A Flexible, Implantable, Bioelectronic Electroporation Device for Targeted Ablation of Seizure Foci in the Mouse Brain
Abstract
1. Introduction
2. Materials and Methods
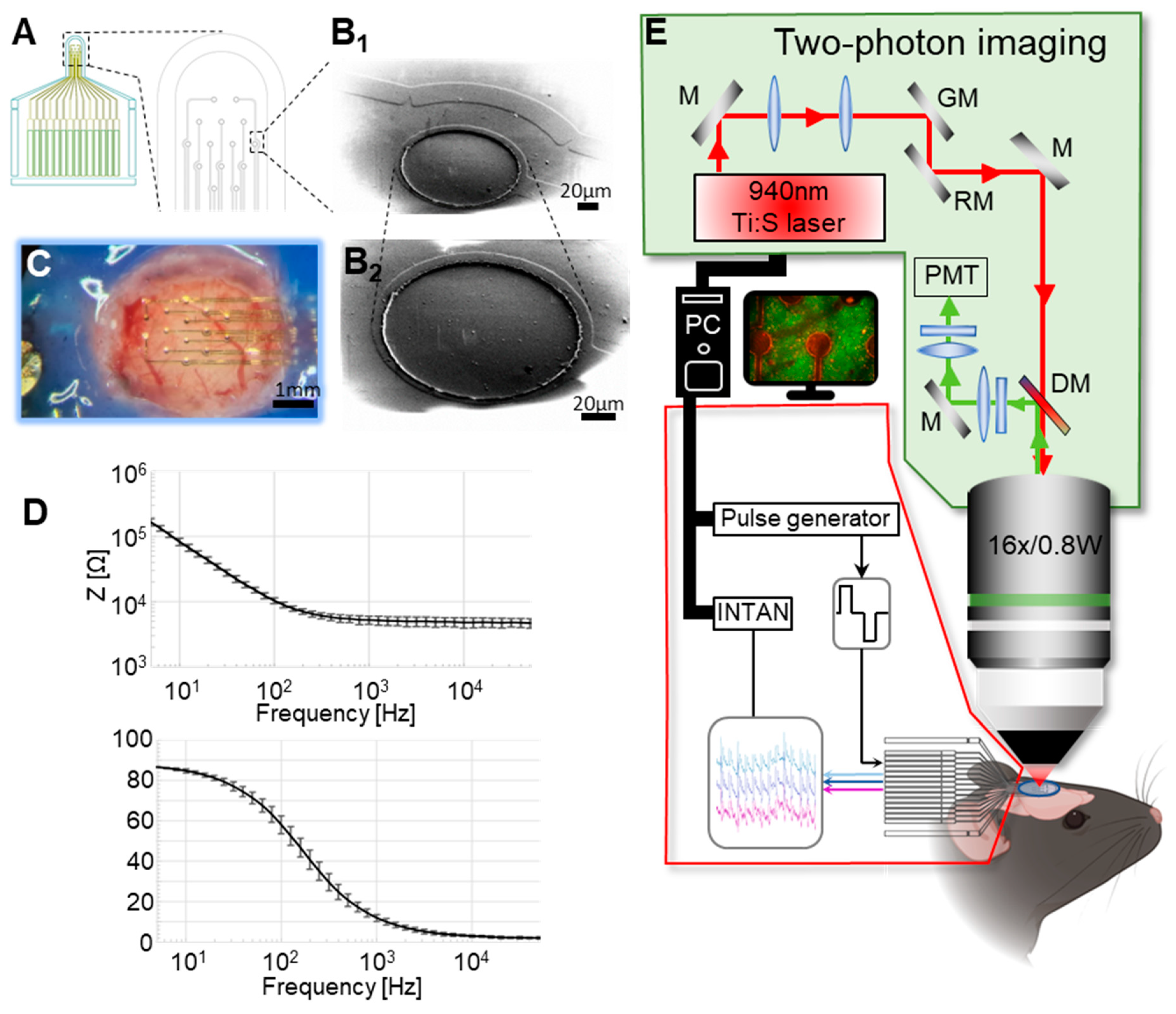
2.1. MEA Fabrication
2.2. Electrochemical Characterization
2.3. Animal Experimentation
2.4. In Vivo Two-Photon Calcium Imaging
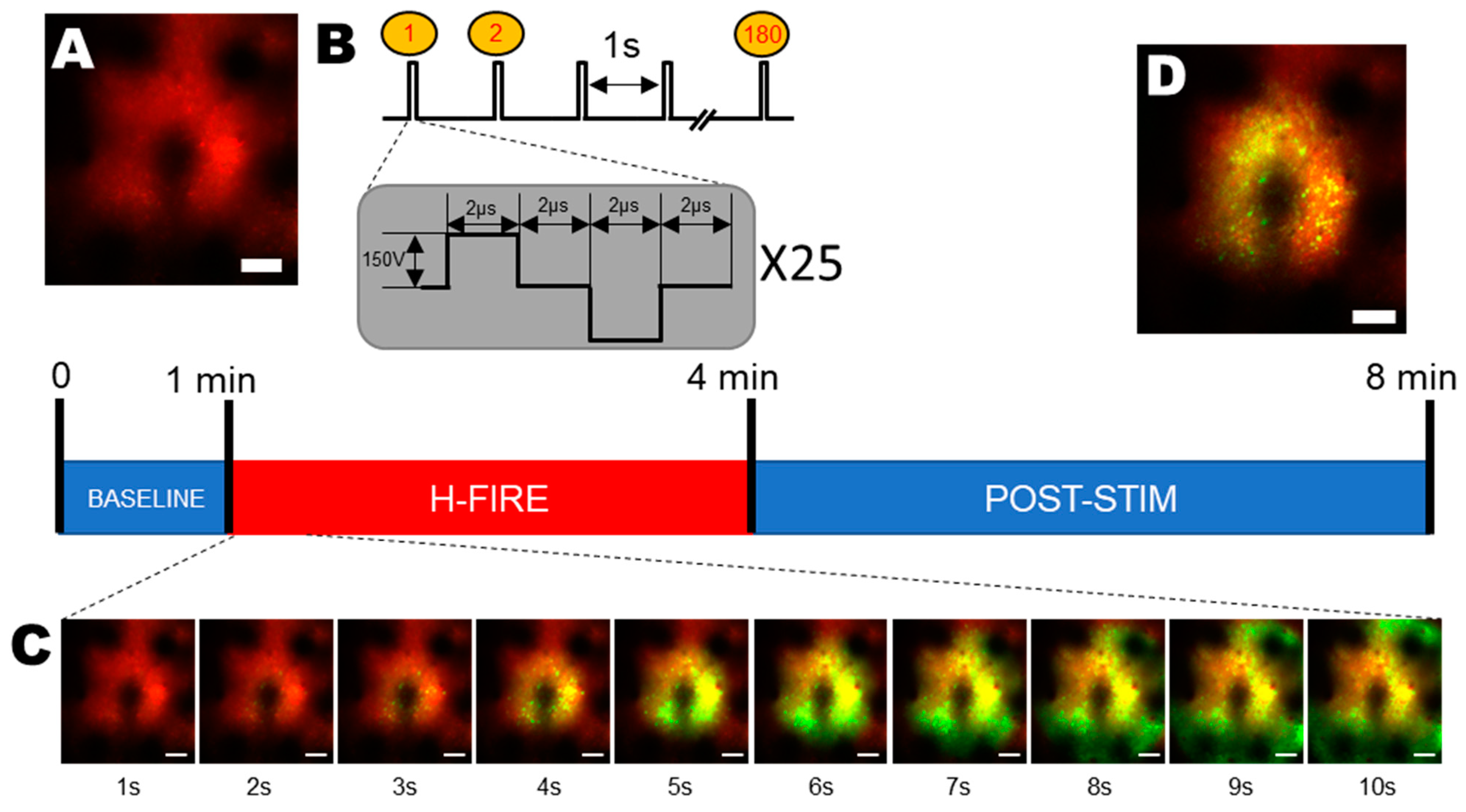
2.5. H-FIRE Protocol
2.6. Electrophysiological Recordings
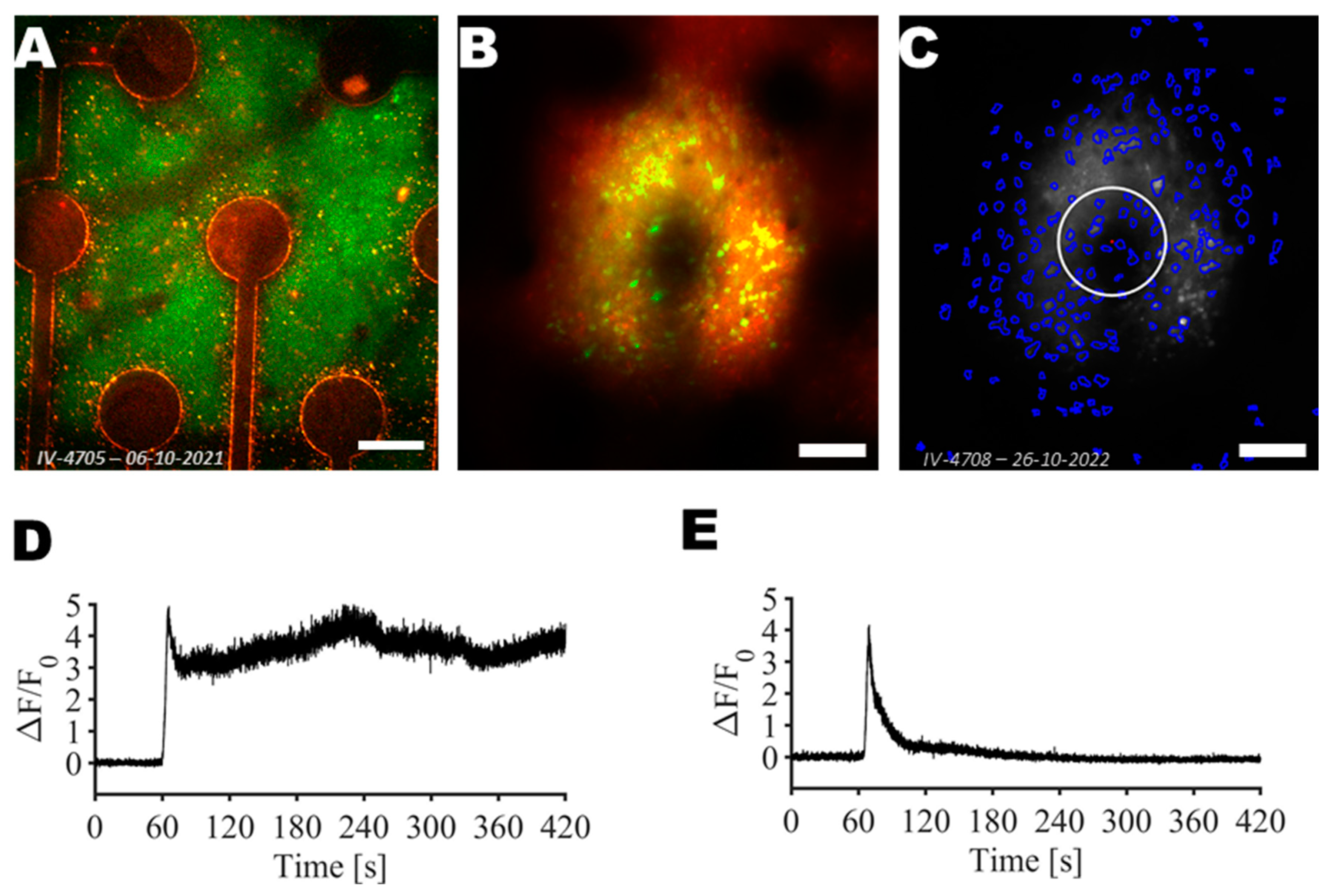
2.7. Segmentation of Neurons in Two-Photon Images
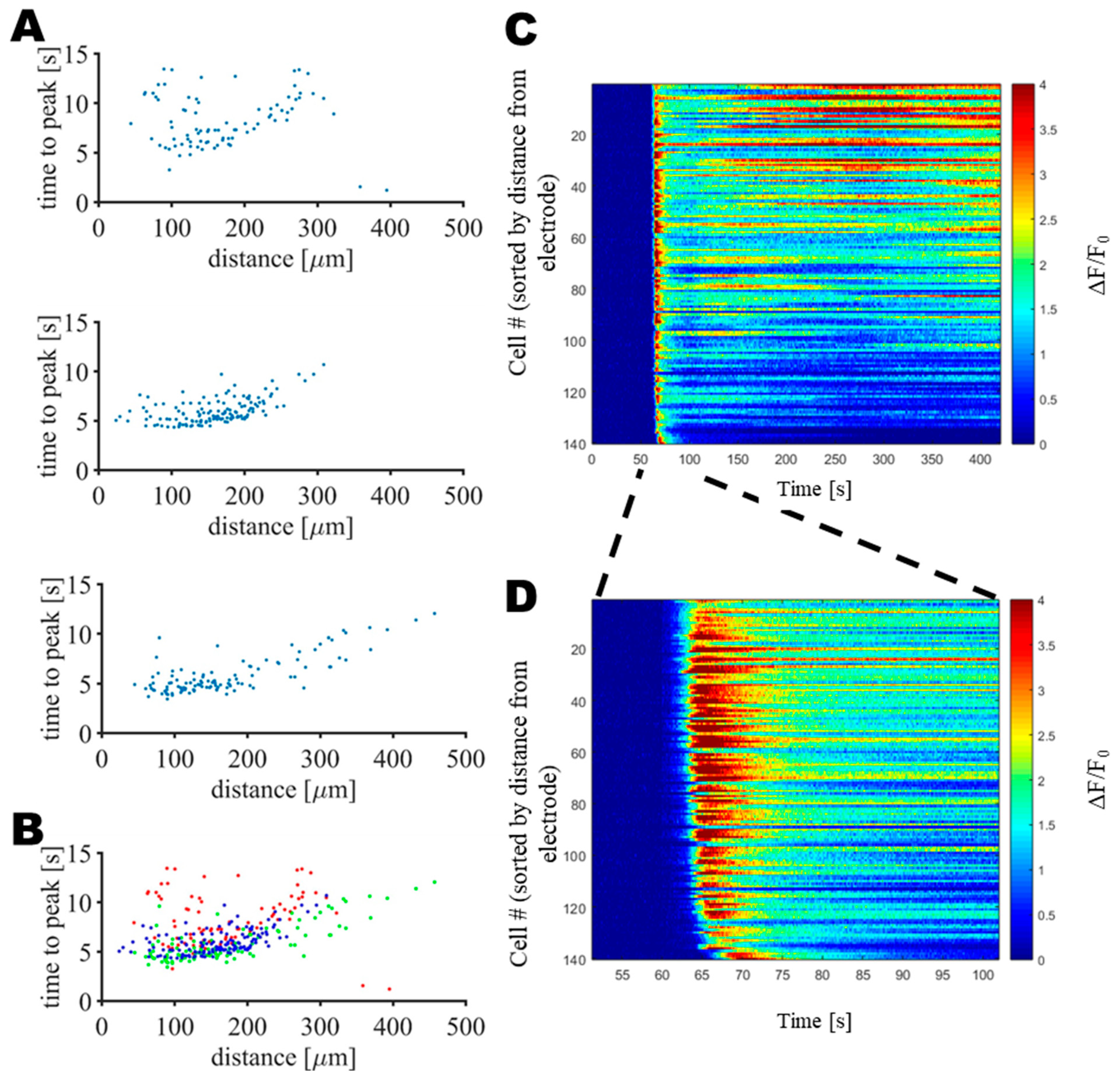
2.8. Data Analysis
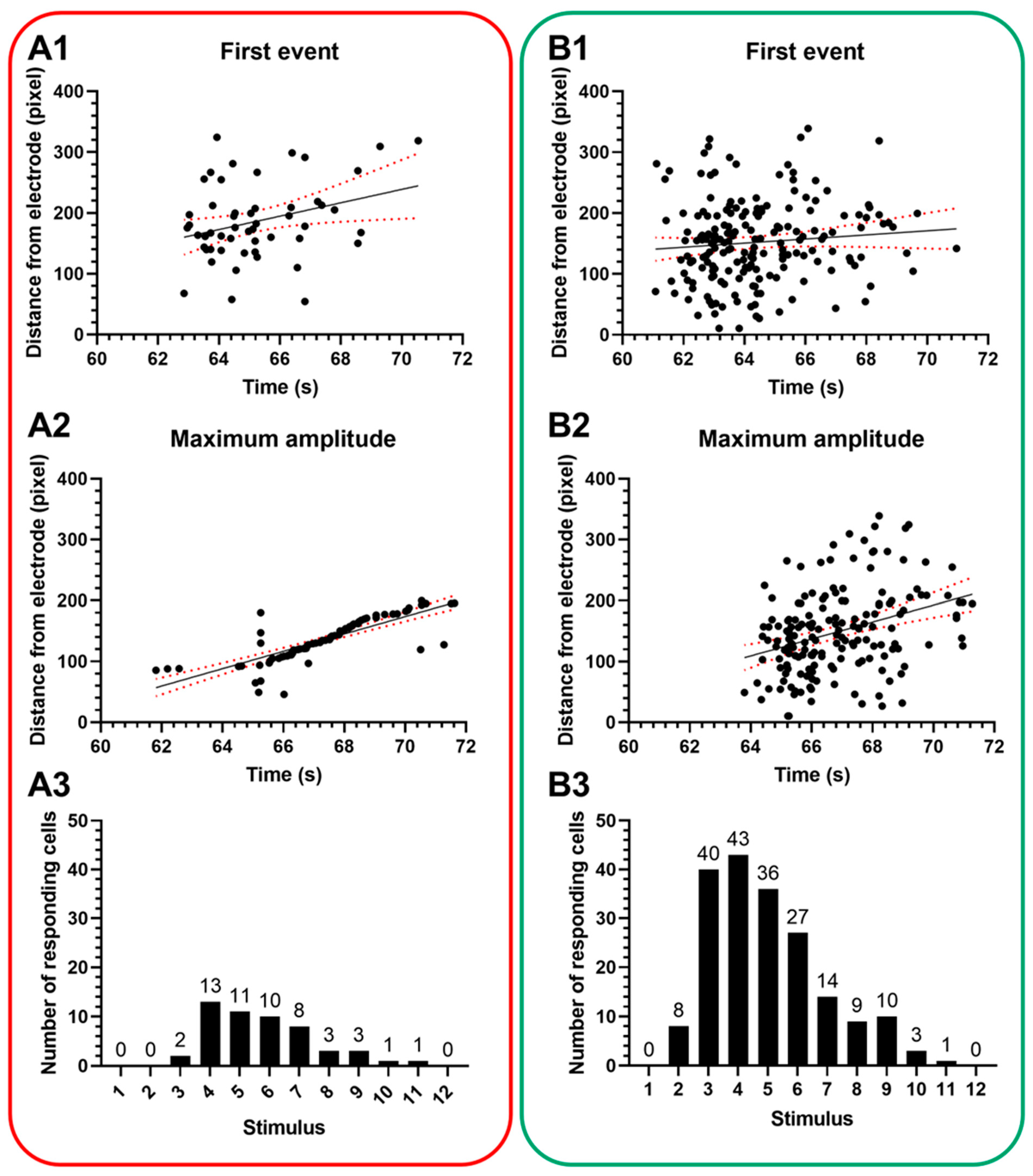
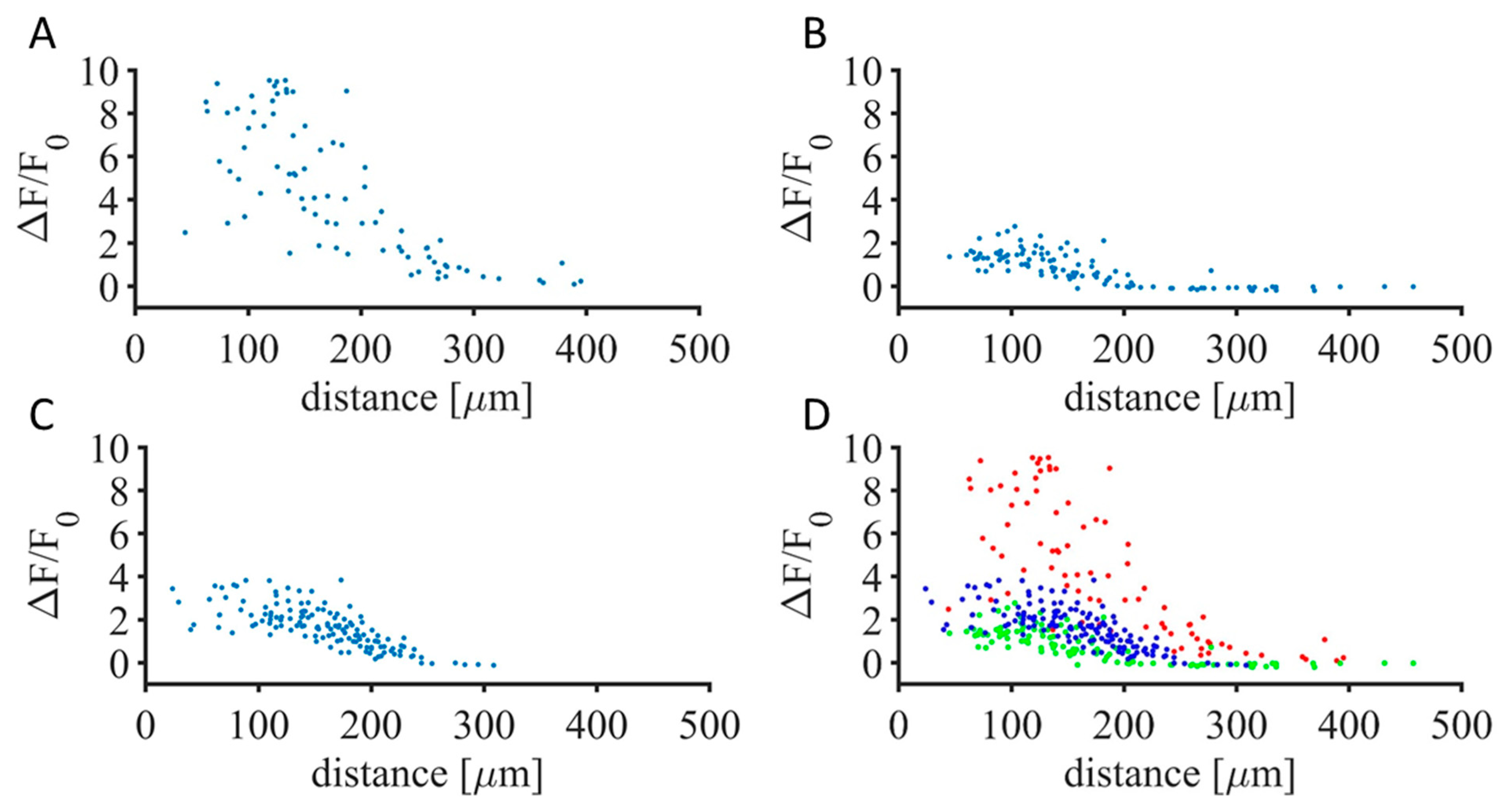
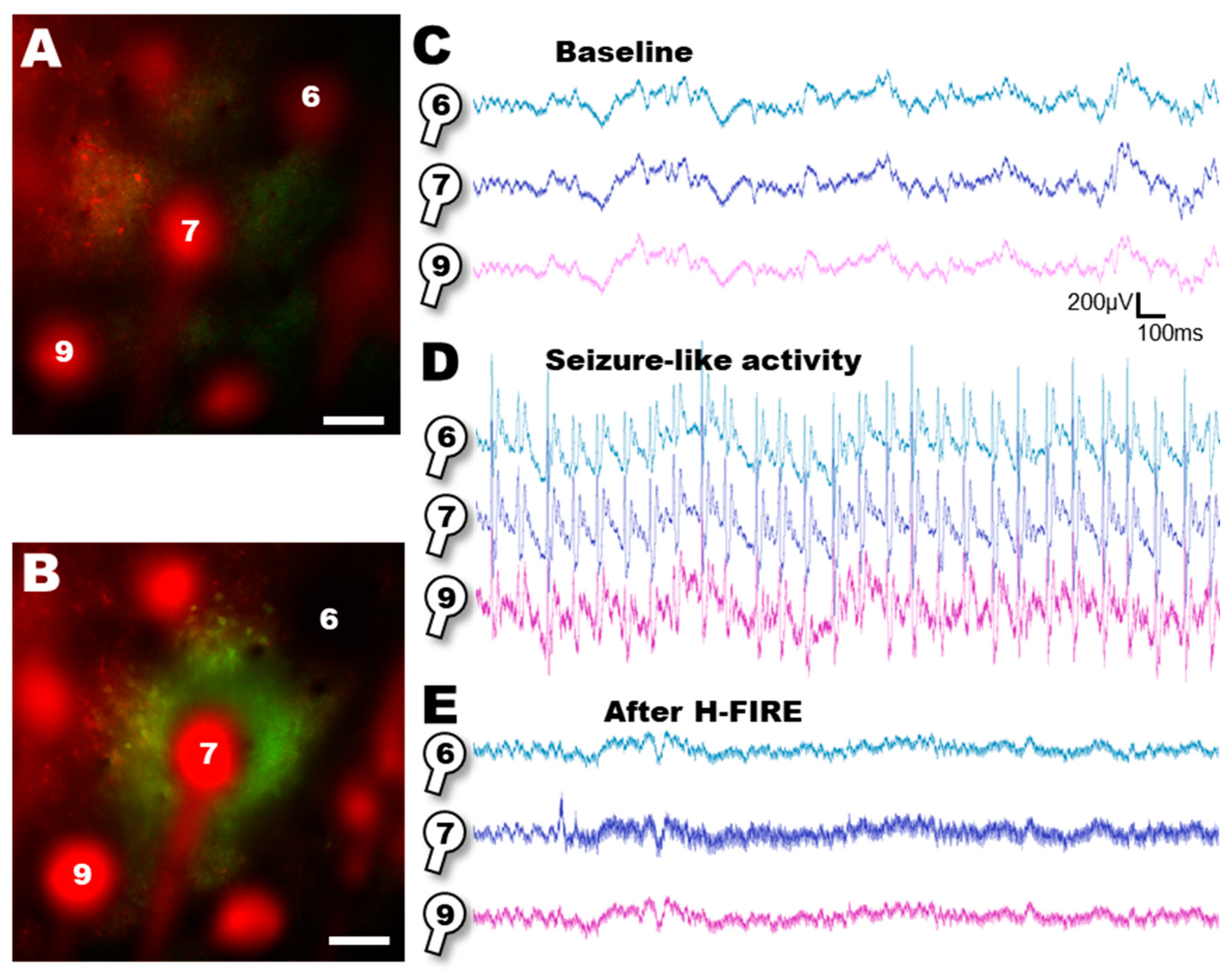
3. Results
4. Discussion
4.1. Targeted Intervention for Epilepsy
4.2. H-FIRE as a Means of Focal Ablation in Epilepsy
4.3. Effective and Verifiable Ablation
4.4. Possible Impact on Other Neurological Diseases
4.5. Scientific Considerations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE Official Report: A Practical Clinical Definition of Epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Ngugi, A.K.; Kariuki, S.M.; Bottomley, C.; Kleinschmidt, I.; Sander, J.W.; Newton, C.R. Incidence of Epilepsy: A Systematic Review and Meta-Analysis. Neurology 2011, 77, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- McCormick, D.A.; Contreras, D. On The Cellular and Network Bases of Epileptic Seizures. Annu. Rev. Physiol. 2001, 63, 815–846. [Google Scholar] [CrossRef] [PubMed]
- McNamara, J.O. Cellular and Molecular Basis of Epilepsy. J. Neurosci. 1994, 14, 3413–3425. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Hartz, A.M.S.; Bauer, B. Drug-Resistant Epilepsy: Multiple Hypotheses, Few Answers. Front. Neurol. 2017, 8, 301. [Google Scholar] [CrossRef]
- Kwon, J.M. Seizures (Epileptic Seizures). Pediatr. Clin. Advis. 2007, 8, 514–515. [Google Scholar] [CrossRef]
- López González, F.J.; Rodríguez Osorio, X.; Gil-Nagel Rein, A.; Carreño Martínez, M.; Serratosa Fernández, J.; Villanueva Haba, V.; Donaire Pedraza, A.J.; Mercadé Cerdá, J.M. Drug-Resistant Epilepsy: Definition and Treatment Alternatives. Neurología 2015, 30, 439–446. [Google Scholar] [CrossRef]
- Fisher, R.S.; Helen Cross, J.; French, J.A.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Mosh, S.L.; Peltola, J.; Roulet Perez, E.; et al. Operational Classification of Seizure Types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 522–530. [Google Scholar] [CrossRef]
- Stafstrom, C.E.; Carmant, L. Seizures and Epilepsy: An Overview for Neuroscientists. Cold Spring Harb. Perspect. Biol. 2015, 5, a022426. [Google Scholar] [CrossRef] [PubMed]
- Goggins, E.; Mitani, S.; Tanaka, S. Clinical Perspectives on Vagus Nerve Stimulation: Present and Future. Clin. Sci. 2022, 136, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Nevitt, S.J.; Sudell, M.; Cividini, S.; Marson, A.G.; Tudur Smith, C. Antiepileptic Drug Monotherapy for Epilepsy: A Network Meta-Analysis of Individual Participant Data. Cochrane Database Syst. Rev. 2022, 4, 1465–1858. [Google Scholar] [CrossRef]
- Lyseng-Williamson, K.A. Levetiracetam: A review of its use in epilepsy. Drugs 2021, 71, 489–514. [Google Scholar] [CrossRef]
- Daneshyar, S.; Ghiasian, M.; Moradi, S.; Khanlarzadeh, E. Efficacy of Levetiracetam, Lamotrigine and Sodium Valproate on Seizure Attacks and EEG Disorders in Patients with Juvenile Myoclonic Epilepsy: A Double Blind Randomized Clinical Trial. Casp. J. Intern. Med. 2022, 13, 617. [Google Scholar] [CrossRef]
- Seymour, N.; Granbichler, C.A.; Polkey, C.E.; Nashef, L. Mortality after Temporal Lobe Epilepsy Surgery. Epilepsia 2012, 53, 267–271. [Google Scholar] [CrossRef]
- Alomar, S.A.; Moshref, R.H.; Moshref, L.H.; Sabbagh, A.J. Outcomes after Laser Interstitial Thermal Ablation for Temporal Lobe Epilepsy: A Systematic Review and Meta-Analysis. Neurosurg. Rev. 2023, 46, 261. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Loshak, H. Laser Interstitial Thermal Therapy for Epilepsy and/or Brain Tumours: A Review of Clinical Effectiveness and Cost-Effectiveness. Cadth 2019, 1, 1–27. [Google Scholar] [PubMed]
- Youngerman, B.E.; Banu, M.A.; Khan, F.; McKhann, G.M.; Schevon, C.A.; Jagid, J.R.; Cajigas, I.; Theodotou, C.B.; Ko, A.; Buckley, R.; et al. Long-Term Outcomes of Mesial Temporal Laser Interstitial Thermal Therapy for Drug-Resistant Epilepsy and Subsequent Surgery for Seizure Recurrence: A Multi-Centre Cohort Study. J. Neurol. Neurosurg. Psychiatry 2023, 94, 879–886. [Google Scholar] [CrossRef]
- LaRiviere, M.J.; Gross, R.E. Stereotactic Laser Ablation for Medically Intractable Epilepsy: The Next Generation of Minimally Invasive Epilepsy Surgery. Front. Surg. 2016, 3, 64. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Y.; Sperling, M.R. Magnetic Resonance Imaging-Guided Laser Interstitial Thermal Therapy for Treatment of Drug-Resistant Epilepsy. Neurotherapeutics 2017, 14, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Kiriakopoulos, E.; Cascino, G.; Britton, J. Types of Epilepsy Surgery|Epilepsy Foundation. Available online: https://www.epilepsy.com/treatment/surgery/types (accessed on 10 September 2024).
- Kerezoudis, P.; Tsayem, I.N.; Lundstrom, B.N.; Van Gompel, J.J. Systematic Review and Patient-Level Meta-Analysis of Radiofrequency Ablation for Medically Refractory Epilepsy: Implications for Clinical Practice and Research. Seizure Eur. J. Epilepsy 2022, 102, 113–119. [Google Scholar] [CrossRef]
- Franzini, A.; Moosa, S.; Servello, D.; Small, I.; DiMeco, F.; Xu, Z.; Elias, W.J.; Franzini, A.; Prada, F. Ablative Brain Surgery: An Overview. Int. J. Hyperth. 2019, 36, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Campelo, S.N.; Huang, P.H.; Buie, C.R.; Davalos, R.V. Recent Advancements in Electroporation Technologies: From Bench to Clinic. Annu. Rev. Biomed. Eng. 2023, 25, 77–100. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.C.; Chizmadzhev, Y.A. Theory of Electroporation: A Review. Bioelectrochem. Bioenerg. 1996, 41, 135–160. [Google Scholar] [CrossRef]
- Arena, C.B.; Sano, M.B.; Rossmeisl, J.H.; Caldwell, J.L.; Garcia, P.A.; Rylander, M.N.; Davalos, R.V. High-Frequency Irreversible Electroporation (H-FIRE) for Non-Thermal Ablation without Muscle Contraction. Biomed. Eng. Online 2011, 10, 102. [Google Scholar] [CrossRef]
- Batista Napotnik, T.; Polajžer, T.; Miklavčič, D. Cell Death Due to Electroporation—A Review. Bioelectrochemistry 2021, 141, 107871. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.R.; Aycock, K.N.; Hay, A.N.; Rossmeisl, J.H.; Davalos, R.V.; Dervisis, N.G. High-Frequency Irreversible Electroporation Brain Tumor Ablation: Exploring the Dynamics of Cell Death and Recovery. Bioelectrochemistry 2022, 144, 108001. [Google Scholar] [CrossRef]
- Anic, A.; Breskovic, T.; Sikiric, I. Pulsed Field Ablation: A Promise That Came True. Curr. Opin. Cardiol. 2021, 36, 10–16. [Google Scholar] [CrossRef]
- Bradley, C.J.; Haines, D.E. Pulsed Field Ablation for Pulmonary Vein Isolation in the Treatment of Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2020, 31, 2136–2147. [Google Scholar] [CrossRef] [PubMed]
- Sharabi, S.; Last, D.; Daniels, D.; Liraz Zaltsman, S.; Mardor, Y. The Effects of Point-Source Electroporation on the Blood-Brain Barrier and Brain Vasculature in Rats: An MRI and Histology Study. Bioelectrochemistry 2020, 134, 107521. [Google Scholar] [CrossRef] [PubMed]
- Rossmeisl, J.H.; Garcia, P.A.; Robertson, J.L.; Davalos, R.V. Irreversible Electroporation for the Treatment of Brain Tumors: Pre-Clinical Results in a Canine Model of Spontaneous Glioma. IFMBE Proc. 2015, 45, 809–812. [Google Scholar] [CrossRef]
- Sharabi, S.; Bresler, Y.; Ravid, O.; Shemesh, C.; Atrakchi, D.; Schnaider-Beeri, M.; Gosselet, F.; Dehouck, L.; Last, D.; Guez, D.; et al. Transient Blood-Brain Barrier Disruption Is Induced by Low Pulsed Electrical Fields in Vitro: An Analysis of Permeability and Trans-Endothelial Electric Resistivity. Drug Deliv. 2019, 26, 459–469. [Google Scholar] [CrossRef]
- Acerbo, E.; Safieddine, S.; Weber, P.; Botzanowski, B.; Missey, F.; Carrère, M.; Gross, R.E.; Bartolomei, F.; Carron, R.; Jirsa, V.; et al. Non-Thermal Electroporation Ablation of Epileptogenic Zones Stops Seizures in Mice While Providing Reduced Vascular Damage and Accelerated Tissue Recovery. Front. Behav. Neurosci. 2021, 15, 774999. [Google Scholar] [CrossRef] [PubMed]
- Aycock, K.N.; Davalos, R.V. Irreversible Electroporation: Background, Theory, and Review of Recent Developments in Clinical Oncology. Bioelectricity 2019, 1, 214–234. [Google Scholar] [CrossRef] [PubMed]
- Campelo, S.N.; Lorenzo, M.F.; Partridge, B.; Alinezhadbalalami, N.; Kani, Y.; Garcia, J.; Saunier, S.; Thomas, S.C.; Hinckley, J.; Verbridge, S.S.; et al. High-Frequency Irreversible Electroporation Improves Survival and Immune Cell Infiltration in Rodents with Malignant Gliomas. Front. Oncol. 2023, 13, 1171278. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.A.; Rossmeisl, J.H.; Ellis, T.L.; Davalos, R.V. Nonthermal Irreversible Electroporation as a Focal Ablation Treatment for Brain Cancer. In Volume 12: Molecular Mechanisms, Children’s Cancer, Treatments, and Radiosurgery; Springer: Berlin/Heidelberg, Germany, 2014; pp. 171–182. [Google Scholar]
- Aycock, K.N.; Vadlamani, R.A.; Jacobs, E.J.; Imran, K.M.; Verbridge, S.S.; Allen, I.C.; Manuchehrabadi, N.; Davalos, R.V. Experimental and Numerical Investigation of Parameters Affecting High-Frequency Irreversible Electroporation for Prostate Cancer Ablation. J. Biomech. Eng. 2022, 144, 061003. [Google Scholar] [CrossRef]
- Wang, H.; Xue, W.; Yan, W.; Yin, L.; Dong, B.; He, B.; Yu, Y.; Shi, W.; Zhou, Z.; Lin, H.; et al. Extended Focal Ablation of Localized Prostate Cancer with High-Frequency Irreversible Electroporation: A Nonrandomized Controlled Trial. JAMA Surg. 2022, 157, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Latouche, E.L.; DeWitt, M.R.; Swet, J.H.; Kirks, R.C.; Baker, E.H.; Iannitti, D.A.; Vrochides, D.; Davalos, R.V.; McKillop, I.H. Induction of Rapid, Reproducible Hepatic Ablations Using next-Generation, High Frequency Irreversible Electroporation (H-FIRE) In Vivo. HPB 2016, 18, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Hay, A.N.; Aycock, K.N.; Lorenzo, M.; David, K.; Coutermarsh-Ott, S.; Salameh, Z.; Campelo, S.N.; Arroyo, J.P.; Ciepluch, B.; Daniel, G.; et al. Investigation of High Frequency Irreversible Electroporation for Canine Spontaneous Primary Lung Tumor Ablation. Biomedicines 2024, 12, 2038. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.; Kim, J.H.; Lee, S.H. Soft Implantable Microelectrodes for Future Medicine: Prosthetics, Neural Signal Recording and Neuromodulation. Lab. Chip 2016, 16, 959–976. [Google Scholar] [CrossRef] [PubMed]
- Fekete, Z.; Zátonyi, A.; Kaszás, A.; Madarász, M.; Slézia, A. Transparent Neural Interfaces: Challenges and Solutions of Microengineered Multimodal Implants Designed to Measure Intact Neuronal Populations Using High-Resolution Electrophysiology and Microscopy Simultaneously. Microsyst. Nanoeng. 2023, 9, 66. [Google Scholar] [CrossRef]
- Fattahi, P.; Yang, G.; Kim, G.; Abidian, M.R. A Review of Organic and Inorganic Biomaterials for Neural Interfaces. Adv. Mater. 2014, 26, 1846–1885. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, M.C.; Dijk, G.; Kaszas, A.; Baca, M.; Moreau, D.; O’Connor, R.P. Integrating Flexible Electronics for Pulsed Electric Field Delivery in a Vascularized 3D Glioblastoma Model. npj Flex. Electron. 2021, 5, 19. [Google Scholar] [CrossRef]
- Szalay, G.; Judak, L.; Katona, G.; Ocsai, K.; Juhasz, G.; Veress, M.; Szadai, Z.; Feher, A.; Tompa, T.; Chiovini, B.; et al. Fast 3D Imaging of Spine, Dendritic, and Neuronal Assemblies in Behaving Animals. Neuron 2016, 92, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates; Elsevier Academic Press: Salt Lake City, UT, USA, 2001; ISBN 9780125476409. [Google Scholar]
- Nimmerjahn, A.; Kirchhoff, F.; Kerr, J.N.D.; Helmchen, F. Sulforhodamine 101 as a Specific Marker of Astroglia in the Neocortex In Vivo. Nat. Methods 2004, 1, 31–37. [Google Scholar] [CrossRef]
- Donahue, M.J.; Kaszas, A.; Turi, G.F.; Rózsa, B.; Slézia, A.; Vanzetta, I.; Katona, G.; Bernard, C.; Malliaras, G.G.; Williamson, A. Multimodal Characterization of Neural Networks Using Highly Transparent Electrode Arrays. eNeuro 2018, 5, ENEURO.0187-18.2018. [Google Scholar] [CrossRef] [PubMed]
- Fedor, F.Z.; Paraczky, C.; Ravasz, L.; Tóth, K.; Borhegyi, Z.; Somogyvári, Z.; Juhász, G.; Fekete, Z. Electrophysiological and Behavioral Properties of 4-Aminopyridine-Induced Epileptic Activity in Mice. Biol. Futur. 2020, 71, 427–434. [Google Scholar] [CrossRef]
- Lim, J.S. Two-Dimensional Signal and Image Processing; Prentice Hall: Kent, OH, USA, 1990; ISBN 9780139353222. [Google Scholar]
- Zuiderveld, K. Contrast Limited Adaptive Histogram Equalization; Elsevier: Amsterdam, The Netherland, 1994. [Google Scholar]
- Otsu, N. Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man. Cybern. 1979, SMC-9, 62–66. [Google Scholar] [CrossRef]
- Meyer, F. Topographic Distance and Watershed Lines. Signal Process. 1994, 38, 113–125. [Google Scholar] [CrossRef]
- Lavielle, M. Using Penalized Contrasts for the Change-Point Problem. Signal Process. 2005, 85, 1501–1510. [Google Scholar] [CrossRef]
- Killick, R.; Fearnhead, P.; Eckley, I.A. Optimal Detection of Changepoints with a Linear Computational Cost. J. Am. Stat. Assoc. 2012, 107, 1590–1598. [Google Scholar] [CrossRef]
- Hjouj, M.; Last, D.; Guez, D.; Daniels, D.; Sharabi, S.; Lavee, J.; Rubinsky, B.; Mardor, Y. MRI Study on Reversible and Irreversible Electroporation Induced Blood Brain Barrier Disruption. PLoS ONE 2012, 7, e42817. [Google Scholar] [CrossRef] [PubMed]
- Paemeleire, K.; Leybaert, L. Ionic Changes Accompanying Astrocytic Intercellular Calcium Waves Triggered by Mechanical Cell Damaging Stimulation. Brain Res. 2000, 857, 235–245. [Google Scholar] [CrossRef]
- Nedergaard, M. Direct Signaling from Astrocytes to Neurons in Cultures of Mammalian Brain Cells. Science 1994, 263, 1768–1771. [Google Scholar] [CrossRef]
- Foutz, T.J.; Wong, M. Brain Stimulation Treatments in Epilepsy: Basic Mechanisms and Clinical Advances. Biomed. J. 2022, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Dana, H.; Chen, T.-W.; Hu, A.; Shields, B.C.; Guo, C.; Looger, L.L.; Kim, D.S.; Svoboda, K. Thy1-GCaMP6 Transgenic Mice for Neuronal Population Imaging In Vivo. PLoS ONE 2014, 9, e108697. [Google Scholar] [CrossRef]
- de Juan-Sanz, J.; Holt, G.T.; Schreiter, E.R.; de Juan, F.; Kim, D.S.; Ryan, T.A. Axonal Endoplasmic Reticulum Ca2+ Content Controls Release Probability in CNS Nerve Terminals. Neuron 2017, 93, 867–881.e6. [Google Scholar] [CrossRef] [PubMed]
- Linsley, J.W.; Shah, K.; Castello, N.; Chan, M.; Haddad, D.; Doric, Z.; Wang, S.; Leks, W.; Mancini, J.; Oza, V.; et al. Genetically Encoded Cell-Death Indicators (GEDI) to Detect an Early Irreversible Commitment to Neurodegeneration. Nat. Commun. 2021, 12, 5284. [Google Scholar] [CrossRef]
- Garcia, M.I.; Chen, J.J.; Boehning, D. Genetically Encoded Calcium Indicators for Studying Long-Term Calcium Dynamics during Apoptosis. Cell Calcium 2017, 61, 44–49. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Piper, R.J.; Richardson, R.M.; Worrell, G.; Carmichael, D.W.; Baldeweg, T.; Litt, B.; Denison, T.; Tisdall, M.M. Towards Network-Guided Neuromodulation for Epilepsy. Brain 2022, 145, 3347–3362. [Google Scholar] [CrossRef]
- Li, C.; Zhao, G.; Zou, W.; Zhang, Z.; Zhao, Y.; Liu, R. Ultrasound-guided Percutaneous High-frequency Irreversible Electroporation in Porcine Livers Using Four Electrode Needles: A Feasibility and Safety Study. Cancer Med. 2024, 13, e7035. [Google Scholar] [CrossRef]
- Krook-Magnuson, E.; Armstrong, C.; Oijala, M.; Soltesz, I. On-Demand Optogenetic Control of Spontaneous Seizures in Temporal Lobe Epilepsy. Nat. Commun. 2013, 4, 1376. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.C.; Liao, Y.S.; Yeh, W.H.; Liang, S.F.; Shaw, F.Z. Directions of Deep Brain Stimulation for Epilepsy and Parkinson’s Disease. Front. Neurosci. 2021, 15, 680938. [Google Scholar] [CrossRef]
- White, M.G.; Luca, L.E.; Nonner, D.; Saleh, O.; Hu, B.; Barrett, E.F.; Barrett, J.N. Cellular Mechanisms of Neuronal Damage from Hyperthermia. Prog. Brain Res. 2007, 162, 347–371. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Connors, B.W. High Temperatures Alter Physiological Properties of Pyramidal Cells and Inhibitory Interneurons in Hippocampus. Front. Cell Neurosci. 2012, 6, 25084. [Google Scholar] [CrossRef] [PubMed]
- Liebregts, M.T.; McLachlan, R.S.; Leung, L.S. Hyperthermia Induces Age-Dependent Changes in Rat Hippocampal Excitability. Ann. Neurol. 2002, 52, 318–326. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhan, X.; Xiong, J.; Peng, S.; Huang, W.; Joshi, R.; Cai, Y.; Liu, Y.; Li, R.; Yuan, K.; et al. Temperature-Dependent Cell Death Patterns Induced by Functionalized Gold Nanoparticle Photothermal Therapy in Melanoma Cells. Sci. Rep. 2018, 8, 8720. [Google Scholar] [CrossRef] [PubMed]
- Joosten, E.A.; Franken, G. Spinal Cord Stimulation in Chronic Neuropathic Pain: Mechanisms of Action, New Locations, New Paradigms. Pain. 2020, 161, S104–S113. [Google Scholar] [CrossRef]
- Anwer, S.; Paxton, T.; Rahimpour, S.; Alshaikh, J.; Ballard, J.; Steffens, J.; Zorn, M.; Kassavetis, P. Utilization of Intraoperative Subthalamic Nucleus Local Field Potential Recordings to Guide Postoperative Deep Brain Stimulation Programming in Parkinson’s Disease (P5-11.013). Neurology 2023, 100, 2796. [Google Scholar] [CrossRef]
- Thom, M.; Mathern, G.W.; Cross, J.H.; Bertram, E.H. Mesial Temporal Lobe Epilepsy: How Do We Improve Surgical Outcome? Ann. Neurol. 2010, 68, 424. [Google Scholar] [CrossRef] [PubMed]
- Bonilha, L.; Keller, S.S. Quantitative MRI in Refractory Temporal Lobe Epilepsy: Relationship with Surgical Outcomes. Quant. Imaging Med. Surg. 2015, 5, 204. [Google Scholar] [CrossRef]
- Bonilha, L.; Yasuda, C.L.; Rorden, C.; Li, L.M.; Tedeschi, H.; De Oliveira, E.; Cendes, F. Does Resection of the Medial Temporal Lobe Improve the Outcome of Temporal Lobe Epilepsy Surgery? Epilepsia 2007, 48, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Boutet, A.; Madhavan, R.; Elias, G.J.B.; Joel, S.E.; Gramer, R.; Ranjan, M.; Paramanandam, V.; Xu, D.; Germann, J.; Loh, A.; et al. Predicting Optimal Deep Brain Stimulation Parameters for Parkinson’s Disease Using Functional MRI and Machine Learning. Nat. Commun. 2021, 12, 3043. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.T.; Morrell, M.J. Closed-Loop Neurostimulation: The Clinical Experience. Neurotherapeutics 2014, 11, 553–563. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matta, R.; Balogh-Lantos, Z.; Fekete, Z.; Baca, M.; Kaszas, A.; Moreau, D.; O’Connor, R.P. A Flexible, Implantable, Bioelectronic Electroporation Device for Targeted Ablation of Seizure Foci in the Mouse Brain. Sensors 2025, 25, 4. https://doi.org/10.3390/s25010004
Matta R, Balogh-Lantos Z, Fekete Z, Baca M, Kaszas A, Moreau D, O’Connor RP. A Flexible, Implantable, Bioelectronic Electroporation Device for Targeted Ablation of Seizure Foci in the Mouse Brain. Sensors. 2025; 25(1):4. https://doi.org/10.3390/s25010004
Chicago/Turabian StyleMatta, Rita, Zsofia Balogh-Lantos, Zoltan Fekete, Martin Baca, Attila Kaszas, David Moreau, and Rodney Philip O’Connor. 2025. "A Flexible, Implantable, Bioelectronic Electroporation Device for Targeted Ablation of Seizure Foci in the Mouse Brain" Sensors 25, no. 1: 4. https://doi.org/10.3390/s25010004
APA StyleMatta, R., Balogh-Lantos, Z., Fekete, Z., Baca, M., Kaszas, A., Moreau, D., & O’Connor, R. P. (2025). A Flexible, Implantable, Bioelectronic Electroporation Device for Targeted Ablation of Seizure Foci in the Mouse Brain. Sensors, 25(1), 4. https://doi.org/10.3390/s25010004






