Gold–Mercury–Platinum Alloy for Light-Enhanced Electrochemical Detection of Hydrogen Peroxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instruments
2.3. Synthesis of AuHgPt/ITO Electrode
2.4. Electrochemical Measurements
3. Results and Discussion
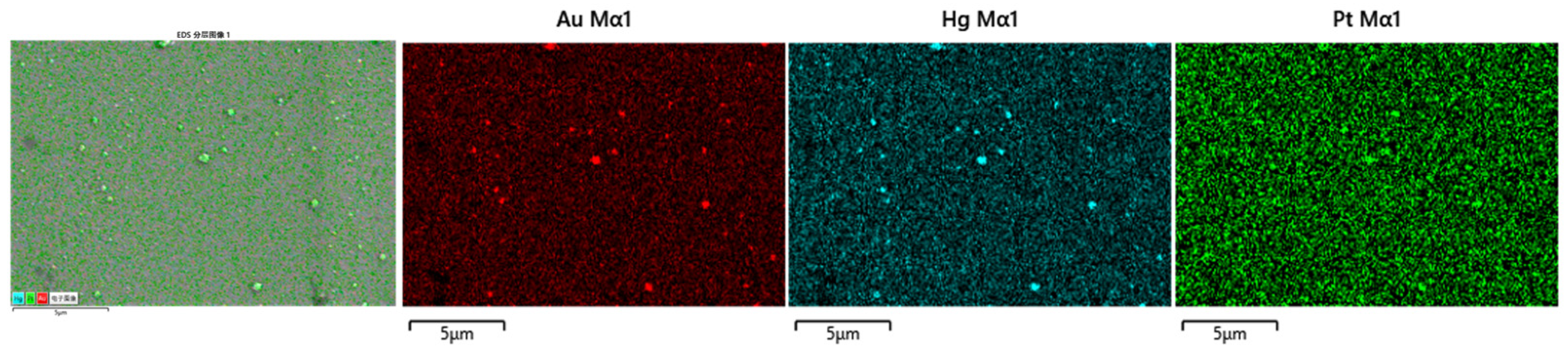
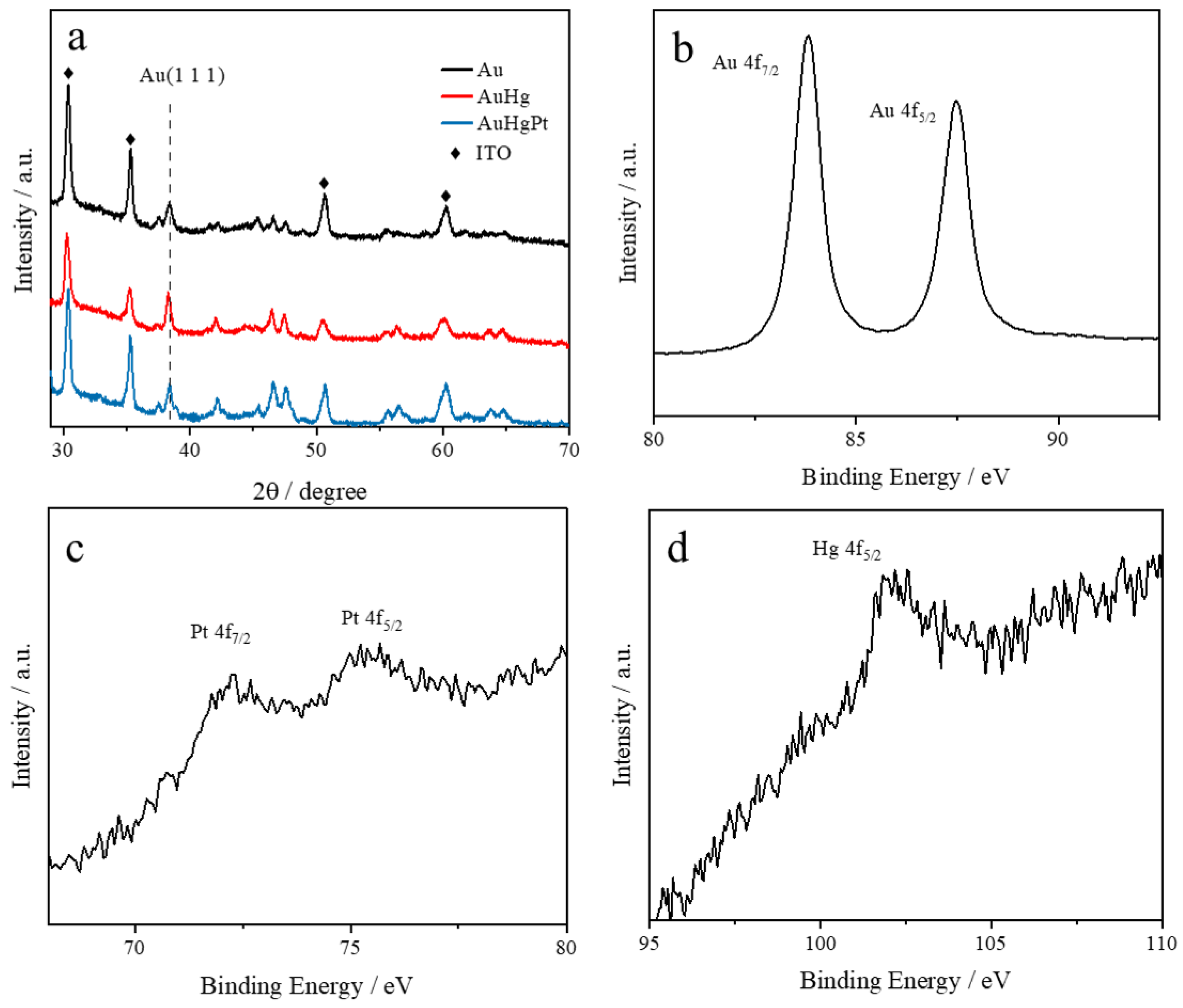
3.1. Structural and Morphological Characterization
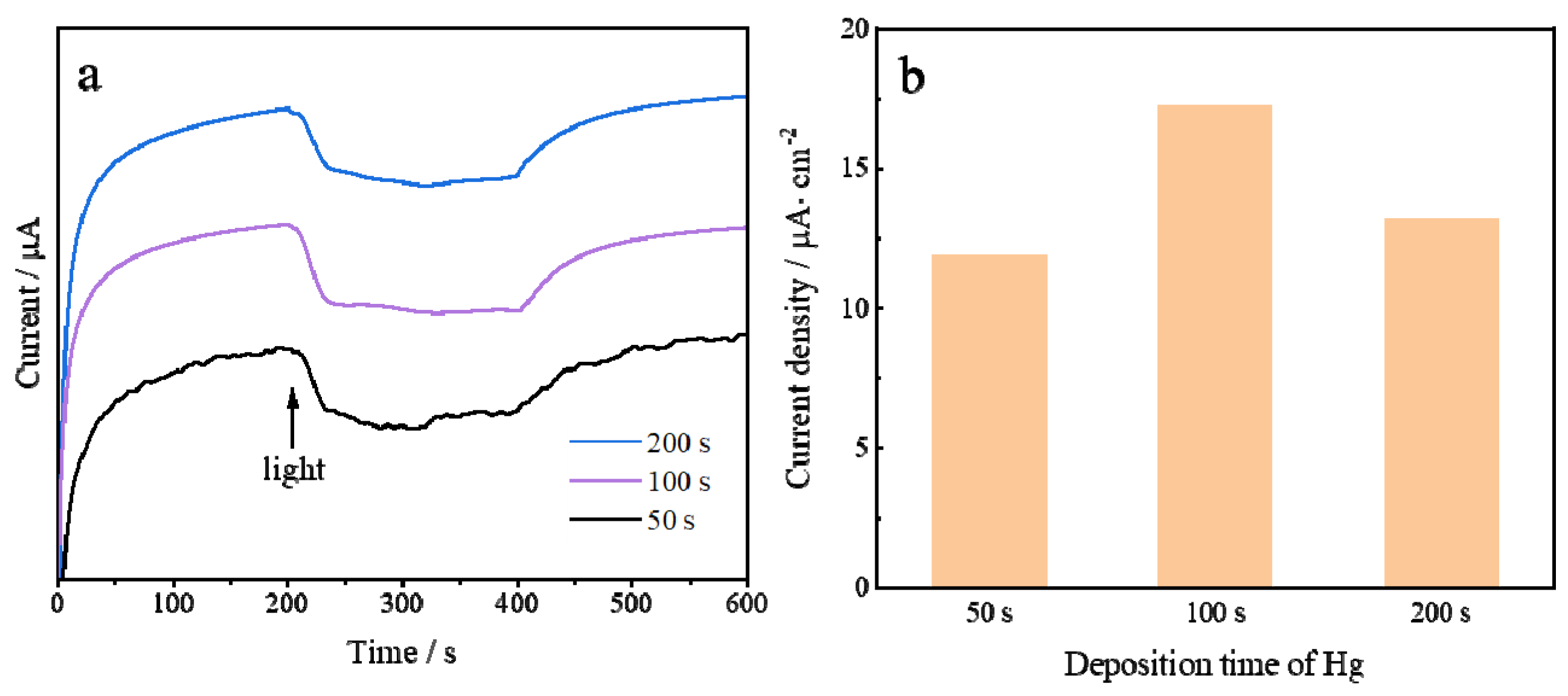
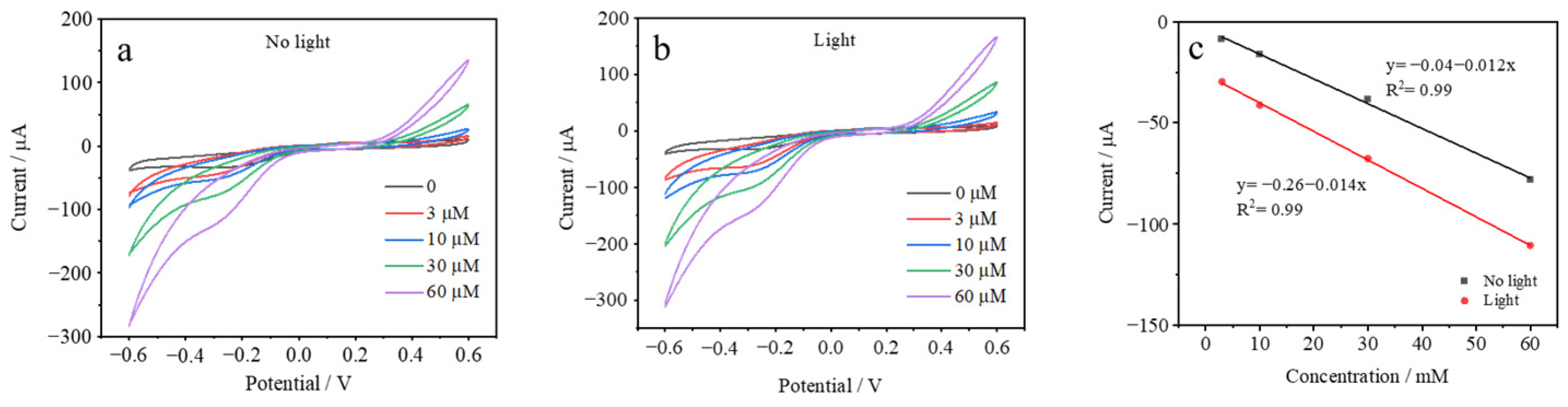
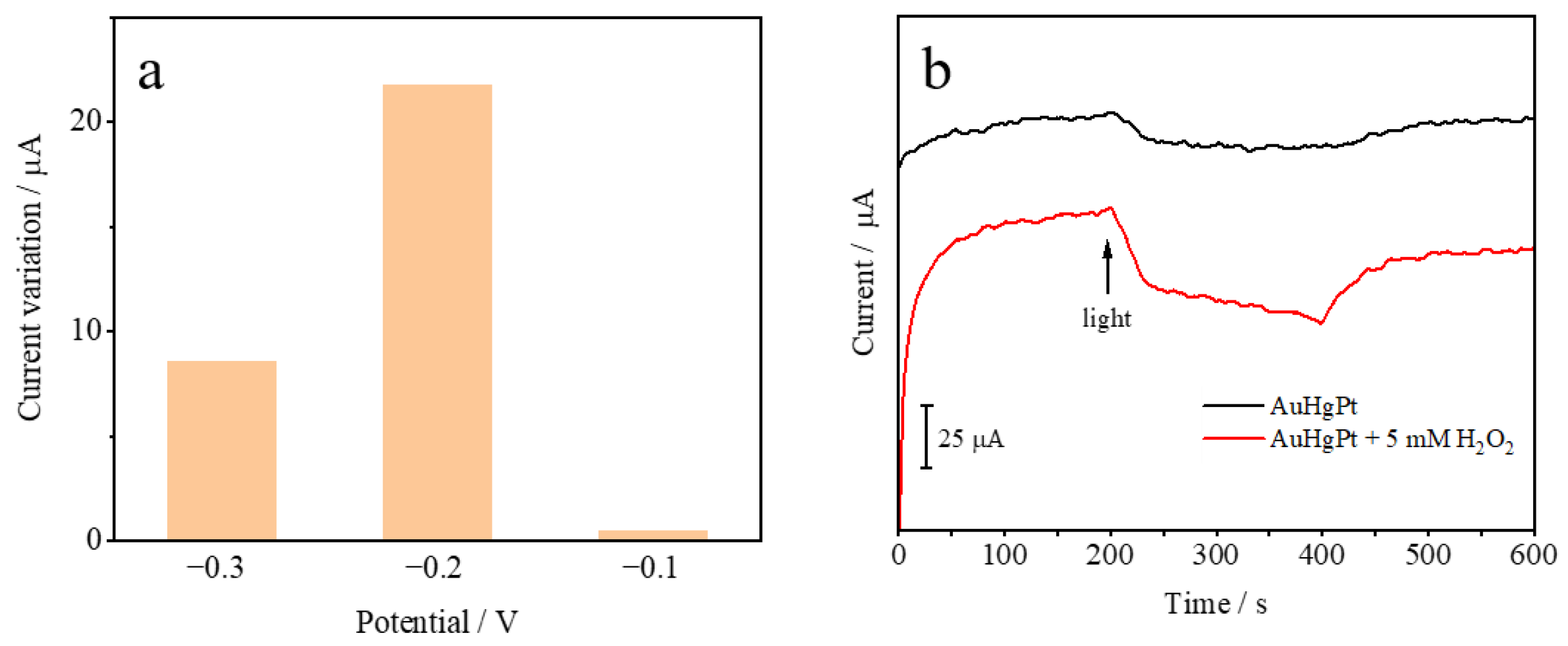
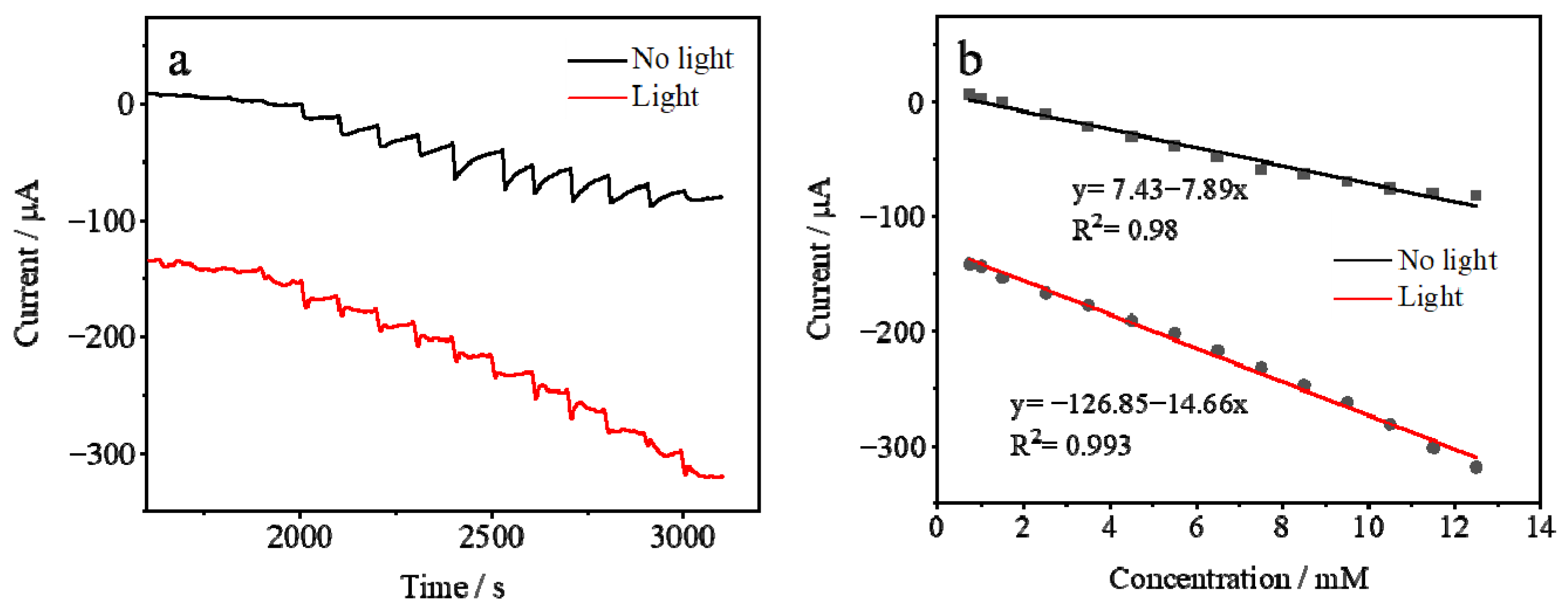
3.2. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kelly, K.L.; Coronado, E. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Kale, M.J.; Avanesian, T. Direct photocatalysis by plasmonic nanostructures. ACS Catal. 2014, 4, 116–128. [Google Scholar] [CrossRef]
- Li, W.; Cheng, S.; Zhang, H.; Yi, Z.; Tang, B.; Ma, C.; Wu, P.; Zeng, Q.; Raza, R. Multi-functional metasurface: Ultra-wideband/multi-band absorption switching by adjusting guided mode resonance and local surface plasmon resonance effects. Commun. Theor. Phys. 2024, 76, 065701. [Google Scholar] [CrossRef]
- Atwater, H.A.; Polman, A. Plasmonics for improved photovoltaic devices. Nat. Mater. 2010, 9, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Robatjazi, H.; Bahauddin, S.M. Direct plasmon-driven photoelectrocatalysis. Nano Lett. 2015, 15, 6155–6161. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.N.; Ai, Y. Plasmon-induced nanolocalized reduction of diazonium salts. ACS Omega 2017, 2, 1947–1955. [Google Scholar] [CrossRef]
- Wang, C.; Nie, X.G. Direct plasmon-accelerated electrochemical reaction on gold nanoparticles. ACS Nano. 2017, 11, 5897–5905. [Google Scholar] [CrossRef] [PubMed]
- Maji, S.K. Plasmon-enhanced electrochemical biosensing of hydrogen peroxide from cancer cells by gold nanorods. ACS Appl. Nano Mater. 2019, 2, 7162–7169. [Google Scholar] [CrossRef]
- Ilayaraja, N.; Prabu, N. Au-Pt graded nano-alloy formation and its manifestation in small organics oxidation reaction. J. Mater. Chem. A. 2013, 1, 4048–4056. [Google Scholar] [CrossRef]
- Wang, C.; Xu, H. Ir-doped Pd nanosheet assemblies as bifunctional electrocatalysts for advanced hydrogen evolution reaction and liquid fuel electrocatalysis. Inorg. Chem. 2020, 59, 3321–3329. [Google Scholar] [CrossRef]
- Gruzeł, G.; Arabasz, S. Conversion of bimetallic PtNi3 nanopolyhedra to ternary PtNiSn nanoframes by galvanic replacement reaction. Nanoscale 2019, 11, 5355–5364. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ren, F. Au-Cu-Pt ternary catalyst fabricated by electrodeposition and galvanic replacement with superior methanol electrooxidation activity. RSC Adv. 2014, 4, 57600–57607. [Google Scholar] [CrossRef]
- Ryu, J.; Choi, J. Morphology-controlled synthesis of ternary Pt-Pd-Cu alloy nanoparticles for efficient electrocatalytic oxygen reduction reactions. Appl. Catal. B 2015, 174–175, 526–532. [Google Scholar] [CrossRef]
- Shviro, M.; Polani, S. Hollow octahedral and cuboctahedral nanocrystals of ternary Pt-Ni-Au alloys. Nanoscale 2015, 7, 13521–13529. [Google Scholar] [CrossRef]
- Wang, N.; Cao, P. Hollow multiple noble metallic nanoalloys by mercury-assisted galvanic replacement reaction for hydrogen evolution. Inorg. Chem. 2021, 60, 3471–3478. [Google Scholar] [CrossRef] [PubMed]
- Labib, M.; Sargent, E.H. Electrochemical methods for the analysis of clinically relevant biomolecules. M. Chem. Rev. 2016, 116, 9001–9090. [Google Scholar] [CrossRef]
- Liu, C.; Ding, Y. Photochemical synthesis of glutathione-stabilized silver nanoclusters for fluorometric determination of hydrogen peroxide. Microchim. Acta 2017, 184, 2497–2503. [Google Scholar] [CrossRef]
- Sheng, Y.; Yang, H. Silver nanoclusters-catalyzed luminol chemiluminescence for hydrogen peroxide and uric acid detection. Talanta 2017, 166, 268–274. [Google Scholar] [CrossRef]
- Xin, Q.; Liu, Q. Electron spin resonance and fluorescence imaging assisted electrochemical approach for accurate and comprehensive monitoring of cellular hydrogen peroxide dynamics. Analyst 2017, 142, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Mahshid, S.S.; Flynn, S.E. The potential application of electrochemical biosensors in the COVID-19 pandemic: A perspective on the rapid diagnostics of SARS-CoV-2. Biosens. Bioelectron. 2021, 176, 112905. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Qi, J. Facile synthesis of Au@TiO2 core–shell hollow spheres for dye-sensitized solar cells with remarkably improved efficiency. Energy Environ. Sci. 2012, 5, 6914–6918. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, Y. Colorimetric response of biogenetic gold nanoparticles to mercury (II) ions. Colloids Surf. A 2016, 508, 360–365. [Google Scholar] [CrossRef]
- Cao, P.F.; Xu, C.C. Amalgamation-based AuHgPt nanochains as electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2024, 12, 33908–33916. [Google Scholar] [CrossRef]
- Luo, J.; Niu, Q. Study on the effects of oxygen-containing functional groups on Hg0 adsorption in simulated flue gas by XAFS and XPS analysis. J. Hazard. Mater. 2019, 376, 21–28. [Google Scholar] [CrossRef]
- Liang, W.; Wang, Y. 3D anisotropic Au@Pt–Pd hemispherical nanostructures as efficient electrocatalysts for methanol, ethanol, and formic acid oxidation reaction. Adv. Mater. 2021, 33, 2100713. [Google Scholar] [CrossRef]
- Cui, X.; Li, Z. Low-potential sensitive hydrogen peroxide detection based on nanotubular TiO2 and platinum composite electrode. Electroanalysis 2008, 9, 970–975. [Google Scholar] [CrossRef]
- Gowthaman, N.S.K.; Arul, P. Free standing Au-Ag nanoparticles on carbon cloth: A non-enzymatic flexible electrochemical sensor for the biomarker of oxidative stress. Appl. Surf. Sci. 2019, 495, 143550. [Google Scholar] [CrossRef]
- Thanh, T.D.; Balamurugan, J. Novel porous gold-palladium nanoalloy network-supported graphene as an advanced catalyst for non-enzymatic hydrogen peroxide sensing. Biosens. Bioelectron. 2016, 85, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Lin, D.Q. Flexible fiber-shaped non-enzymatic sensors with a graphene-metal heterostructure based on graphene fibres decorated with gold nanosheets. Carbon 2018, 136, 329–336. [Google Scholar] [CrossRef]


| Material | Linear Range (μM) | Detection Limit (μM) | Ref. |
|---|---|---|---|
| Pt-TiO2 nanotube arrays | 40–1.25 × 103 | 4.0 | [26] |
| Au–Ag NPs | 0.5–2 × 103 | 0.059 | [27] |
| Au–Pd/graphene | 5–11.5 × 103 | 1.0 | [28] |
| Au nanosheet | 9.4–13 × 103 | 1.62 | [29] |
| AuHgPt | 0.5–5 × 103 | 0.17 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Li, R.; Lin, M. Gold–Mercury–Platinum Alloy for Light-Enhanced Electrochemical Detection of Hydrogen Peroxide. Sensors 2025, 25, 135. https://doi.org/10.3390/s25010135
Wei Y, Li R, Lin M. Gold–Mercury–Platinum Alloy for Light-Enhanced Electrochemical Detection of Hydrogen Peroxide. Sensors. 2025; 25(1):135. https://doi.org/10.3390/s25010135
Chicago/Turabian StyleWei, Yunping, Runze Li, and Meng Lin. 2025. "Gold–Mercury–Platinum Alloy for Light-Enhanced Electrochemical Detection of Hydrogen Peroxide" Sensors 25, no. 1: 135. https://doi.org/10.3390/s25010135
APA StyleWei, Y., Li, R., & Lin, M. (2025). Gold–Mercury–Platinum Alloy for Light-Enhanced Electrochemical Detection of Hydrogen Peroxide. Sensors, 25(1), 135. https://doi.org/10.3390/s25010135






