Short-Peptide-Modified Copper Nanoclusters as a Fluorescent Probe for the Specific Detection of Ascorbic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
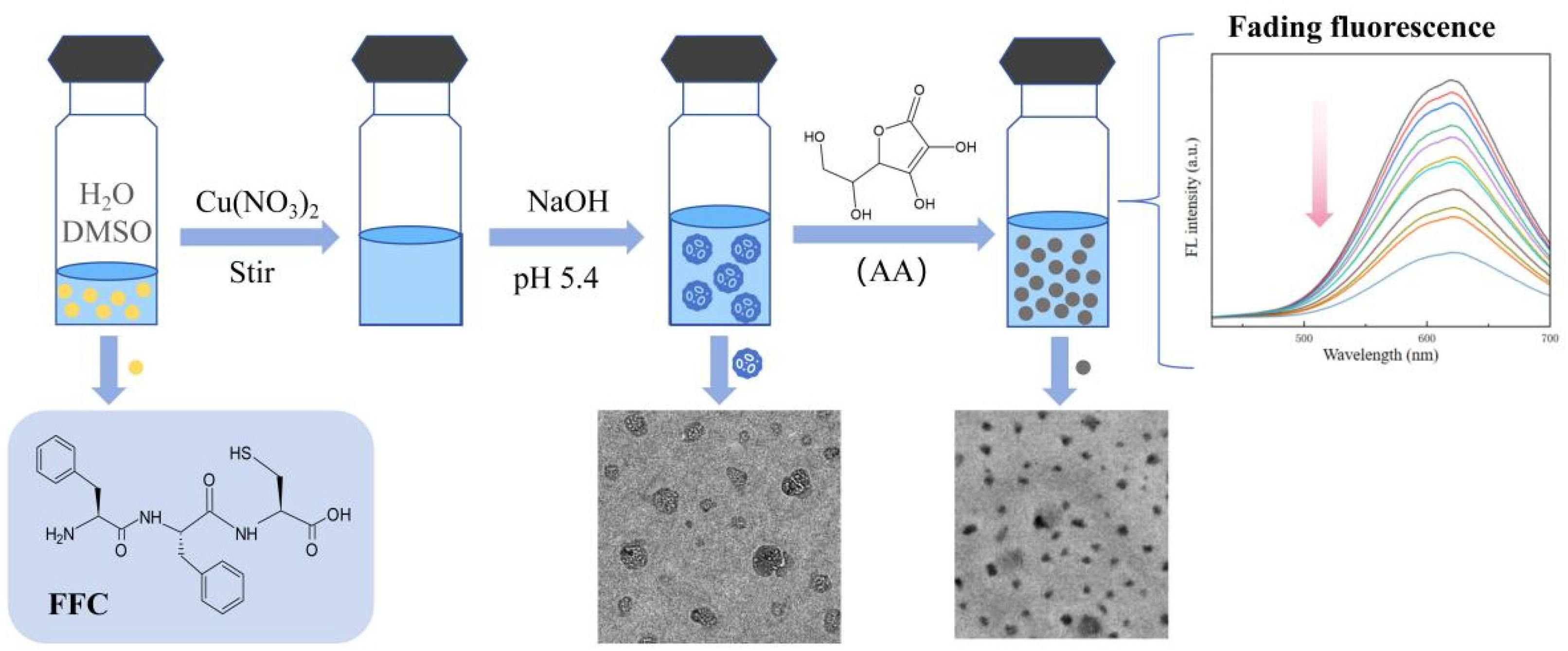
2.3. The Assembly of Cu NCs
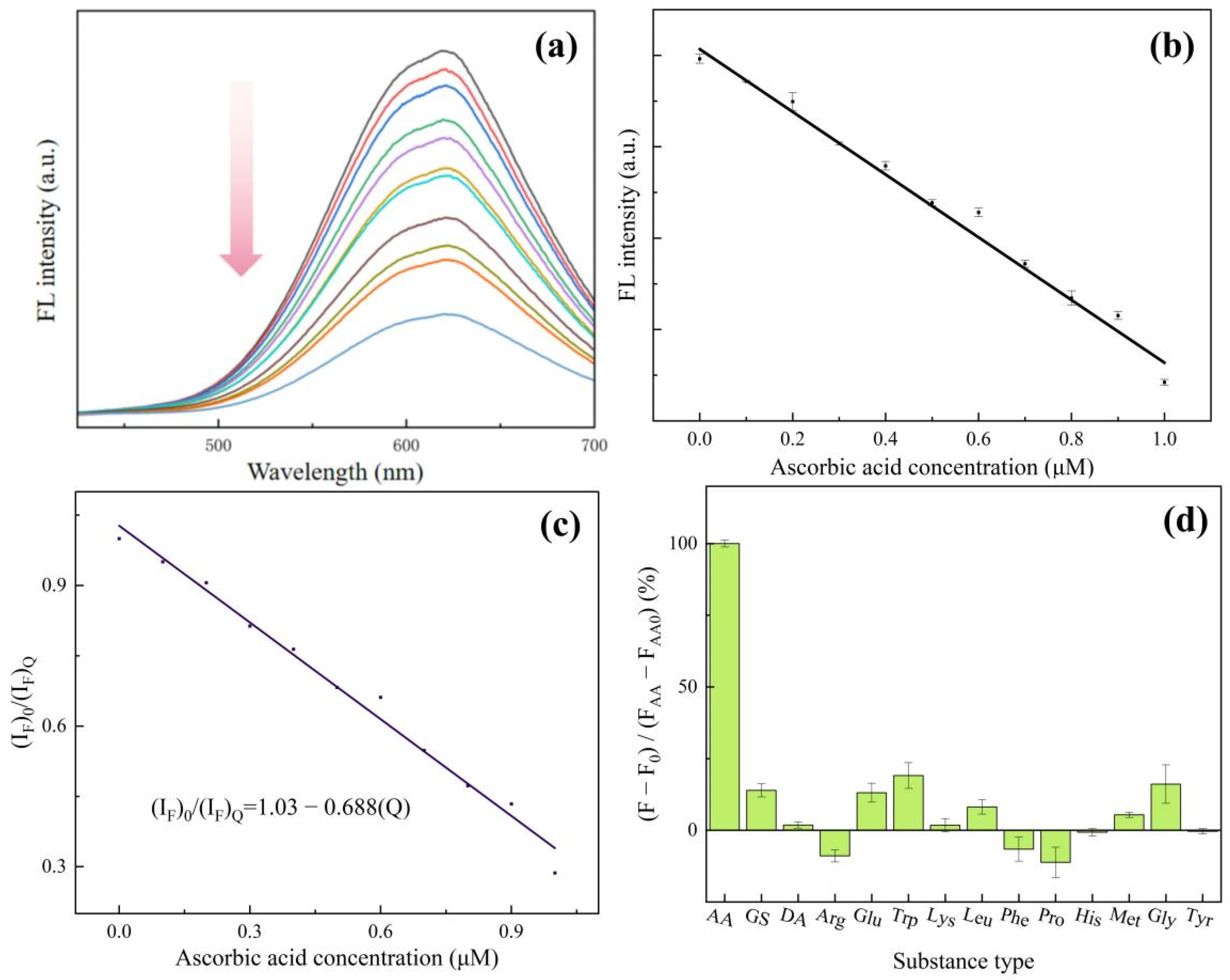
2.4. The Detection of Ascorbic Acid
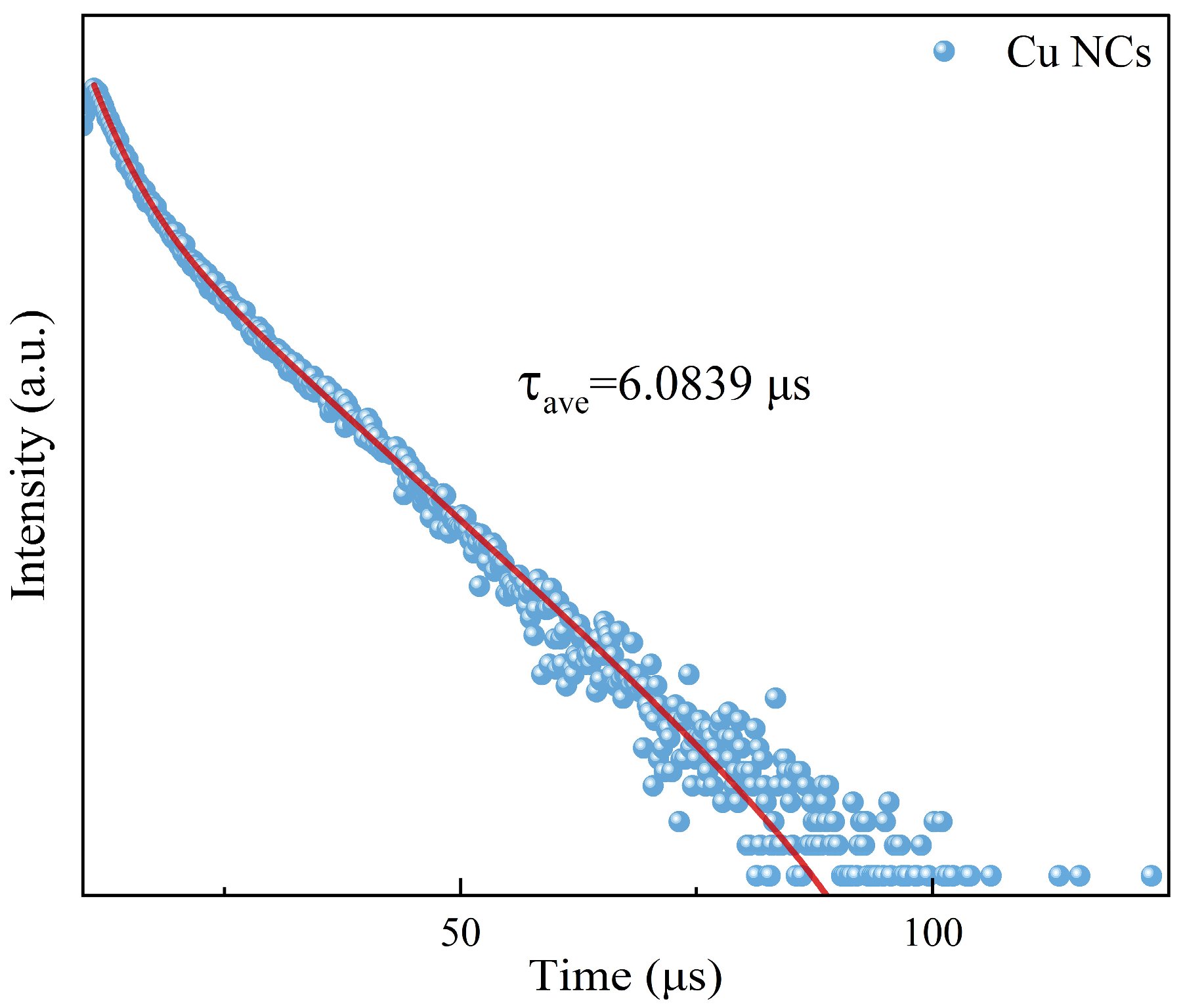
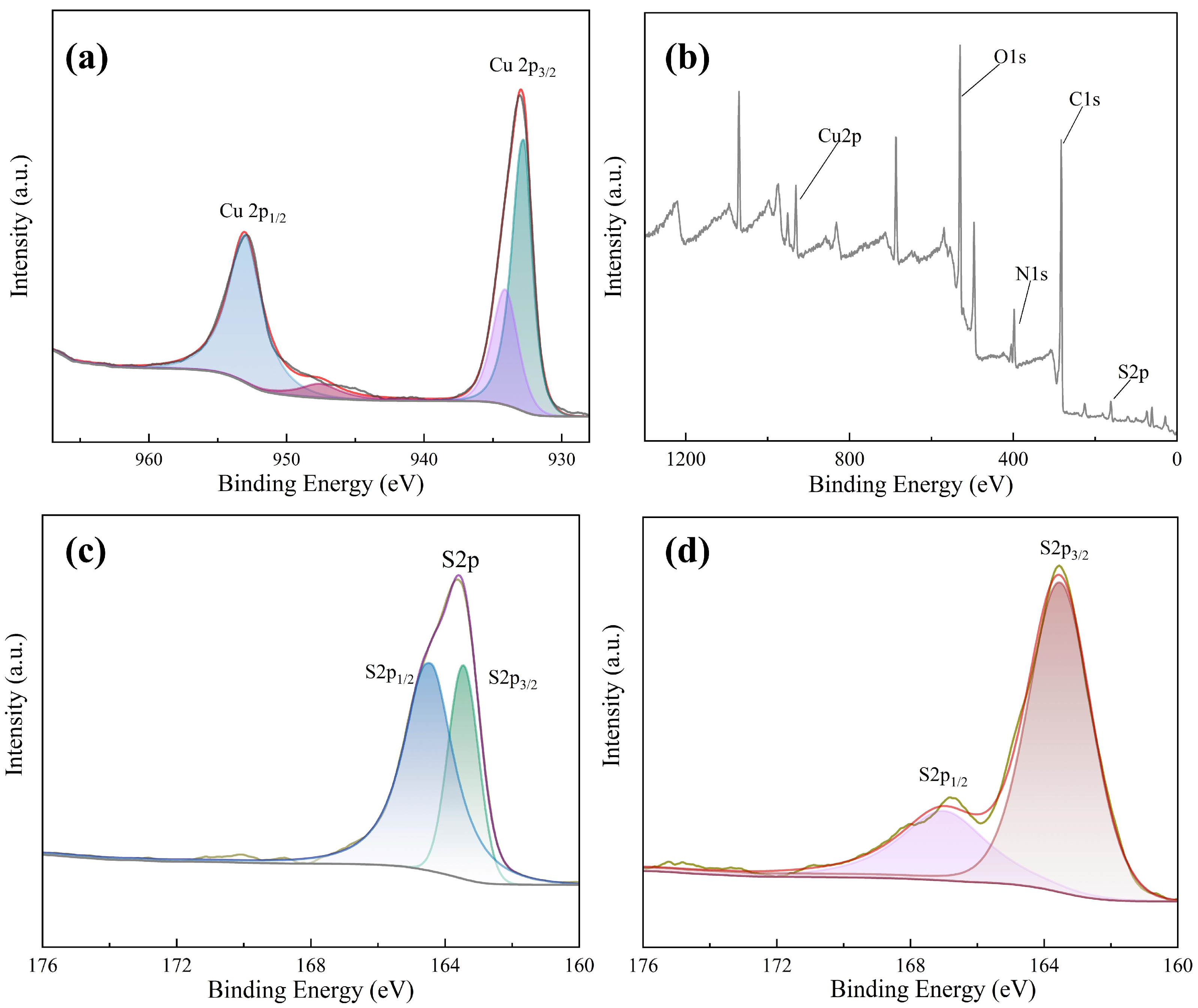
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, J.X.; Feng, Y.; Hu, Q.Q.; Kuang, J.H.; Cheng, Z.J. A ratio fluorescence method based on dual emissive copper nanoclusters for the detection of vanillin. J. Fluoresc. 2024, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Lao, S.Y.; Ding, W.J.; Zhang, Z.D.; Liu, S.Y. A novel ratiometric fluorescent probe for detection of iron ions and zinc ions based on dual-emission carbon dots. Sens. Actuators B Chem. 2019, 284, 186–192. [Google Scholar] [CrossRef]
- Guo, Y.M.; Zhang, L.F.; Zhang, S.S.; Yang, Y.; Chen, H.X.; Zhang, M.C. Fluorescent carbon nanoparticles for the fluorescent detection of metal ions. Biosens. Bioelectron. 2015, 63, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.H.; Zhang, Z.H.; Tang, A.W.; Li, C.; Shen, Y.H. Synthesis of yeast extract-stabilized Cu nanoclusters for sensitive fluorescent detection of sulfide ions in water. Biosens. Bioelectron. 2016, 79, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ling, L.; Yao, Y.G.; Song, Q.J. One-step synthesis of fluorescent smart thermo-responsive copper clusters: A potential nanothermometer in living cells. Nano Res. 2015, 8, 1975–1986. [Google Scholar] [CrossRef]
- Qiao, Y.Y.; Xu, T.; Zhang, Y.; Zhang, C.H.; Shi, L.H.; Zhang, G.M.; Shuang, S.M.; Dong, C. Green synthesis of fluorescent copper nanoclusters for reversible pH-sensors. Sens. Actuators B Chem. 2015, 220, 1064–1069. [Google Scholar] [CrossRef]
- Hong, S.; Kuo, Y.-A.; Nguyen, T.; Chen, Y.-I.; Liu, Y.-L.; Shankar, P.; Yeh, H. Array-based differential sensing of cancer cells using DNA-templated silver nanoclusters. In Proceedings of the Nanoscale Imaging, Sensing, and Actuation for Biomedical Applications XVII, San Francisco, CA, USA, 2–3 February 2020; Volume 11254, pp. 75–82. [Google Scholar]
- Zhang, M.M.; Qiao, J.; Zhang, S.F.; Qi, L. Copper nanoclusters as probes for turn-on fluorescence sensing of L-lysine. Talanta 2018, 182, 595–599. [Google Scholar] [CrossRef]
- Pandit, S.; Kundu, S. 2-Methods of synthesis of metal nanoclusters. In Luminescent Metal Nanoclusters; Woodhead Publishing: Cambridge, UK, 2022; pp. 17–55. [Google Scholar]
- Asif, N.; Amir, M.; Fatma, T. Recent advances in the synthesis, characterization and biomedical applications of zinc oxide nanoparticles. Bioprocess Biosyst. Eng. 2023, 46, 1377–1398. [Google Scholar] [CrossRef]
- Yang, J.; Jin, R.C. New advances in atomically precise silver nanoclusters. ACS Mater. Lett. 2019, 1, 482–489. [Google Scholar] [CrossRef]
- Park, M.; Im, J.; Shin, M.; Min, Y.; Park, J.; Cho, H.; Park, S.; Shim, M.-B.; Jeon, S.; Chung, D.-Y.; et al. Highly stretchable electric circuits from a composite material of silver nanoparticles and elastomeric fibres. Nat. Nanotechnol. 2012, 7, 803–809. [Google Scholar] [CrossRef]
- Park, S.Y.; Lytton-Jean, A.K.R.; Lee, B.; Weigand, S.; Schatz, G.C.; Mirkin, C.A. DNA-programmable nanoparticle crystallization. Nature 2008, 451, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, R.J.; Lee, B.; Jones, M.R.; Harris, N.; Schatz, G.C.; Mirkin, C.A. Nanoparticle superlattice engineering with DNA. Science 2011, 334, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.D.; Zhang, J.Q.; Ekman, J.M.; Kenis, P.J.A.; Lu, Y. DNA-mediated control of metal nanoparticle shape: One-pot synthesis and cellular uptake of highly stable and functional gold nanoflowers. Nano Lett. 2010, 10, 1886–1891. [Google Scholar] [CrossRef]
- Adler-Abramovich, L.; Gazit, E. The physical properties of supramolecular peptide assemblies: From building block association to technological applications. Chem. Soc. Rev. 2014, 43, 6881–6893. [Google Scholar] [CrossRef] [PubMed]
- Fichman, G.; Gazit, E. Self-assembly of short peptides to form hydrogels: Design of building blocks, physical properties and technological applications. Acta Biomater. 2014, 10, 1671–1682. [Google Scholar] [CrossRef]
- Ding, S.C.; Zhang, N.; Lyu, Z.Y.; Zhu, W.L.; Chang, Y.C.; Hu, X.L.; Du, D.; Lin, Y.H. Protein-based nanomaterials and nanosystems for biomedical applications: A review. Mater. Today 2021, 43, 166–184. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J.; Tveden-nyborg, P. The pharmacokinetics of vitamin C. Nutrients 2019, 11, 2412. [Google Scholar] [CrossRef]
- Zheng, X.Z.; Gong, M.; Zhang, Q.D.; Tan, H.Q.; Li, L.P.; Tang, Y.W.; Li, Z.G.; Peng, M.C.; Deng, W. Metabolism and regulation of ascorbic acid in fruits. Plants 2022, 11, 1602. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef]
- Ding, W.C.; Liu, Y.; Li, Y.J.; Shi, Q.R.; Li, H.S.; Xia, H.B.; Wang, D.Y.; Tao, X.T. Water-soluble gold nanoclusters with pH-dependent fluorescence and high colloidal stability over a wide pH range via co-reduction of glutathione and citrate. RSC Adv. 2014, 4, 22651–22659. [Google Scholar] [CrossRef]
- Souza, T.G.F.; Ciminelli, V.S.T.; Mohallem, N.D.S. A comparison of TEM and DLS methods to characterize size distribution of ceramic nanoparticles. J. Phys. Conf. Ser. 2016, 733, 012039. [Google Scholar] [CrossRef]
- Pandit, S.; Kundu, S. pH-Dependent reversible emission behaviour of lysozyme coated fluorescent copper nanoclusters. J. Lumin. 2020, 228, 117607. [Google Scholar] [CrossRef]
- Liao, X.Q.; Li, R.Y.; Long, X.H.; Li, Z.J. Ultra sensitive and wide-range pH sensor based on the BSA-capped Cu nanoclusters fabricated by fast synthesis through the use of hydrogen peroxide additive. RSC Adv. 2015, 5, 48835–48841. [Google Scholar]
- Kawasaki, H.; Hamaguchi, K.; Osaka, I.; Arakawa, R. ph-Dependent synthesis of pepsin-mediated gold nanoclusters with blue green and red fluorescent emission. Adv. Funct. Mater. 2011, 21, 3508–3515. [Google Scholar] [CrossRef]
- Gou, S.Y.; Shi, Y.-E.; Li, P.; Wang, H.G.; Li, T.Z.; Zhuang, X.M.; Li, W.; Wang, Z.G. Stimuli-responsive luminescent copper nanoclusters in alginate and their sensing ability for glucose. ACS Appl. Mater. Interfaces 2019, 11, 6561–6567. [Google Scholar] [CrossRef]
- Stenspil, G.S.; Laursen, B.W. Photophysics of fluorescent nanoparticles based on organic dyes-challenges and design principles. Chem. Sci. 2024, 15, 8625–8638. [Google Scholar] [CrossRef]
- Athina, P.; Green, R.J.; Frazier, R.A. Interaction of flavonoids with bovine serum albumin: A fluorescence quenching study. J. Agric. Food Chem. 2004, 53, 158–163. [Google Scholar]
- Porto, I.S.A.; Santos Neto, J.H.; Santos, L.O.D.; Gomes, A.A.; Ferreira, S.L.C. Determination of ascorbic acid in natural fruit juices using digital image colorimetry. Microchem. J. 2019, 149, 104031. [Google Scholar] [CrossRef]
- He, J.; He, D.X.; Yang, L.; Wu, G.L.; Tian, J.M.; Liu, Y.; Wang, W.G. Preparation of urchin-like Pd-Pt-Ir nanozymes and their application for the detection of ascorbic acid and hydrogen peroxide. Mater. Lett. 2022, 314, 131851. [Google Scholar] [CrossRef]
- Fernandes, D.S.; Carmo, D.R. Silsesquioxane modifified with PAMAM dendrimer and a bimetallic complex for electrochemical detection of ascorbic acid. Electroanalysis 2021, 33, 365–374. [Google Scholar] [CrossRef]
- Huang, D.Q.; Li, X.; Chen, M.M.; Rui, R.; Wang, R.; Fan, S.H.; Wu, H. An electrochemical sensor based on a porphyrin dye-functionalized multi-walled carbon nanotubes hybrid for the sensitive determination of ascorbic acid. Electroanal. Chem. 2019, 841, 101–106. [Google Scholar] [CrossRef]
- Shi, H.; Chen, L.G.; Niu, N. An off-on fluorescent probe based on graphene quantum dots intercalated hydrotalcite for determination of ascorbic acid and phytase. Sens. Actuators B Chem. 2021, 345, 130353. [Google Scholar] [CrossRef]
- Huang, D.; Qi, H.Y.; Jing, J.; Sami, R.; Jing, T.; Alsufyani, S.J.; Benajiba, N.; Madkhali, N. A continuously tunable full-color emission nitrogen-doped carbon dots and for ultrasensitive and highly selective detection of ascorbic acid. Nanomaterials 2022, 12, 693. [Google Scholar] [CrossRef]
- Xu, S.F.; Ye, S.Q.; Xu, Y.H.; Liu, F.F.; Zhou, Y.S.; Yang, Q.; Peng, H.L.; Xiong, H.; Zhang, Z. Microwave-assisted synthesis of N, S-co-carbon dots as switch-on fluorescent sensor for rapid and sensitive detection of ascorbic acid in processed fruit juice. Anal. Sci. 2020, 36, 353–360. [Google Scholar] [CrossRef]
- Pirot, S.M.; Omer, K.M.; Alshatteri, A.H.; Ali, G.K.; Shatery, O.B.A. Dual-template molecularly surface imprinted polymer on fluorescent metal-organic frameworks functionalized with carbon dots for ascorbic acid and uric acid detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 291, 122340. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Lan, X.; Liu, X. Short-Peptide-Modified Copper Nanoclusters as a Fluorescent Probe for the Specific Detection of Ascorbic Acid. Sensors 2024, 24, 6974. https://doi.org/10.3390/s24216974
Li J, Lan X, Liu X. Short-Peptide-Modified Copper Nanoclusters as a Fluorescent Probe for the Specific Detection of Ascorbic Acid. Sensors. 2024; 24(21):6974. https://doi.org/10.3390/s24216974
Chicago/Turabian StyleLi, Jiataiqi, Xin Lan, and Xingcen Liu. 2024. "Short-Peptide-Modified Copper Nanoclusters as a Fluorescent Probe for the Specific Detection of Ascorbic Acid" Sensors 24, no. 21: 6974. https://doi.org/10.3390/s24216974
APA StyleLi, J., Lan, X., & Liu, X. (2024). Short-Peptide-Modified Copper Nanoclusters as a Fluorescent Probe for the Specific Detection of Ascorbic Acid. Sensors, 24(21), 6974. https://doi.org/10.3390/s24216974




