α-Fe2O3/TiO2/Ti3C2Tx Nanocomposites for Enhanced Acetone Gas Sensors
Abstract
1. Introduction
2. Materials and Methods
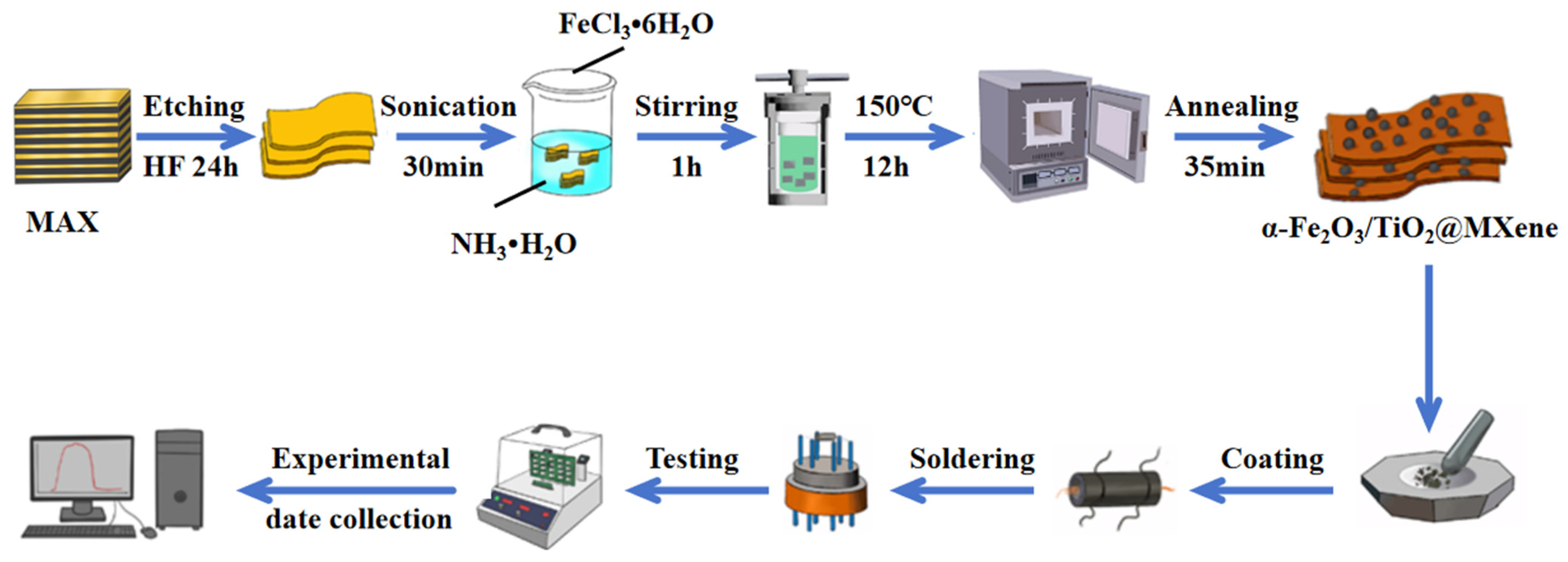
2.1. Synthesis of Ti3C2Tx MXene
2.2. Synthesis of α-Fe2O3
2.3. Synthesis of α-Fe2O3/TiO2@Ti3C2Tx Nanocomposites
2.4. Structures and Morphology Characterizations
2.5. Evaluation of Gas-Sensing Performance
3. Results
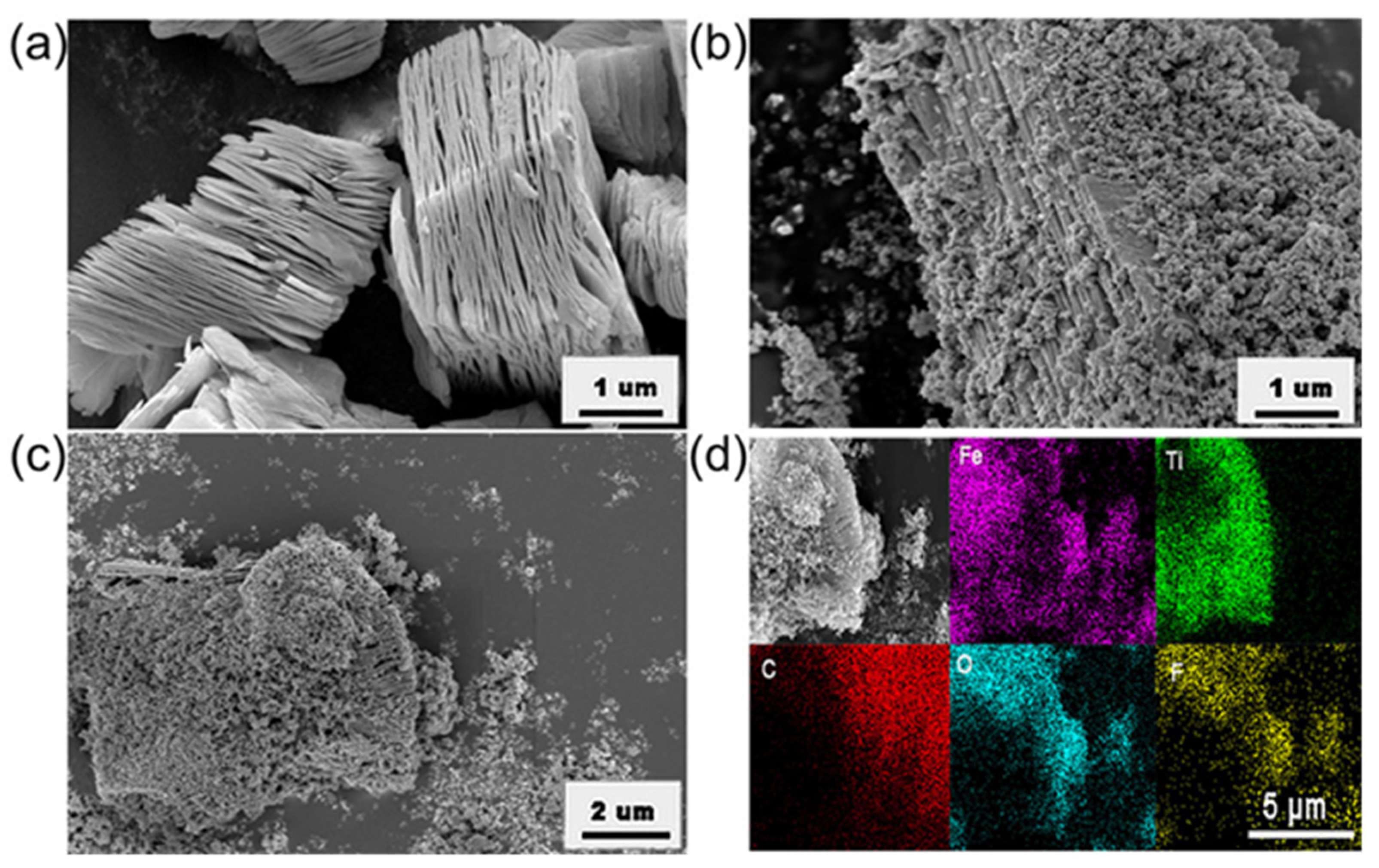
3.1. Structures and Morphology of Samples
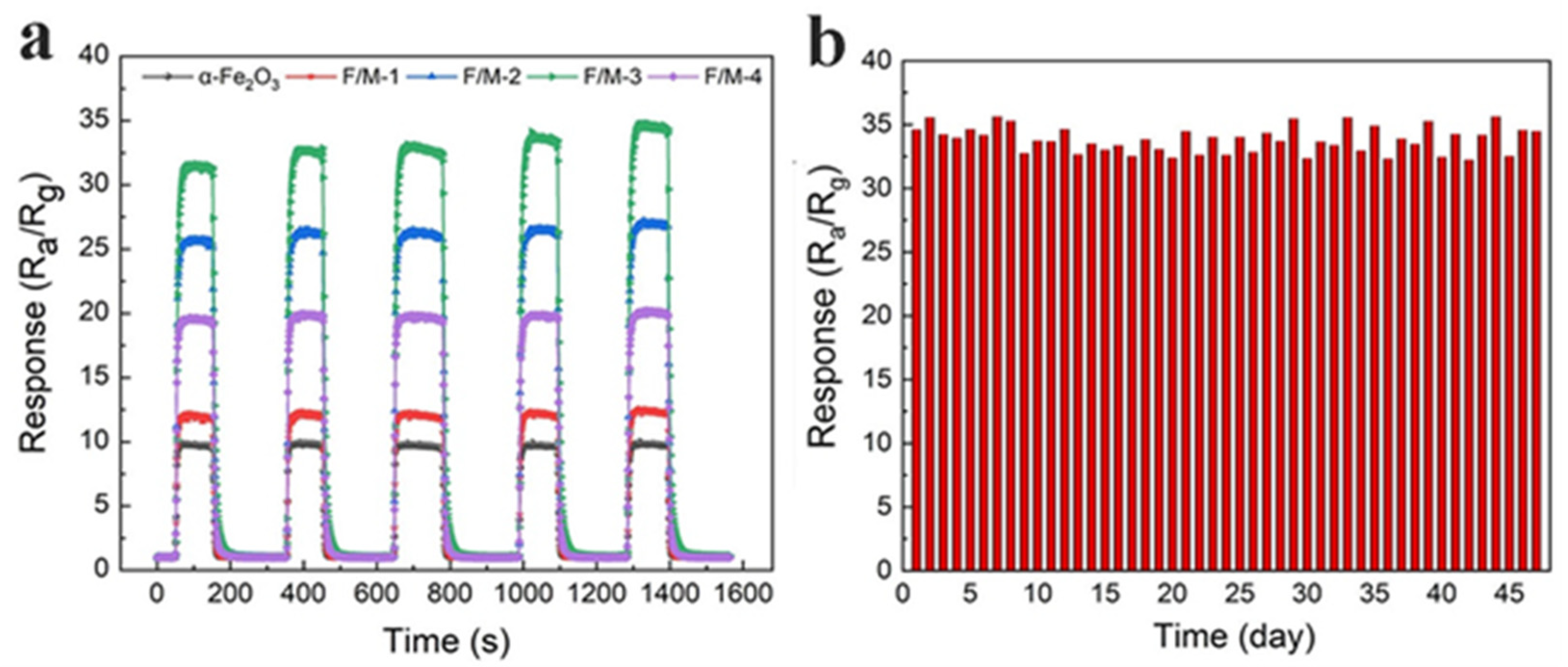
3.2. Gas-Sensing Performance
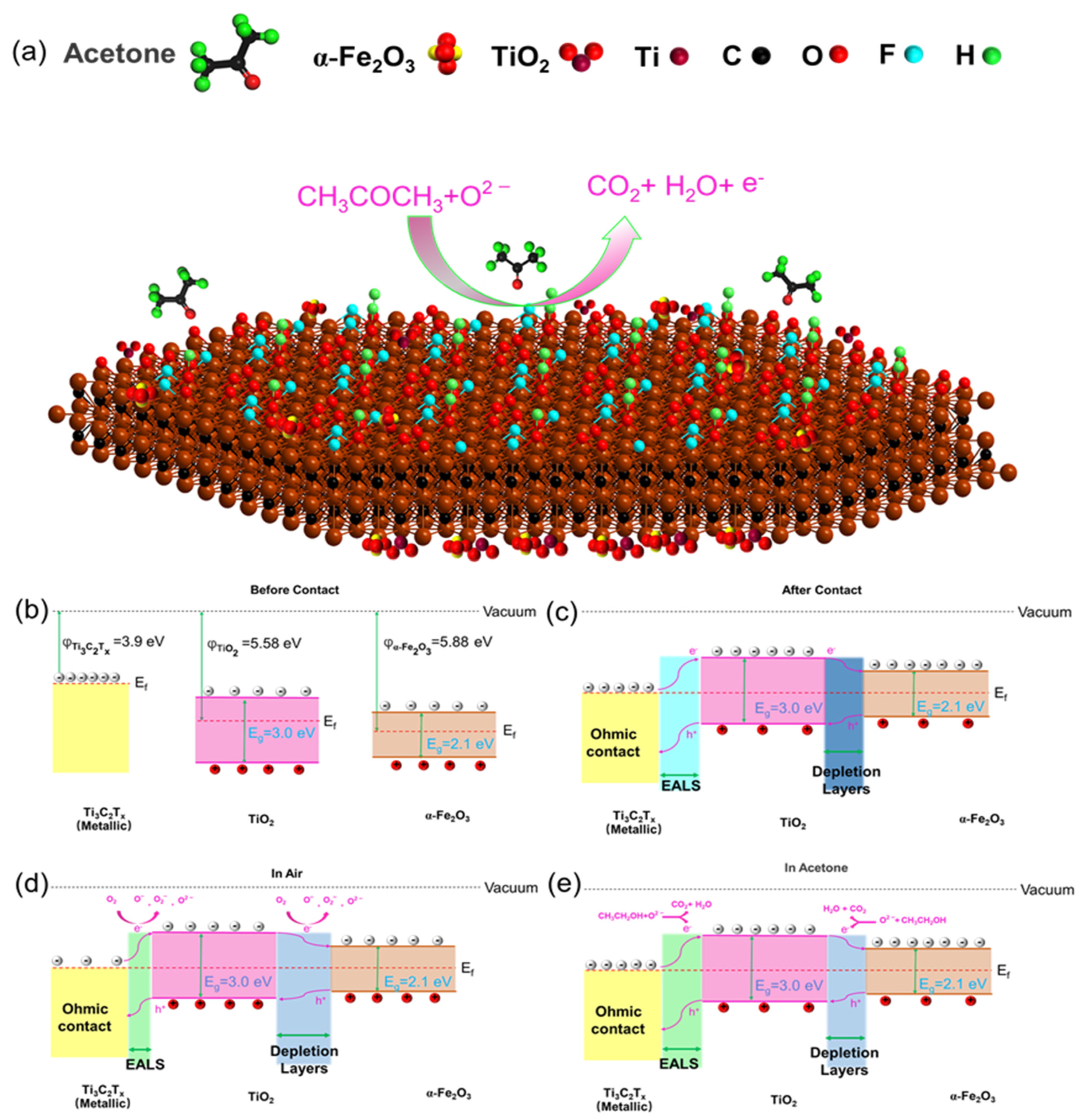
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lupan, O.; Postica, V.; Wolff, N.; Polonskyi, O.; Duppel, V.; Kaidas, V.; Lazari, E.; Ababii, A.; Faupel, F.; Kienle, L.; et al. Localized synthesis of iron oxide nanowires and fabrication of high performance nanosensors based on a single Fe2O3 nanowire. Small 2017, 13, 1602868. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Ma, W.; Jiang, F.; Cao, E.S.; Sun, K.M.; Cheng, L.; Song, X.Z. Prussian Blue analogue derived porous NiFe2O4 nanocubes for low-concentration acetone sensing at low working temperature. Chem. Eng. J. 2018, 338, 504–512. [Google Scholar] [CrossRef]
- Makisimovich, N.; Vorotyntsev, V.; Nikitina, N.; Kaskevich, O.; Karabun, P.; Martynenko, F. Adsorption semiconductor sensor for diabetic ketoacidosis diagnosis. Sens. Actuators B Chem. 1996, 36, 419–421. [Google Scholar] [CrossRef]
- Park, S. Acetone gas detection using TiO2 nanoparticles functionalized In2O3 nanowires for diagnosis of diabetes. J. Alloys Compd. 2017, 696, 655–662. [Google Scholar] [CrossRef]
- Galassetti, P.R.; Novak, B.; Nemet, D.; Rose-Gottron, C.; Cooper, D.M.; Meinardi, S.; Newcomb, R.; Zaldivar, F.; Blake, D.R. Breath ethanol and acetone as indicators of serum glucose levels: An initial report. Diabetes Technol. Ther. 2005, 7, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Yamazoe, N. Toward innovations of gas sensor technology. Sens. Actuators B Chem. 2005, 108, 2–14. [Google Scholar] [CrossRef]
- Wu, C.; Yin, P.; Zhu, X.; Zhu, X.; OuYang, C.; Xie, Y. Synthesis of hematite (α-Fe2O3) nanorods: Diameter-size and shape effects on their applications in magnetism, lithium ion battery, and gas sensors. J. Phys. Chem. B 2006, 110, 17806–17812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Fu, W.; Meng, X.; Meng, X.; Ruan, A.; Su, P.; Yang, H. Enhanced ethanol sensing properties based on spherical-coral-like SnO2 nanorods decorated with α-Fe2O3 nanocrystallites. Sens. Actuators B Chem. 2018, 261, 505–514. [Google Scholar] [CrossRef]
- Cao, J.; Wang, S.; Zhang, H.; Zhang, T. Facile construction of Co3O4 porous microspheres with enhanced acetone gas sensing performances. Mater. Sci. Semicond. Process. 2019, 101, 10–15. [Google Scholar] [CrossRef]
- Kou, X.; Xie, N.; Chen, F.; Wang, T.; Guo, L.; Wang, C.; Wang, Q.; Ma, J.; Sun, Y.; Zhang, H.; et al. Superior acetone gas sensor based on electrospun SnO2 nanofibers by Rh doping. Sens. Actuators B Chem. 2018, 256, 861–869. [Google Scholar] [CrossRef]
- Song, Y.G.; Park, J.Y.; Suh, J.M.; Shim, Y.S.; Yi, S.Y.; Jang, H.W.; Kim, S.; Yuk, J.M.; Ju, B.K.; Kang, C.Y. Heterojunction based on Rh-decorated WO3 nanorods for morphological change and gas sensor application using the transition effect. Chem. Mater. 2018, 31, 207–215. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, S.; Qu, F.; Gong, S.; Wang, C.; Qiu, L.; Yang, M.; Cheng, W. High performance acetone sensor based on ZnO nanorods modified by Au nanoparticles. J. Alloys Compd. 2019, 797, 246–252. [Google Scholar] [CrossRef]
- Kim, D.H.; Shim, Y.S.; Jeon, J.M.; Jeong, H.Y.; Park, S.S.; Kim, Y.W.; Kim, J.S.; Lee, J.H.; Jang, H.W. Vertically ordered hematite nanotube array as an ultrasensitive and rapid response acetone sensor. ACS Appl. Mater. Interfaces 2014, 6, 14779–14784. [Google Scholar] [CrossRef] [PubMed]
- Picasso, G.; Quintilla, A.; Pina, M.P.; Herguido, J. Total combustion of methyl-ethyl ketone over Fe2O3 based catalytic membrane reactors. Appl. Catal. B Environ. 2003, 46, 133–143. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, T.; Zheng, L.; Zhao, Y.; Liu, X.; Zhang, J. Heterostructures of hematite-sensitized W18O49 hollow spheres for improved acetone detection with ultralow detection limit. Sens. Actuators B Chem. 2019, 288, 432–441. [Google Scholar] [CrossRef]
- Wang, X.; Wang, T.; Si, G.; Li, Y.; Zhang, S.; Deng, X.; Xu, X. Oxygen vacancy defects engineering on Ce-doped α-Fe2O3 gas sensor for reducing gases. Sens. Actuators B Chem. 2020, 302, 127165. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Zhao, P.; Li, W.; Wang, Q.; Sun, P.; Chuai, X.; Lu, G. Porous α-Fe2O3 microflowers: Synthesis, structure, and enhanced acetone sensing performances. J. Colloid Interface Sci. 2017, 505, 1039–1046. [Google Scholar] [CrossRef]
- Guo, L.; Kou, X.; Ding, M.; Wang, C.; Dong, L.; Zhang, H.; Feng, C.; Sun, Y.; Gao, Y.; Sun, P.; et al. Reduced graphene oxide/α-Fe2O3 composite nanofibers for application in gas sensors. Sens. Actuators B Chem. 2017, 244, 233–242. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, K.; Wei, W.; Wang, L.; Han, W. High-performance flexible sensing devices based on polyaniline/MXene nanocomposites. InfoMat 2019, 1, 407–416. [Google Scholar] [CrossRef]
- Lee, E.; VahidMohammadi, A.; Yoon, Y.S.; Beidaghi, M.; Kim, D.J. Two-dimensional vanadium carbide MXene for gas sensors with ultrahigh sensitivity toward nonpolar gases. ACS Sens. 2019, 4, 1603–1611. [Google Scholar] [CrossRef]
- Zhang, S.; Song, P.; Sun, J.; Ding, Y.; Wang, Q. MoO3/Ti3C2Tx MXene nanocomposites with rapid response for enhanced ethanol-sensing at a low temperature. Sens. Actuators B Chem. 2023, 378, 133216. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; Cheng, J.; Liu, Z.; Li, Q.; Li, W.; Yang, X.; Xiao, B. Monolayer Ti2CO2: A promising candidate for NH3 sensor or capturer with high sensitivity and selectivity. ACS Appl. Mater. Interfaces 2015, 7, 13707–13713. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; VahidMohammadi, A.; Prorok, B.C.; Yoon, Y.S.; Beidaghi, M.; Kim, D.J. Room temperature gas sensing of two-dimensional titanium carbide (MXene). ACS Appl. Mater. Interfaces 2017, 9, 37184–37190. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ji, J.; Song, P.; Wang, J.; Wang, Q. Sensing performance of α-Fe2O3/Ti3C2Tx MXene nanocomposites to NH3 at room temperature. J. Alloys Compd. 2022, 898, 162812. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, A.; Wang, C.; Liu, F.; He, J.; Li, S.; Wang, J.; You, R.; Yan, X.; Sun, P.; et al. Improvement of gas and humidity sensing properties of organ-like MXene by alkaline treatment. ACS Sens. 2019, 4, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, J.L.; Jiang, Y.D.; He, Z.Z.; Liu, B.H.; Xie, H.K.; Li, H.; Li, Z.M.; Wang, Y.; Tai, H.L. Toward agricultural ammonia volatilization monitoring: A flexible polyaniline/Ti3C2Tx hybrid sensitive films based gas sensor. Sens. Actuators B-Chem. 2020, 316, 11. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, C.; Jiang, F.; Liu, J.; Ma, X.; Liu, P.; Xu, J.; Wang, L.; Huang, R. Flexible and lightweight Ti3C2Tx MXene@Pd colloidal nanoclusters paper film as novel H2 sensor. J. Hazard. Mater. 2020, 399, 123054. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, M.; Chang, X.; Jiang, Y.; Zhang, D.; Wang, D.; Zhang, Y.; Lei, Y. W18O49/Ti3C2Tx Mxene nanocomposites for highly sensitive acetone gas sensor with low detection limit. Sens. Actuators B Chem. 2020, 304, 127274. [Google Scholar] [CrossRef]
- Xie, Q.; Ding, Y.; Wang, Q.; Song, P. Fabrication of 1D/2D In2O3 nanofibers/Ti3C2TX MXene composites for high performance detection of trimethylamine at low temperature. Sens. Actuators B Chem. 2024, 405, 135338. [Google Scholar] [CrossRef]
- Liu, M.; Ji, J.; Song, P.; Liu, M.; Wang, Q. α-Fe2O3 nanocubes/Ti3C2Tx MXene composites for improvement of acetone sensing performance at room temperature. Sens. Actuators B Chem. 2021, 349, 130782. [Google Scholar] [CrossRef]
- Hermawan, A.; Zhang, B.; Taufik, A.; Asakura, Y.; Hasegawa, T.; Zhu, J.; Shi, P.; Yin, S. CuO nanoparticles/Ti3C2Tx MXene hybrid nanocomposites for detection of toluene gas. ACS Appl. Nano Mater. 2020, 3, 4755–4766. [Google Scholar] [CrossRef]
- Chen, P.; Zhao, Z.; Shao, Z.; Tian, Y.; Li, B.; Huang, B.; Zhang, S.; Liu, C.; Shen, X. Highly selective NH3 gas sensor based on polypyrrole/Ti3C2Tx nanocomposites operating at room temperature. J. Mater. Sci. Mater. Electron. 2022, 33, 6168–6177. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Sun, D.; Zhang, Y.; Liu, B.; Hu, Q.; Zhou, A. Synthesis and thermal stability of two-dimensional carbide MXene Ti3C2. Mater. Sci. Eng. B 2015, 191, 33–40. [Google Scholar] [CrossRef]
- Jia, X.; Cheng, C.; Feng, S.; Yu, X.; Xia, L.; Song, H. Hierarchical porous nanorod@ core-shell α-Fe2O3/TiO2 microspheres: Synthesis, characterization, and gas-sensing applications. Appl. Surf. Sci. 2019, 481, 1001–1010. [Google Scholar] [CrossRef]
- Pazniak, H.; Plugin, I.A.; Loes, M.J.; Inerbaev, T.M.; Burmistrov, I.N.; Gorshenkov, M.; Polcak, J.; Varezhnikov, A.S.; Kuznetsov, D.Z.; Bruns, M.; et al. Partially oxidized Ti3C2Tx MXenes for fast and selective detection of organic vapors at part-per-million concentrations. ACS Appl. Nano Mater. 2020, 3, 3195–3204. [Google Scholar] [CrossRef]
- Liang, S.; Li, J.; Wang, F.; Qin, J.; Lai, X.; Jiang, X. Highly sensitive acetone gas sensor based on ultrafine α-Fe2O3 nanoparticles. Sens. Actuators B Chem. 2017, 238, 923–927. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, J.; Cui, X.; Wang, Y.; Gao, Y.; Sun, P.; Liu, F.; Shimanoe, K.; Yamazoe, N.; Lu, G. Enhanced gas sensing properties to acetone vapor achieved by α-Fe2O3 particles ameliorated with reduced graphene oxide sheets. Sens. Actuators B Chem. 2017, 241, 904–914. [Google Scholar] [CrossRef]
- Sun, B.; Ding, Y.; Wang, Q.; Song, P. Rational design of 1D/2D heterostructured ZnSnO3/ZnO/Ti3C2TX MXene nanocomposites for enhanced acetone gas sensing performance. Sens. Actuators B Chem. 2024, 409, 135541. [Google Scholar] [CrossRef]
- He, T.; Liu, W.; Lv, T.; Ma, M.; Liu, Z.; Vasiliev, A.; Li, X. MXene/SnO2 heterojunction based chemical gas sensors. Sens. Actuators B Chem. 2021, 329, 129275. [Google Scholar] [CrossRef]
- Kim, S.J.; Koh, H.J.; Ren, C.E.; Kwon, O.; Maleski, K.; Cho, S.Y.; Anasori, B.; Kim, C.K.; Choi, Y.K.; Kim, J.; et al. Metallic Ti3C2Tx MXene gas sensors with ultrahigh signal-to-noise ratio. ACS Nano 2018, 12, 986–993. [Google Scholar] [CrossRef]
- Jia, Q.; Ji, H.; Zhang, Y.; Chen, Y.; Sun, X.; Jin, Z. Rapid and selective detection of acetone using hierarchical ZnO gas sensor for hazardous odor markers application. J. Hazard. Mater. 2014, 276, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, H.; Tong, X.; Shen, W.; Chen, X.; Bloch, J.F. A low operating temperature and high performance sensor for H2S detection based on Ti3C2Tx/TiO2 heterojunction nanoparticles composite. J. Mater. Sci. Mater. Electron. 2019, 30, 12695–12709. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, F.; Hermawan, A.; Asakura, Y.; Hasegawa, T.; Kumagai, H.; Kato, H.; Kakihana, M.; Zhu, J.; Yin, S. SnO-SnO2 modified two-dimensional MXene Ti3C2Tx for acetone gas sensor working at room temperature. J. Mater. Sci. Technol. 2021, 73, 128–138. [Google Scholar] [CrossRef]



| Concentration | 5 ppm | 15 ppm | 25 ppm | 35 ppm | 50 ppm | 100 ppm |
|---|---|---|---|---|---|---|
| Response time (s) | 17 | 11 | 10 | 9 | 10 | 9 |
| Recovery time (s) | 9 | 9 | 10 | 10 | 12 | 9 |
| Sensing Material | Acetone (ppm) | T Sens (°C) | Response | Res/Rec (s/s) | Ref. |
|---|---|---|---|---|---|
| α-Fe2O3 | 100 | 340 °C | 9.1 | / | [36] |
| rGO/α-Fe2O3 | 100 | 225 °C | 13.9 | / | [37] |
| ZnSnO3/ZnO/Ti3C2TX | 100 | 120 | 15.68 | 5/12 | [38] |
| MXene/SnO2 heterojunctions | 50 | 23.5 | 0.8% | / | [39] |
| Partially oxidized Ti3C2Tx | 2 | 350 | 180% | / | [35] |
| Metallic Ti3C2Tx | 100 | RT | 0.9~0.1% | / | [40] |
| α-Fe2O3/TiO2@Ti3C2Tx nanocomposites | 100 | 220 | 34.66 | 10/7 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Lv, Z.; Chen, Z.; Zhou, B.; Shao, Z. α-Fe2O3/TiO2/Ti3C2Tx Nanocomposites for Enhanced Acetone Gas Sensors. Sensors 2024, 24, 2604. https://doi.org/10.3390/s24082604
Zhao Z, Lv Z, Chen Z, Zhou B, Shao Z. α-Fe2O3/TiO2/Ti3C2Tx Nanocomposites for Enhanced Acetone Gas Sensors. Sensors. 2024; 24(8):2604. https://doi.org/10.3390/s24082604
Chicago/Turabian StyleZhao, Zhihua, Zhenli Lv, Zhuo Chen, Baocang Zhou, and Zhigang Shao. 2024. "α-Fe2O3/TiO2/Ti3C2Tx Nanocomposites for Enhanced Acetone Gas Sensors" Sensors 24, no. 8: 2604. https://doi.org/10.3390/s24082604
APA StyleZhao, Z., Lv, Z., Chen, Z., Zhou, B., & Shao, Z. (2024). α-Fe2O3/TiO2/Ti3C2Tx Nanocomposites for Enhanced Acetone Gas Sensors. Sensors, 24(8), 2604. https://doi.org/10.3390/s24082604






