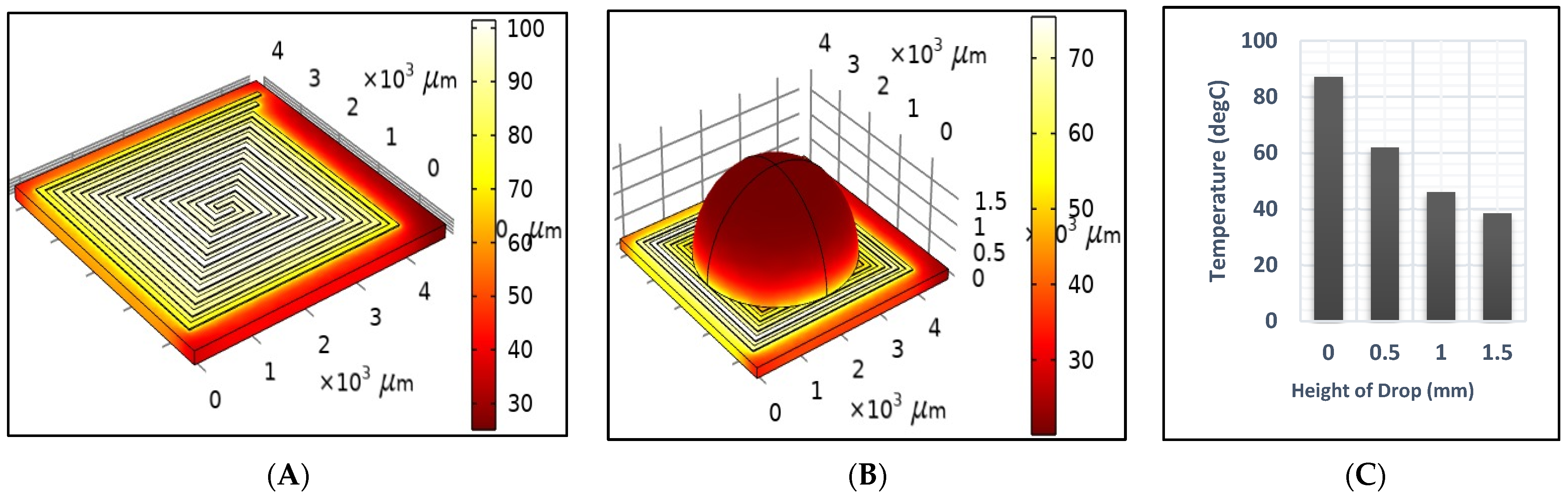
- Dry test: The DFM was tested at an ambient temperature, which was represented by the convection heat flux. The convection heat flux of air was described by the heat transfer coefficient as 5 [W/(m2·K)], and the ambient temperature is set to 20 °C.
- Sample test: A droplet of saline was used as an example in a half-sphere shape. A 20 µL drop is preferred for PCB technology to be suitable for the heat generated by the DFM.
- Cooling test: An initial 1 s duration pulse was applied to heat the DFM to 95 °C. Then, it was turned off to study the cooling rate of the DFM at room temperature for an additional four seconds.
2.3.4. Meshing
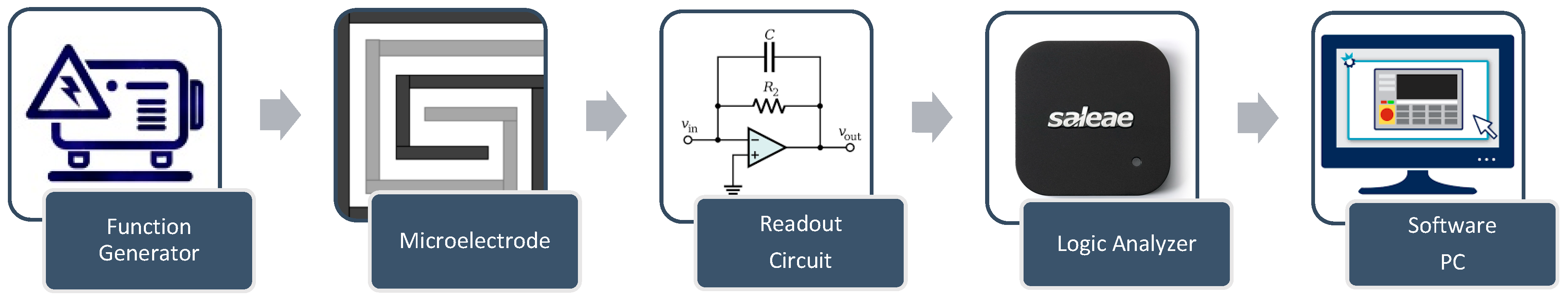
2.4. Experimental Setup
2.4.1. DFM Chip
2.4.2. Control System
- Function generator: The function generator is implemented using Analog Devices AD9851 synthesizers. The AD9851 digital synthesizer generates the required signals with a 5 MHz bandwidth.
- The design features wrapped electrodes with dual synchronized functions (Figure 3). A DC signal heats the sample, while a control circuit with two relays switches between heating and impedance microscopy modes during the pulsed signal’s off time, enabling real-time aggregation monitoring.
- Temperature sensor: The generated temperature was measured using a Fluke 87 V Industrial Multimeter, which was pre-calibrated by the manufacturer. According to the manufacturer’s specifications, the multimeter is capable of measuring the temperature with an error margin of ±2 °C at an input of 100 °C and ±1 °C at 0 °C [35].
- Software plays a crucial role in estimating impedance, serving as the final step in the process. Various tasks must be executed to achieve accurate impedance estimation. In version 17.0 of LabVIEW, a user interface is designed to read the input AC signal from the function generator and the output AC signal from the readout circuit. It determines the phase shift between the input and output signals and exports the amplitude and phase shift data in an Excel format. Additionally, Version R2023b of MATLAB was utilized to estimate impedance by implementing a script based on the transfer function of the readout circuit, which is defined as follows:
2.4.3. Preparing Sample
Nanoprobe
Synthetic Targets
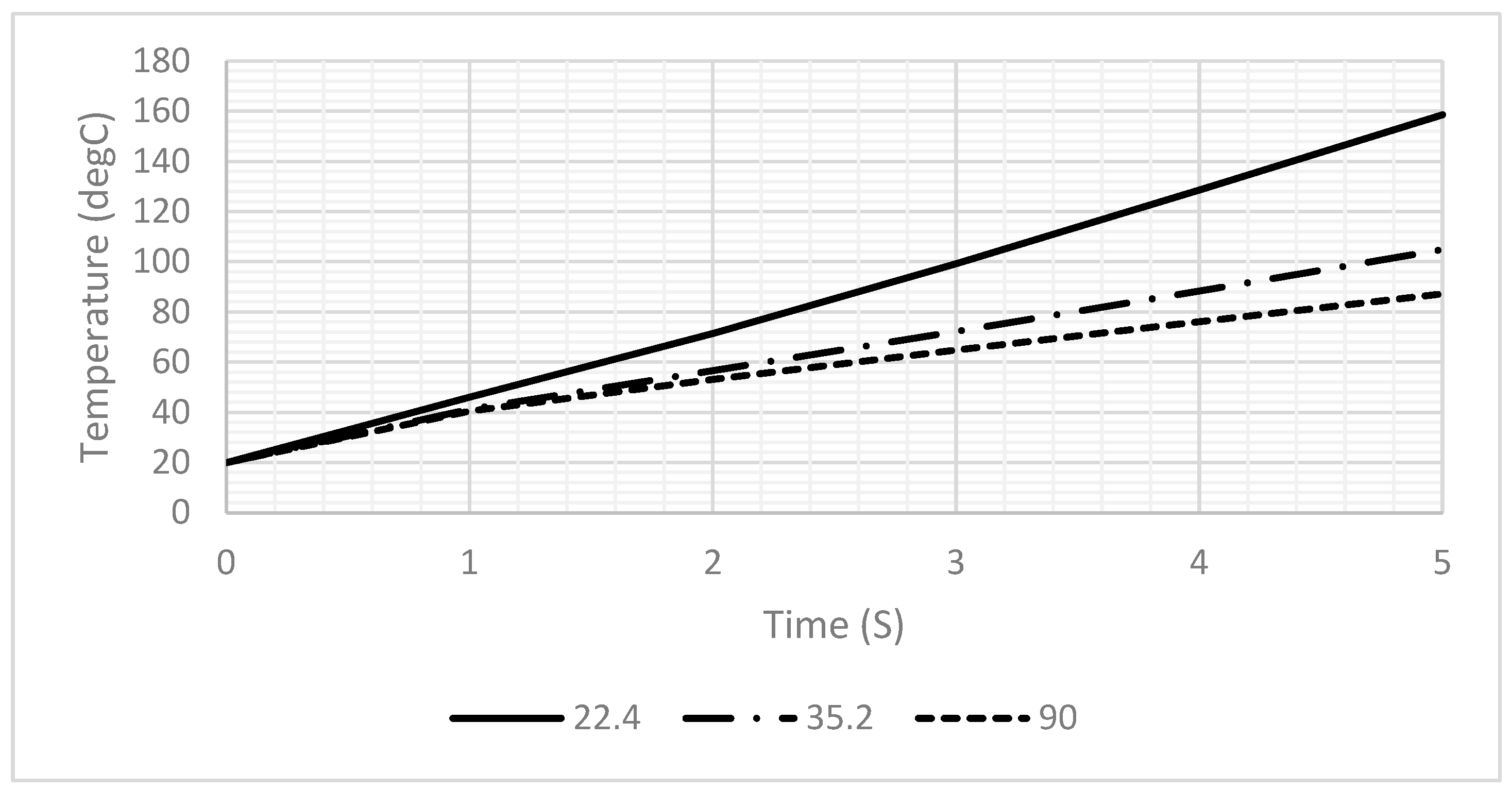
3. Results
3.1. Simulation
3.2. Experimental Results
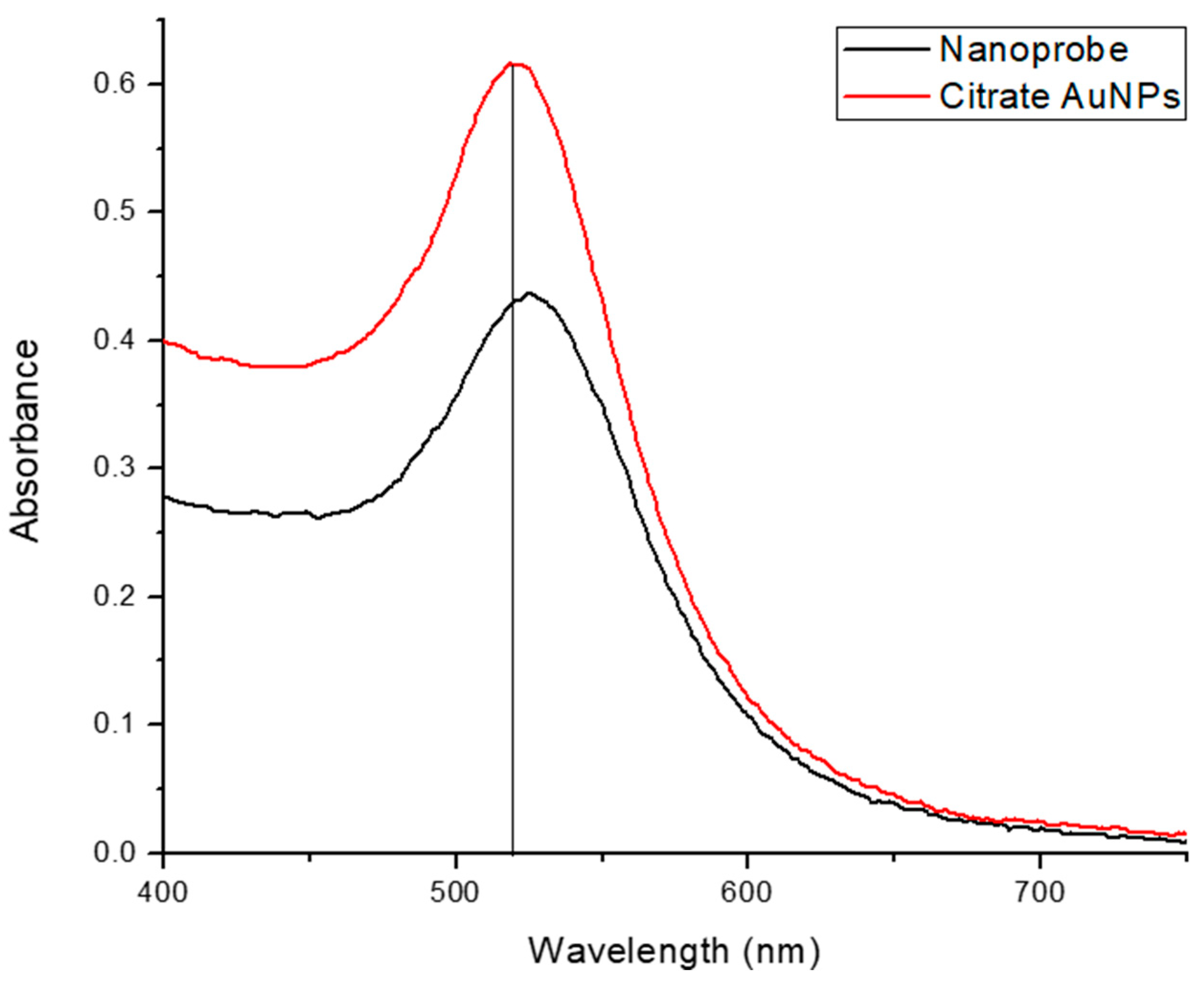
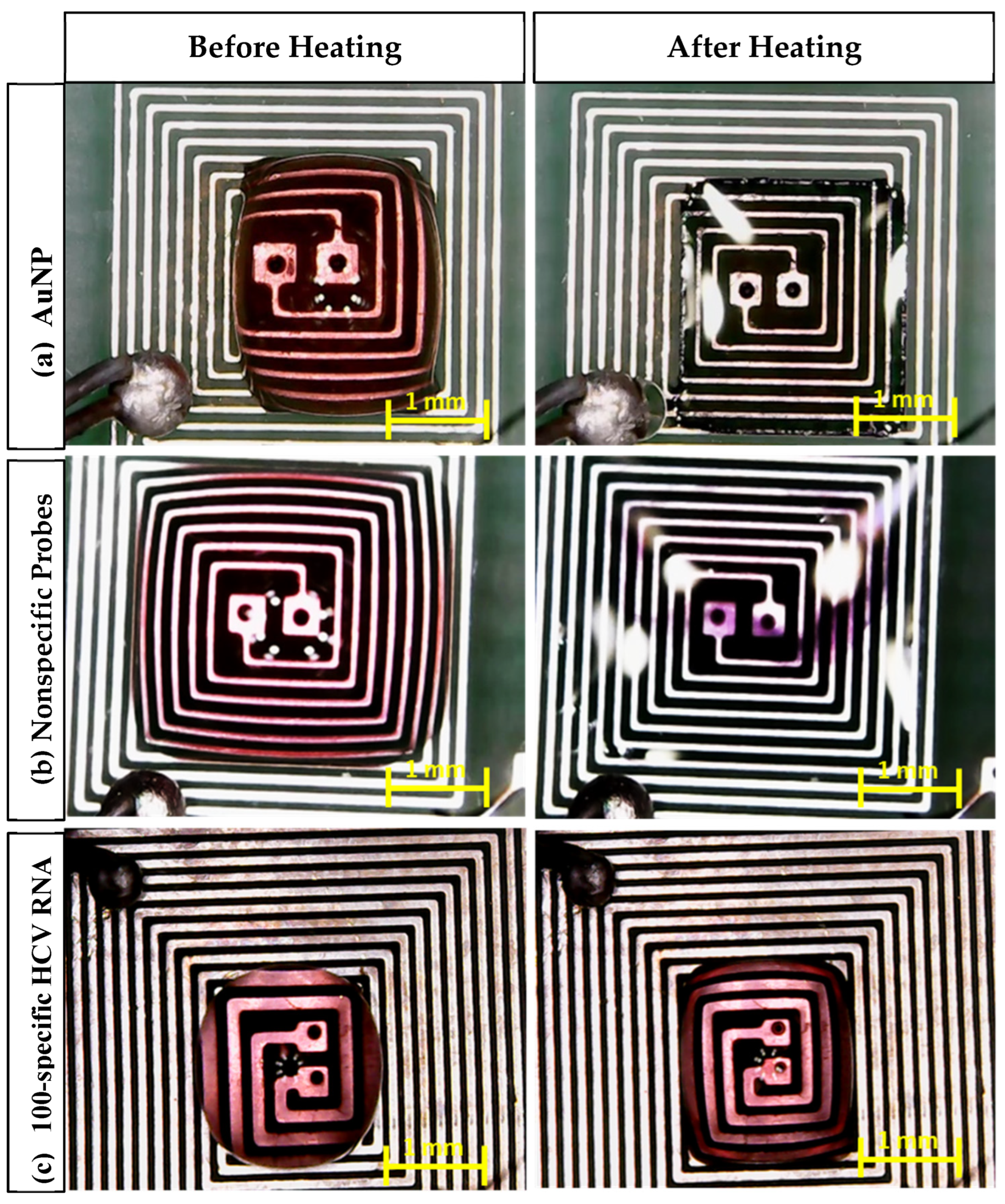
- In the case of AuNPs alone, the sample color changed from red to transparent, which indicates that the nanoparticles remained dispersed and did not aggregate, which is a crucial factor for the observed color change.
- In the case of the probe + nonspecific RNA sample, the sample color changed from red to violet, indicating AuNP aggregation caused by nonspecific target interactions.
- In the case of the probe + target HCV RNA sample, the sample color remained unchanged. This stability is attributed to the specific interaction between the AuNPs and the target RNA, preventing aggregation.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AuNPs | Gold nanoparticles |
| RNA | Ribonucleic Acid |
| HCV | Hepatitis C virus |
| PCB | Printed circuit board |
| AC | Alternating current |
| DFM | Dual-function microelectrode |
| FEM | Finite element method |
| ATP | Adenosine triphosphate |
| JHM | Joule heating microelectrode |
References
- Dias, R.A. Towards a Comprehensive Definition of Pandemics and Strategies for Prevention: A Historical Review and Future Perspectives. Microorganisms 2024, 12, 1802. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Haq, A.; Ali, S.; Imran, M.; Riaz, F.; Riaz, F.; Aimen, W.; Hassan, M.B. Nano-Vaccines and Therapeutics: Emerging Horizons to Better Fight Infectious Diseases. In Expanding Nanobiotechnology: Applications and Commercialization; CRC Press: Boca Raton, FL, USA, 2025; pp. 1–23. [Google Scholar]
- Parkhe, V.S.; Tiwari, A.P. Gold nanoparticles-based biosensors: Pioneering solutions for bacterial and viral pathogen detection—A comprehensive review. World J. Microbiol. Biotechnol. 2024, 40, 269. [Google Scholar] [CrossRef]
- Chauhan, S.; Mittal, R.; Kumar, M.; Mittal, A.; Kushwah, A.S. Gold nanoparticle-based biosensors for point-of-care diagnostics: A review of sensing nanoparticle applications and future prospects. Comb. Chem. High Throughput Screen. 2025, 28, 417–434. [Google Scholar] [CrossRef]
- Shamim Ali, S.; Ali, T.; Sharma, H.; Kishor, B.N.; Jha, S.K. Recent Advances in Monodisperse Gold Nanoparticle Delivery, Synthesis, and Emerging Applications in Cancer Therapy. Plasmonics 2025, 1–21. [Google Scholar] [CrossRef]
- Kumalasari, M.R.; Alfanaar, R.; Andreani, A.S. Gold nanoparticles (AuNPs): A versatile material for biosensor application. Talanta Open 2024, 9, 100327. [Google Scholar] [CrossRef]
- Ghafari, Y.; Asefnejad, A.; Ogbemudia, D.O. Gold nanoparticles in biomedicine: Advancements in cancer therapy, drug delivery, diagnostics, and tissue regeneration. Sci. Hypotheses 2024, 1, 21–35. [Google Scholar] [CrossRef]
- Jayeoye, T.J.; Nwude, E.F.; Singh, S.; Prajapati, B.G.; Kapoor, D.U.; Muangsin, N. Sustainable synthesis of gold nanoparticles for drug delivery and cosmeceutical applications: A review. BioNanoScience 2024, 14, 3355–3384. [Google Scholar] [CrossRef]
- Palomino-Vizcaino, G.; Reséndiz, D.G.; Benítez-Hess, M.L.; Martínez-Acuña, N.; Tapia-Vieyra, J.V.; Bahena, D.; Díaz-Sánchez, M.; García-González, O.P.; Alvarez-Sandoval, B.A.; Alvarez-Salas, L.M. Effect of HPV16 L1 virus-like particles on the aggregation of non-functionalized gold nanoparticles. Biosens. Bioelectron. 2018, 100, 176–183. [Google Scholar] [CrossRef]
- Xu, X.; Ji, J.; Chen, P.; Wu, J.; Jin, Y.; Zhang, L.; Du, S. Salt-induced gold nanoparticles aggregation lights up fluorescence of DNA-silver nanoclusters to monitor dual cancer markers carcinoembryonic antigen and carbohydrate antigen 125. Anal. Chim. Acta 2020, 1125, 41–49. [Google Scholar] [CrossRef]
- Wu, M.; Xiao, Y.; Wu, R.; Lei, J.; Li, T.; Zheng, Y. Aggregable gold nanoparticles for cancer photothermal therapy. J. Mater. Chem. B 2024, 12, 8048–8061. [Google Scholar] [CrossRef]
- Basu, S.; Ghosh, S.K.; Kundu, S.; Panigrahi, S.; Praharaj, S.; Pande, S.; Jana, S.; Pal, T. Biomolecule induced nanoparticle aggregation: Effect of particle size on interparticle coupling. J. Colloid Interface science 2007, 313, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Dürvanger, Z.; Bencs, F.; Menyhárd, D.K.; Horváth, D.; Perczel, A. Solvent induced amyloid polymorphism and the uncovering of the elusive class 3 amyloid topology. Commun. Biol. 2024, 7, 968. [Google Scholar] [CrossRef] [PubMed]
- Breausche, F.E.; Somerlot, A.; Walder, J.; Osei, K.; Okyem, S.; Driskell, J.D. Immobilization of thiol-modified horseradish peroxidase on gold nanoparticles enhances enzyme stability and prevents proteolytic digestion. Langmuir 2024, 40, 13957–13967. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Luo, F.; Guo, L.; Qiu, B.; Lin, Z. Target-triggered aggregation of gold nanoparticles for photothermal quantitative detection of adenosine using a thermometer as readout. Anal. Chim. Acta 2020, 1110, 151–157. [Google Scholar] [CrossRef]
- Dutta, A.; Paul, A.; Chattopadhyay, A. The effect of temperature on the aggregation kinetics of partially bare gold nanoparticles. RSC Adv. 2016, 6, 82138–82149. [Google Scholar] [CrossRef]
- Bano, A.; Dawood, A.; Rida Saira, F.; Malik, A.; Alkholief, M.; Ahmad, H.; Khan, M.A.; Ahmad, Z.; Bazighifan, O. Enhancing catalytic activity of gold nanoparticles in a standard redox reaction by investigating the impact of AuNPs size, temperature and reductant concentrations. Sci. Rep. 2023, 13, 12359. [Google Scholar] [CrossRef]
- Duman, H.; Akdaşçi, E.; Eker, F.; Bechelany, M.; Karav, S. Gold Nanoparticles: Multifunctional Properties, Synthesis, and Future Prospects. Nanomaterials 2024, 14, 1805. [Google Scholar] [CrossRef]
- Yamagata, H.; Kobayashi, A.; Tsunedomi, R.; Seki, T.; Kobayashi, M.; Hagiwara, K.; Chen, C.; Uchida, S.; Okada, G.; Fuchikami, M.; et al. Optimized protocol for the extraction of RNA and DNA from frozen whole blood sample stored in a single EDTA tube. Sci. Rep. 2021, 11, 17075. [Google Scholar] [CrossRef]
- Spurlock, N.; Alfaro, R.; Gabella, W.E.; Chugh, K.; Pask, M.E.; Baudenbacher, F.; Haselton, F.R. Achievement of 15-Minute Adaptive PCR Benchmark with 1370 nm Laser Heating. Biosensors 2025, 15, 258. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Zheng, Q.; Xu, Y.; Kou, X.; Li, T. Formation of gold hollow spheres by rapid heating–cooling process. Gold Bull. 2022, 55, 115–121. [Google Scholar] [CrossRef]
- Malekanfard, A.; Liu, Z.; Song, L.; Kale, A.; Zhang, C.; Yu, L.; Song, Y.; Xuan, X. Joule heating-enabled electrothermal enrichment of nanoparticles in insulator-based dielectrophoretic microdevices. Electrophoresis 2021, 42, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, H.J.; Chen, X.; Alfadhl, Y.; Yu, J.; Wen, D. Electromagnetic heating effect of aggregated gold nanoparticle colloids. J. Appl. Phys. 2014, 115, 094903. [Google Scholar] [CrossRef]
- Abdelbaset, R.; Shawky, S.M.; Abdullah, M.A.; Morsy, O.E.; Yahia, Y.A.; Ghallab, Y.H.; Matboli, M.; Ismail, Y. A new label free spiral sensor using impedance spectroscopy to characterize hepatocellular carcinoma in tissue and serum samples. Sci. Rep. 2024, 14, 13155. [Google Scholar] [CrossRef]
- MacKay, S.; Abdelrasoul, G.N.; Tamura, M.; Lin, D.; Yan, Z.; Chen, J. Using impedance measurements to characterize surface modified with gold nanoparticles. Sensors 2017, 17, 2141. [Google Scholar] [CrossRef]
- Zhou, Y.; Wan, Y.; He, M.; Li, Y.; Wu, Q.; Yao, H. Determination of Vascular Endothelial Growth Factor (VEGF) in Cell Culture Medium by Gold-Coated Magnetic Nanoparticle Based Label-Free Electrochemical Impedance Spectroscopy (EIS). Anal. Lett. 2022, 55, 596–608. [Google Scholar] [CrossRef]
- Perdigones, F.; Quero, J.M. Printed circuit boards: The layers’ functions for electronic and biomedical engineering. Micromachines 2022, 13, 460. [Google Scholar] [CrossRef]
- Carnerero, J.M.; Jimenez-Ruiz, A.; Grueso, E.M.; Prado-Gotor, R. Understanding and improving aggregated gold nanoparticle/dsDNA interactions by molecular spectroscopy and deconvolution methods. Phys. Chem. Chem. Phys. 2017, 19, 16113–16123. [Google Scholar] [CrossRef]
- Shawky, S.M.; Awad, A.M.; Allam, W.; Alkordi, M.H.; El-Khamisy, S.F. Gold aggregating gold: A novel nanoparticle biosensor approach for the direct quantification of hepatitis C virus RNA in clinical samples. Biosens. Bioelectron. 2017, 92, 349–356. [Google Scholar] [CrossRef]
- Torres, C.M.; Arce, P.E.; Justel, F.J.; Romero, L.; Ghorbani, Y. Joule heating effects in electrokinetic remediation: Role of non-uniform soil environments: Temperature profile behavior and hydrodynamics. Environments 2018, 5, 92. [Google Scholar] [CrossRef]
- Simoncelli, M.; Marzari, N.; Cepellotti, A. Generalization of Fourier’s law into viscous heat equations. Phys. Rev. X 2020, 10, 011019. [Google Scholar] [CrossRef]
- Ma, B.; Zhou, X.Y.; Liu, J.; You, Z.; Wei, K.; Huang, X.F. Determination of specific heat capacity on composite shape-stabilized phase change materials and asphalt mixtures by heat exchange system. Materials 2016, 9, 389. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, D.P. Observation of Resistive Transitions in Copper Due to Proximity to a Frustrated Antiferromagnetic Insulator. J. Low Temp. Phys. 2025, 218, 414–422. [Google Scholar] [CrossRef]
- Morsy, O.E.; Abo-Elela, M.; Ghallab, Y.H.; Hammad, A.; Ismail, Y.; El-Ghitani, H. Fully automated digital control system for Lab-on-chip applications. In Proceedings of the 2018 35th National Radio Science Conference (NRSC), Cairo, Egypt, 20–22 March 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 369–376. [Google Scholar] [CrossRef]
- Fluke (n.d.). Available online: https://www.fluke.com/en-us/product/electrical-testing/digital-multimeters/fluke-87v# (accessed on 11 May 2025).
- Hill, H.D.; Mirkin, C.A. The bio-barcode assay for the detection of protein and nucleic acid targets using DTT-induced ligand exchange. Nat. Protoc. 2006, 1, 324–336. [Google Scholar] [CrossRef]
- Pluchery, O.; Prado, Y.; Watkins, W. A complete explanation of the plasmonic colours of gold nanoparticles and of the bichromatic effect. J. Mater. Chem. C 2023, 11, 15824–15832. [Google Scholar] [CrossRef]
- Patil, T.; Gambhir, R.; Vibhute, A.; Tiwari, A.P. Gold nanoparticles: Synthesis methods, functionalization and biological applications. J. Clust. Sci. 2023, 34, 705–725. [Google Scholar] [CrossRef]
- Morales, R.E.; Harke, K.J.; Tringe, J.W.; Stobbe, D.M.; Murray, T.W. Real-time laser ultrasonic monitoring of laser-induced thermal processes. Sci. Rep. 2022, 12, 9865. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Li, S.; Gao, S.; Shen, Y.; Wang, D.; Yang, P. Experimental system and method of aerobic thermal environment simulation based on laser heating. Sci. Rep. 2024, 14, 16318. [Google Scholar] [CrossRef]
- Li, L.; Zhang, R.; Guo, Y.; Ge, J.; Lan, S.; Tian, F.; Long, Y.T.; You, H.; Fang, J. Updated Insights Into the Mechanism of Salt-Induced Aggregation-Based Single-Molecule Surface-Enhanced Raman Spectroscopy. Adv. Sci. 2025, 12, 2417025. [Google Scholar] [CrossRef]
- Allahyartorkaman, M.; Chan, T.H.; Chen, E.H.; Ng, S.T.; Chen, Y.A.; Wen, J.K.; Ho, M.R.; Yen, H.Y.; Kuan, Y.S.; Kuo, M.H.; et al. Phosphorylation-Induced Self-Coacervation versus RNA-Assisted Complex Coacervation of Tau Proteins. J. Am. Chem. Soc. 2025, 147, 10172–10187. [Google Scholar] [CrossRef]

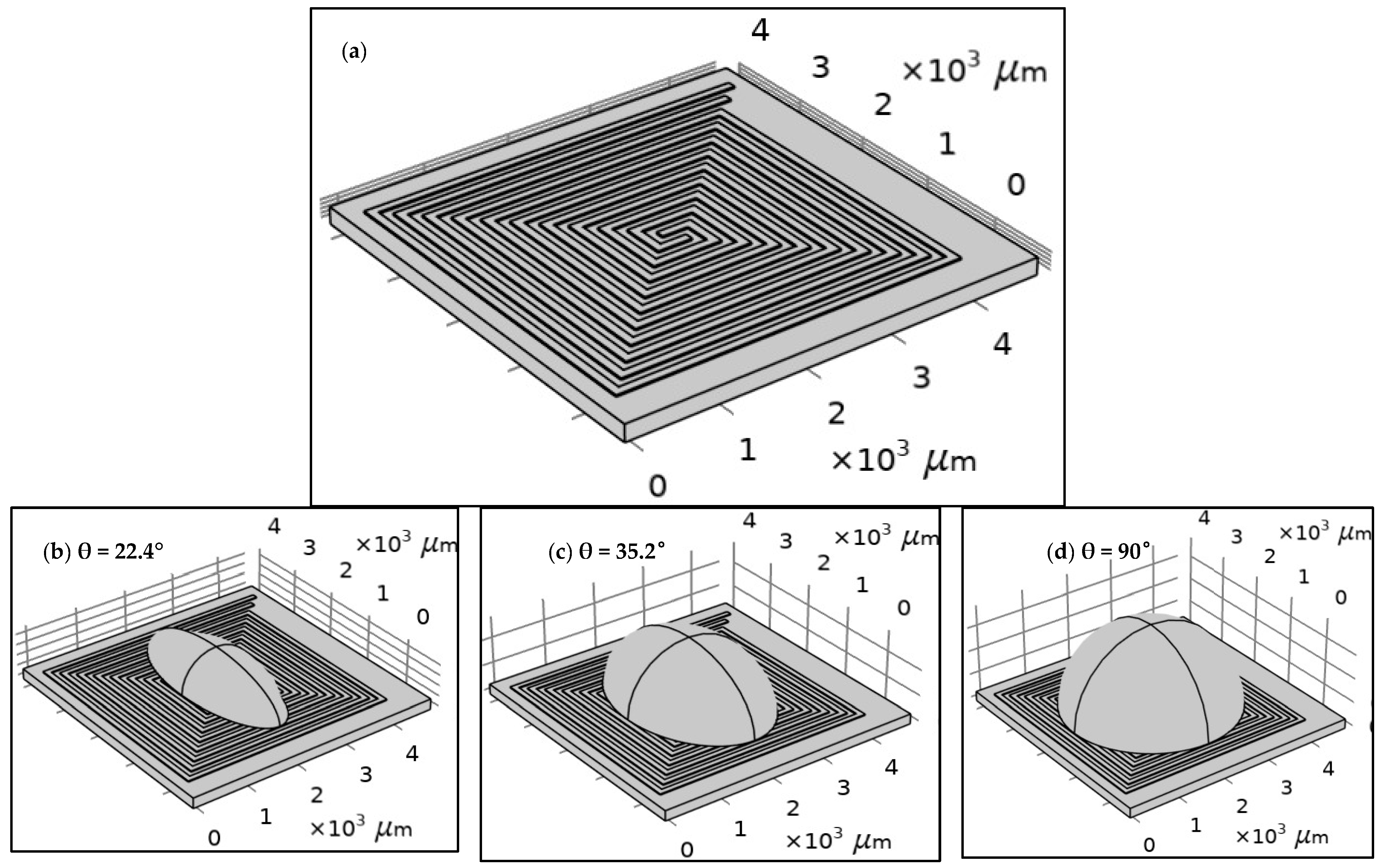




| Item | The Total Length (µm) | The Total Resistance (Ω) | |
|---|---|---|---|
| Mathematical | FEM | ||
| DFM | 48,400 | 41.624 | 38.746 |
| Features | Normal | Fine |
|---|---|---|
| Max element size [μm] | 480 | 384 |
| Min element size [μm] | 86.4 | 48 |
| Growth rate | 1.5 | 1.45 |
| Computational time | 25 s | 3 min 36 s |
| Readings | ||
| Time [s] | Temp [°C] | Temp [°C] |
| 0 | 20.00775 | 20.00937 |
| 1 | 96.48499 | 96.96841 |
| 2 | 164.9275 | 165.6715 |
| 3 | 230.1362 | 231.3351 |
| 4 | 291.6131 | 293.4089 |
| 5 | 349.301 | 351.7258 |
| Max Difference % | 0.694% at 5 s < 1% | |
| Feature Description | Feature Description |
|---|---|
| No. of channels | No. of channels: Eight analog inputs (shared with digital channels) |
| Maximum Sample Rate | Maximum Sample Rate Analog: 50 MSPS @ 3 channels, 12.5 MSPS @ 8 channels |
| Analog Resolution | Analog Resolution 12 bits, 4.88 mV per LSB |
| Analog Input Range | Analog Input Range –10 V to 10 V |
| Capture Buffer | Capture Buffer Length is limited by installed memory. When recording analog at 50 MSPS, captures 10–60 s. |
| Analog Bandwidth | Analog Bandwidth (–3 dB) 5 MHz |
| Shape | Size [μm] | Voltage | Heating | |
|---|---|---|---|---|
| [22] | triangle | 250 | 1400 V AC | 70 °C in 1 min |
| This work | spiral | 100 | 2.5 V DC | 75 °C in 0.5 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelbaset, R.; Morsy, O.E.; Hossam Eldin, M.; Shawky, S.M.; Ghallab, Y.H.; Ismail, Y. A Novel Microelectrode Based on Joule Heating and Impedance Spectroscopy for Inducing and Monitoring the Aggregation of HCV-Specific Probes. Sensors 2025, 25, 3312. https://doi.org/10.3390/s25113312
Abdelbaset R, Morsy OE, Hossam Eldin M, Shawky SM, Ghallab YH, Ismail Y. A Novel Microelectrode Based on Joule Heating and Impedance Spectroscopy for Inducing and Monitoring the Aggregation of HCV-Specific Probes. Sensors. 2025; 25(11):3312. https://doi.org/10.3390/s25113312
Chicago/Turabian StyleAbdelbaset, Reda, Omar E. Morsy, Mariam Hossam Eldin, Sherif M. Shawky, Yehya H. Ghallab, and Yehea Ismail. 2025. "A Novel Microelectrode Based on Joule Heating and Impedance Spectroscopy for Inducing and Monitoring the Aggregation of HCV-Specific Probes" Sensors 25, no. 11: 3312. https://doi.org/10.3390/s25113312
APA StyleAbdelbaset, R., Morsy, O. E., Hossam Eldin, M., Shawky, S. M., Ghallab, Y. H., & Ismail, Y. (2025). A Novel Microelectrode Based on Joule Heating and Impedance Spectroscopy for Inducing and Monitoring the Aggregation of HCV-Specific Probes. Sensors, 25(11), 3312. https://doi.org/10.3390/s25113312






