1. Introduction
In 2019, cardiovascular diseases (CVDs) accounted for 32% of global deaths, totalling 17.9 million lives lost [
1]. Atherosclerosis, hypertension, myocardial infarction, and stroke are among the CVDs that become more prevalent with ageing, profoundly impacting the heart and vascular system [
2,
3,
4,
5]. Primarily, vascular ageing contributes to CVD, marked by alterations in the mechanical and structural features of the vascular wall. These changes lead to a decline in arterial elasticity and reduced arterial compliance [
6,
7,
8]. The ageing process prompts the formation of collagen bridges, preventing elongation by replacing the elastin fibres, giving the vessel walls a less elastic structure, and resulting in arterial stiffness [
9,
10,
11]. Therefore, the measurement and assessment of arterial stiffness becomes imperative in diagnosing vascular ageing.
Current non-invasive assessment techniques, such as pulse transit time (PTT), vascular ultrasound, and magnetic resonance imaging (MRI), are confined to clinical settings due to the expertise required for the imaging techniques or the need for multiple measurement points, as in the case of PTT [
12,
13]. More precise methods, such as angiography and computerised tomography angiography (CTA), require the injection of contrast agents [
14]. While these procedures are accessible within clinical environments, referrals by a General Practitioner (GP) may introduce delays in cardiovascular health assessment. Therefore, the demand for more innovative technologies capable of the accurate and non-invasive assessment of vascular ageing is clear.
In vitro studies simulating vascular ageing in a controlled environment offer a promising path for developing new non-invasive technologies and signal processing algorithms. Previously, it has been discussed that PPG sensor technology has the potential to provide information that is directly related to vascular ageing [
15]. Previously, researchers have created artificial vessels through techniques such as 3D bioprinting [
16,
17,
18], microfluidics based on laminar flow [
19,
20], and coaxial scale-up printing [
21]. Although these approaches have shown encouraging results, they have limitations. For example, they often rely heavily on specific types of inks for printing, and the technology involved in laminar flow-based microfluidics can be complex [
22].
This paper describes the fabrication, mechanical testing, and PPG feature extraction of custom silicone vessels and tissue phantoms, utilising silicone additives to simulate the human vascular system in an in vitro setup. This builds upon previous methods outlined by The Research Centre for Biomedical Engineering, City, University of London. Previously, polydimethylsiloxane (PDMS) (Sylgard 184) (Dow Silicones, Barry, Wales, UK) was explored to create the initial vessels [
23]. While this was successful, the formulations in PDMS silicone cannot be adjusted for customisable vessel elasticity. In this study, vessel fabrication and mechanical testing are performed using PlatSil Gel-10 (Polytek Development Corp., Easton, PA, USA) silicone, which shows more versatility in modifying vessel properties. This paper investigates the effectiveness of the PlatSil elastomer in vessel stiffness customisation to determine the precision of formulation adjustments and the range of elasticities possible, which is greater than previously reported [
24]. Furthermore, this silicone is composed of a scatterer, which diffuses sensor light more similarly to human tissue. Furthermore, compared with the work published by Ferizoli et al. [
25], this study explores mechanical tests on the fabricated vessels, including hardness, thickness, and elasticity measurements. The vascular properties for the full range of elastomer mixtures possible using the PlatSil silicone are presented. The fabricated custom vessels were embedded within silicone to mimic the surrounding tissue, creating vessel–tissue phantoms. The resulting phantoms were integrated into an in vitro model that emulated a cardiovascular system of the lower body, comprising a pulsatile pump and silicone tubing, for the measurement of PPG analysis and statistical analysis [
26]. The setup was used to pump fluid around the system and observe differences in PPG signals corresponding to varying levels of arterial stiffness. This study aims to utilise the vessels produced and the in vitro setup constructed to investigate the utility of PPG sensors in capturing differences in arterial stiffness, as well as determine the precision and maximum range of elasticities attainable through silicone dip-coating.
4. Discussion
The research presented here puts forward an innovative way to investigate the effect of arterial stiffness on PPGs utilising novel vascular tissue phantoms. The process outlined in this paper can be adapted to introduce pathologies by modifying elastomer properties to mimic both healthy and diseased vessels. The results demonstrate that the vessel wall thickness can be regulated using different retarder concentrations. Additionally, the softness and hardness of the vessels and surrounding tissue can be adjusted by incorporating a deadener or hardener. Preliminary PPG signal analysis confirmed that variations in vessel stiffness within phantoms lead to significant amplitude changes.
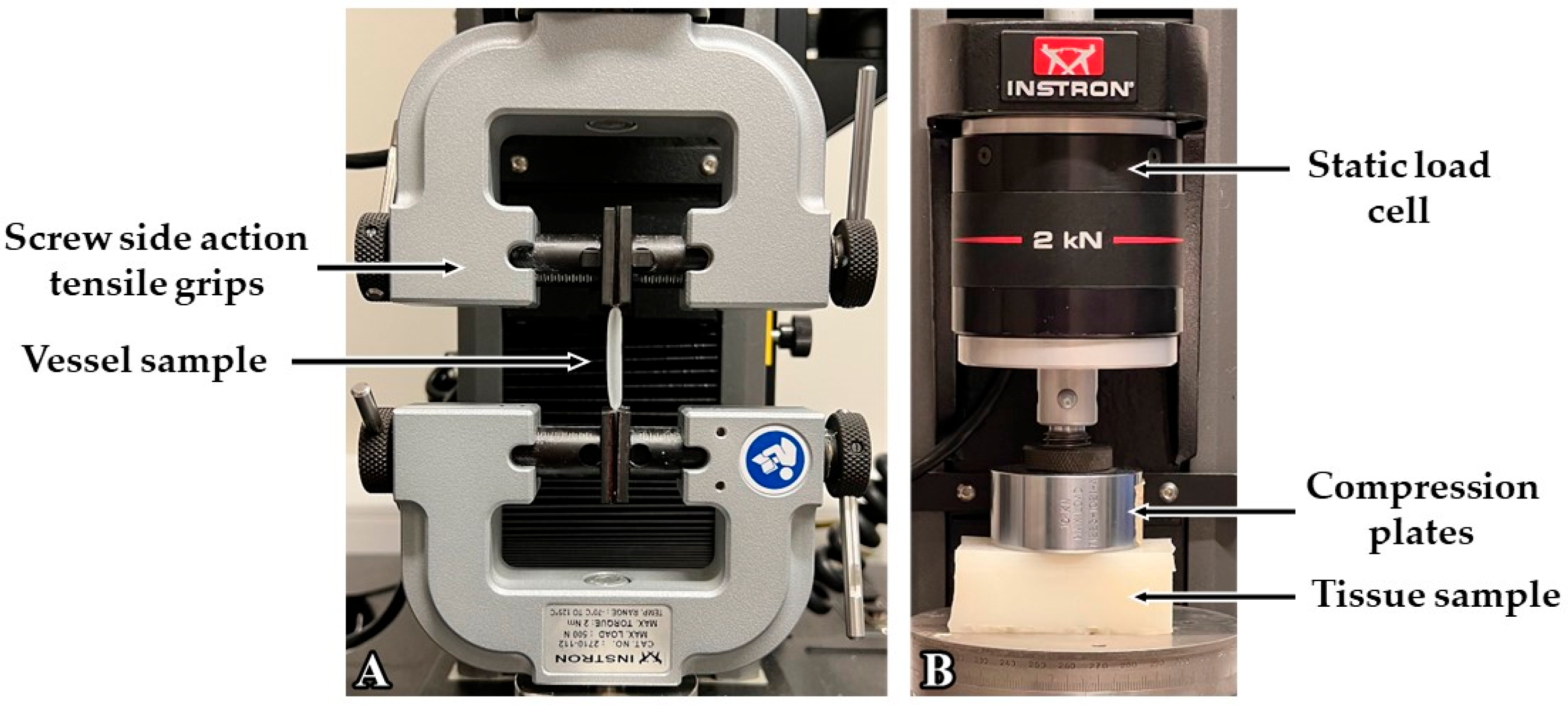
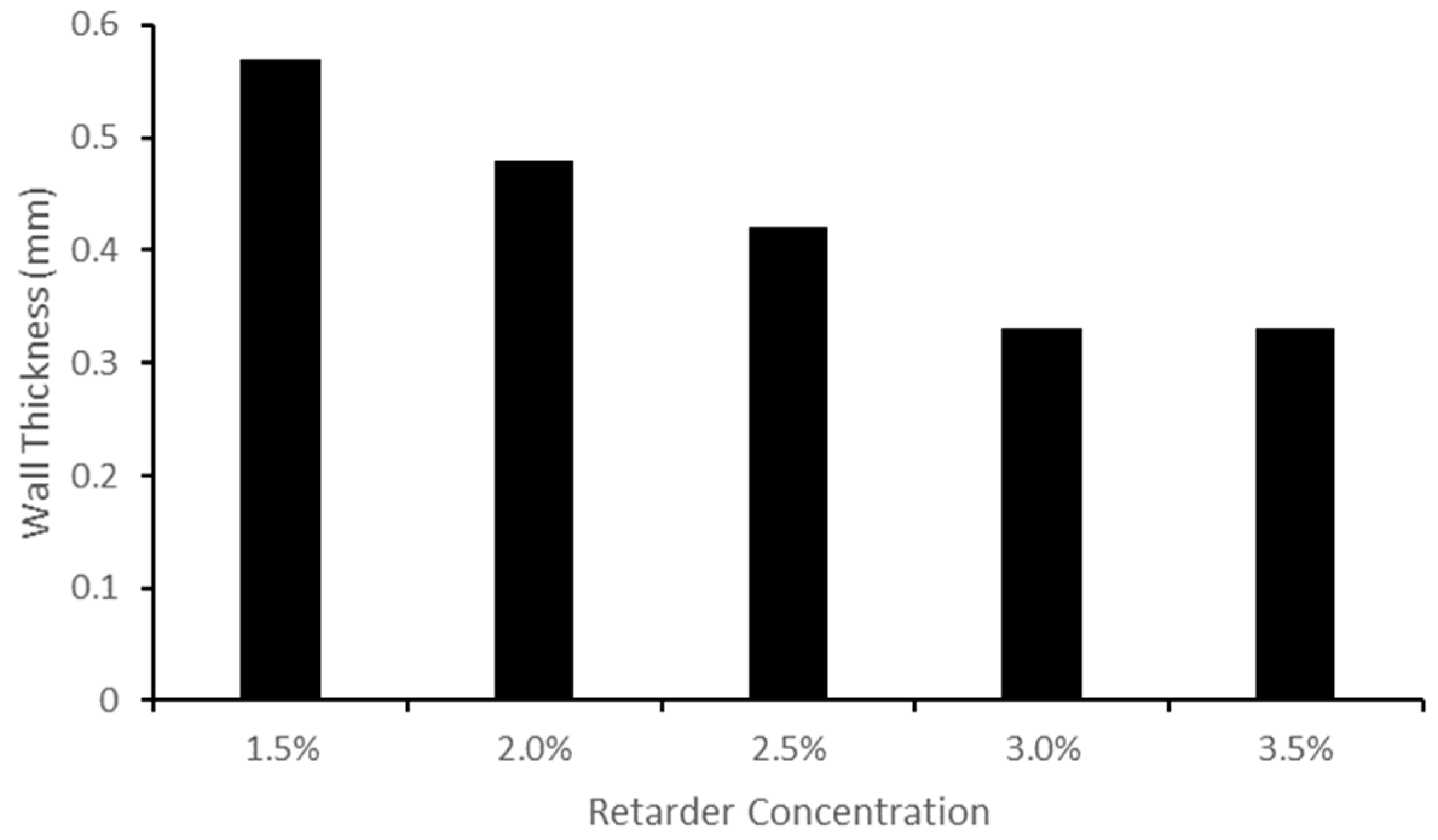
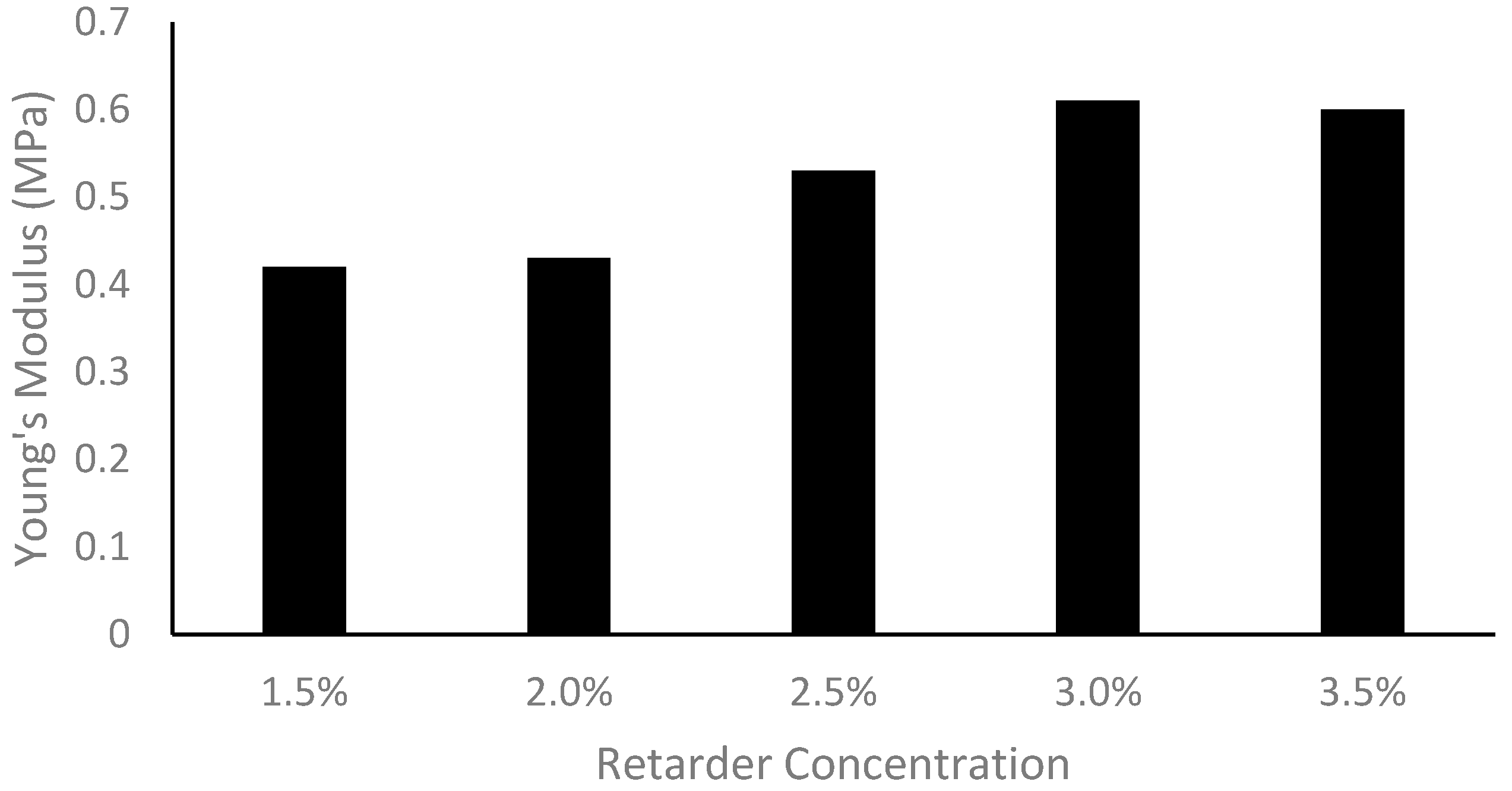
Increasing the retarder concentration reduced the thickness of the custom vessels. This change in thickness is linked to the cure time, which is determined by the retarder level. When the retarder concentration was increased, the silicone coating had more time to descend, resulting in a thinner dip-coated vessel before curing. It should be noted that the retarder level did have an impact on vessel elasticity, as explained by the change in thickness. It was found that the Young’s modulus was thickness-dependent; as the thickness decreased, the Young’s modulus increased [
37].
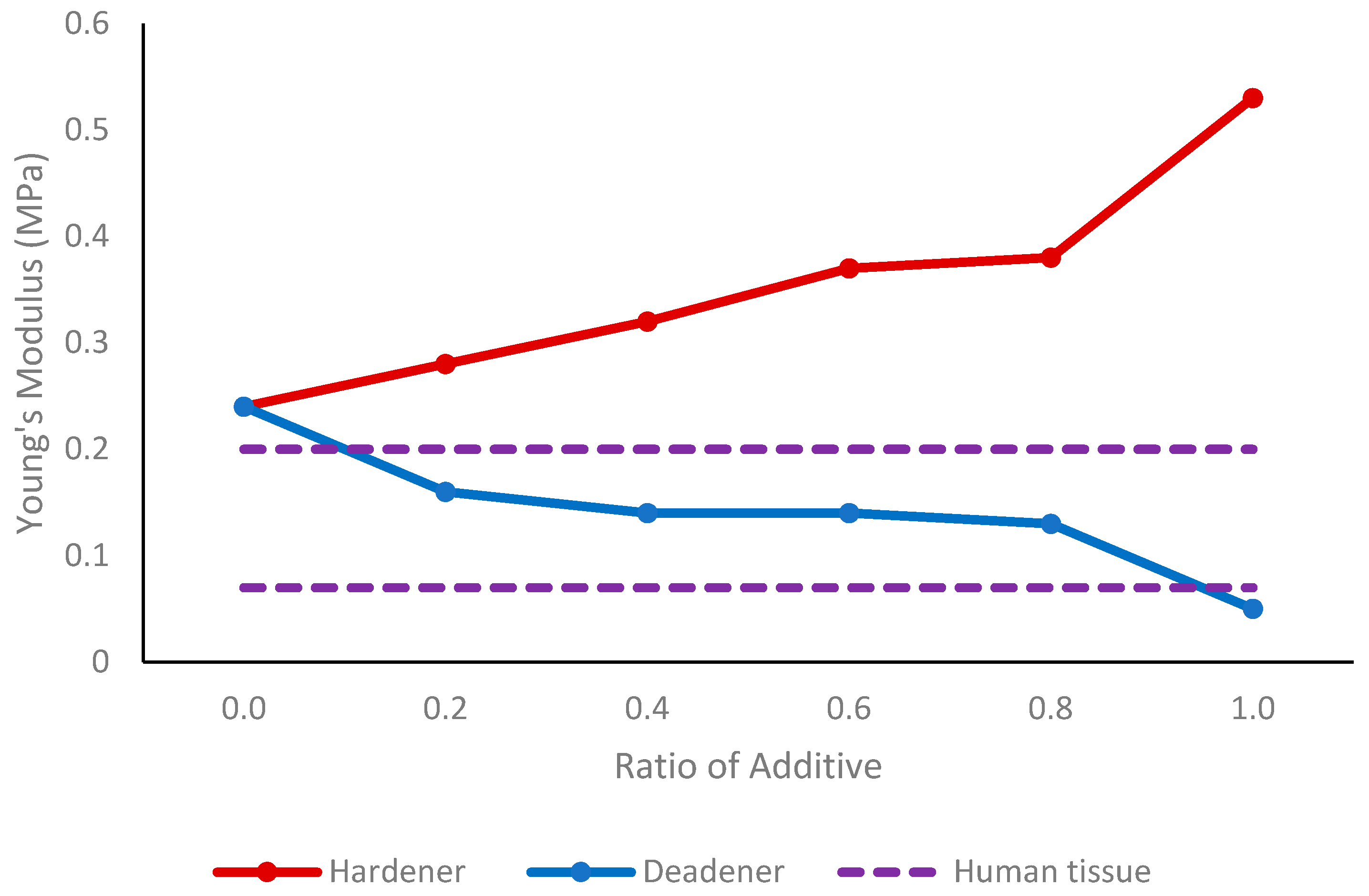
Deadener was used to produce softer and more elastic vessels, simulating the properties of healthy human blood vessels. When the concentration increased to a maximum of 0.6 parts deadener, Young’s modulus decreased from 0.52 MPa to 0.20 MPa, indicating an increase in elasticity. As expected, the wall thickness remained between 0.53 mm and 0.58 mm, showing that the deadener primarily impacted the material’s stiffness. When creating stiffer materials, hardener was utilised to decrease elasticity, mimicking unhealthy vessels. The Young’s modulus obtained using hardener ranged from 0.52 MPa to 1.22 MPa with one-part hardener, representing reduced elasticity. Again, the wall thickness did not alter significantly with the hardener, remaining between 0.50 mm and 0.54 mm.
In the silicone tissue, the deadener was found to have the same effect—making the silicone softer, as indicated by a decrease in shore hardness. Adding hardener had the opposite effect, causing the tissue to become stiffer, as shown by the increase in shore hardness. Therefore, the Young’s modulus and tissue hardness are proportional, as also found by Sun et al. [
38]—as the hardness increased, the Young’s modulus also increased. The dorsal thigh exhibits a shore hardness ranging from shore OO 25 to shore OO 31 [
36]. To replicate this, equal amounts of Part A, Part B, and deadener can be incorporated. The tissue in a finger has an average elasticity of between 0.07 MPa and 0.2 MPa [
35]. By using a ratio of 0.2 to 0.8 deadener to equal amounts of Part A and Part B, it is feasible to create tissue of a similar elasticity to that of the human finger.
Peripheral arterial disease (PAD) is a restriction in the arteries, which usually occurs in the lower extremities [
39]. As vessels age, their longitudinal and circumferential elasticity gradually diminishes [
40]. The results of this study were compared with those of the common femoral artery. According to Soneye et al. [
41], the wall thickness of a healthy left common femoral artery is 0.55 ± 0.05 mm. The common femoral artery’s vessel diameter ranged from 3.9 mm to 8.9 mm, with an average value of 6.6 mm [
42]. A healthy femoral artery has been found to have a Young’s modulus of 0.80 MPa, 0.79 MPa, and 0.82 MPa for the intimate, adventitia, and radial strain, respectively [
34]. From the results obtained in this study, the femoral artery can be mimicked with a 1A:1B:0.6H mixture with 1.5% retarder. If commercial silicone tubing with an OD of 2.6 mm is utilised as a form, a wall thickness of 0.51 mm can be attained. The Young’s modulus of 0.82 MPa is within the range for radial strain, and the diameter would be 3.96 mm, which is also within the normal range. A clogged femoral artery, known as an atherosclerotic femoral artery, has a Young’s modulus of 3.6 MPa and 2.11 MPa in the adventitia and radial strain, respectively [
34]. As such, to mimic an atherosclerotic femoral artery, a higher ratio of hardener must be used than 1A:1B:1H to obtain a less elastic artery with a higher Young’s modulus. In this case, other materials, such as PlatSil Gel-25 (Polytek Development Corp., Easton, PA, USA), should be investigated to analyse the feasibility of creating stiffer vessels.
PPG signals were recorded from three phantoms composed of vessels with varying elasticities to determine if the elasticity influenced the PPG waveform, particularly the amplitude. The softer vessel phantom was created using a ratio of 0.2 deadener (10% of total weight) and the stiffer vessel phantom was made with hardener at a ratio of 0.2 (10% of total weight). The third vessel phantom did not contain additives. Red and infrared PPG signals were recorded and analysed on a cycle-by-cycle basis, which showed that as vessel stiffness increased, PPG amplitude decreased. Kruskal–Wallis analysis followed by a Dunn test found this change to be statistically significant (p < 0.001). This confirms the hypothesis that PPG signals can indicate changes in arterial stiffness, particularly through amplitude changes. This trend is as expected considering the physiology of blood vessels and PPG. Stiffer vessels are less elastic and therefore are less able to expand when the blood pulse wave arrives at the PPG sensor. As PPG is a volumetric technique, and the reduced expansion results in a lower change in the volume of blood at the site, the amplitude of the signal is lower.
Accompanying pressure signals confirmed that the morphology of the PPG wave was induced by changes in pressure. As with the PPG signals, the pressure signals obtained conformed to the expected physiological morphology. Interestingly, the varying vessel stiffness revealed a difference in the PPG morphology such as amplitude, while the pressure waveform remained constant across phantoms. This may be because the pressure sensors were placed after at the end of the vessel phantom, while the PPG sensors were positioned in the middle, possibly providing more information about blood flow changes within the vessel. While pressure waveforms serve as a secondary measure to validate the results, exploring additional methods, such as Doppler ultrasound, could provide further confirmation of the changes in volume.
To extend the findings outlined in this study to clinical measurements, it is crucial to assess potential influences on the PPG waveform. One such factor is sensor contact. While a secure and strong contact has proven to yield high-quality signals, peripheral vasoconstriction may result in signals of lower quality [
15,
43]. In the in vitro setup, the phantoms and sensors were housed within a custom casing that prevented movement and ensured consistent contact force during readings. Another factor to consider is hydrostatic effects. The positioning of a limb in relation to the heart has an impact on arterial flow and venous return, influenced by the hydrostatic effect. The research indicated that lowering the hand below heart level results in decreased amplitudes of both alternating current (AC) and direct current (DC) components of PPG, which has been attributed to venous distension. In contrast, raising the arm above heart level leads to an increase in AC and DC PPG amplitudes [
44]. Therefore, it is concluded that the position of the hand is crucial in in vivo studies due to hydrostatic effects. The in vitro setup was aligned horizontally and phantom placement was kept constant. As such, minimal hydrostatic impacts were observed.
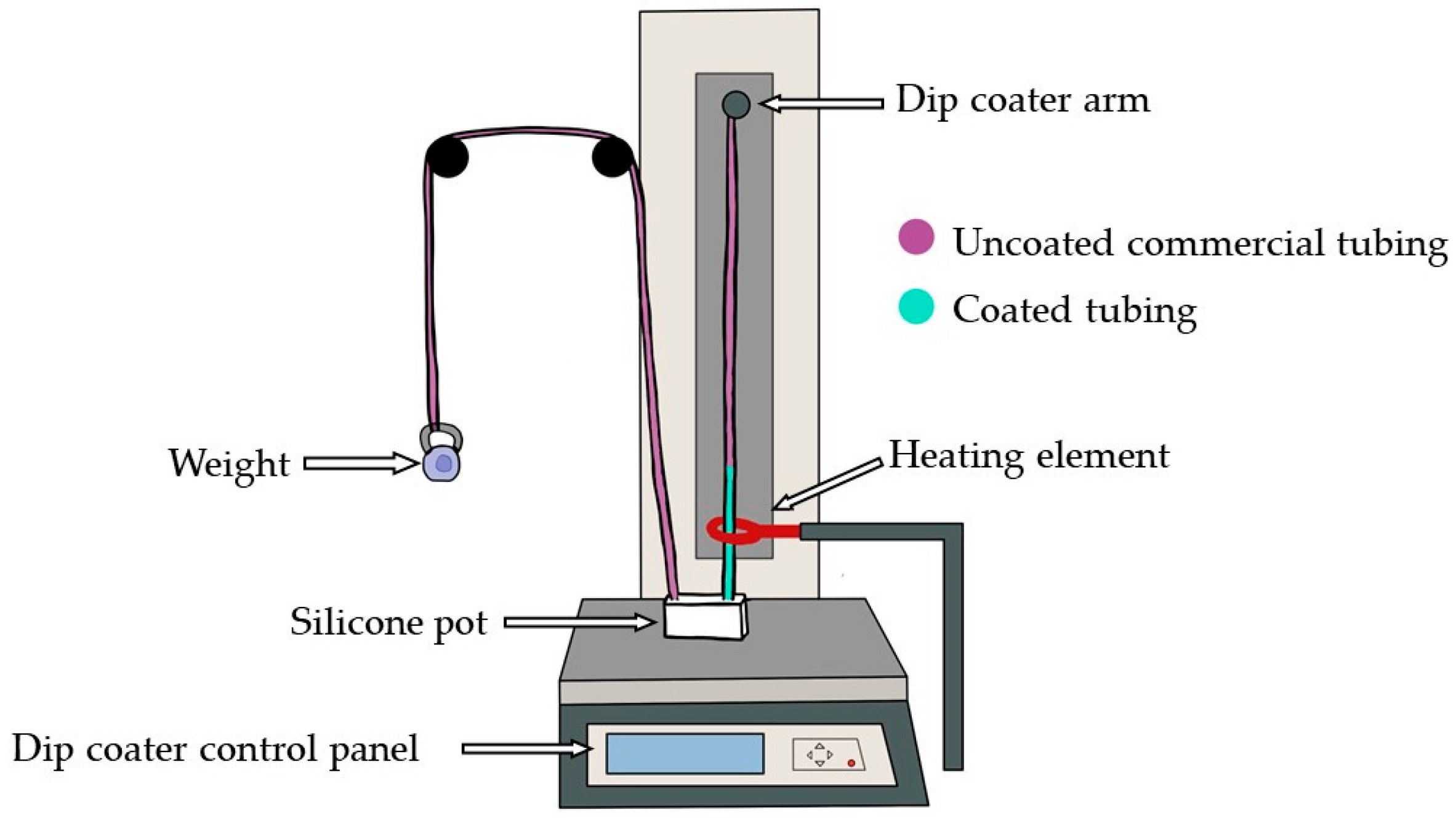
When fabricating custom vessels, some limitations were identified. Due to the delicate nature of the vessels, a ratio of deadener up to 1A:1B:0.6D is possible. It was observed that beyond this ratio, the custom vessel would adhere to the internal commercial silicone tubing being used as the form, making removal challenging. Furthermore, a minimum retarder of 1.5% should be used when the dip coater’s speed is set to 40 mm/min to allow for sufficient time to pull the vessel to the maximum height. Future work can explore the impact of varying the dip coater’s speed. One hypothesis suggests that increasing the speed may reduce the requirement for as much retarder, as it allows the uncured silicone to reach the maximum height quicker, thus shortening the need for a long curing time. Moreover, if the dip coater’s speed is increased while using the same amount of retarder, it is anticipated that the custom vessel will reach its maximum height more rapidly, potentially providing more time for the uncured silicone to descend due to gravity, resulting in thinner custom vessels. Furthermore, additional research should be carried out with a higher ratio of hardener to mimic the stiffness of an atherosclerotic femoral artery. The current experimental procedure was carried out using a deionised water-based fluid, representative of the absorption spectra of the blood [
45]. In vitro investigations utilising non-Newtonian fluids would be beneficial to simulate the behaviour of human blood as it is composed of Casson fluid flow [
46]. Signal analysis can be expanded upon by exploring additional parameters during PPG feature extraction. Features such as half peak width, area under the curve, upslope, and downslope are beneficial, as these characteristics may be indicative of arterial stiffness, providing a deeper understanding of the dynamics and mechanical properties of the custom vessels.
5. Conclusions
With recent developments in PPG, assessment of vascular ageing and CVD would benefit from an in vitro setup that can simulate both healthy and diseased vascular systems. This paper describes the construction of customisable novel silicone vessels, produced through a dip-coating procedure, to be used in an in vitro vascular system. The desired vessel thickness and stiffness attributes can be obtained by adjusting the elastomer formulations. By deliberately altering the formulations to influence the vessel properties, pathologies can be introduced into the vessels and compared to healthy vessels through PPG analysis. This can be employed in conjunction with in vivo studies.
This study aimed to create custom vessels with varying levels of stiffness for use in an in vitro model. In this model, PPG signals were recorded and analysed to detect morphological changes induced by increased vessel stiffness. The waveform of the resulting PPG signals varied depending on the stiffness of the vessel within the phantom. Statistically significant changes in amplitude were detected, indicating the possibility of measuring arterial stiffness with PPG technology.
This paper provides a procedure for making custom vessels and illustrates the changes in the morphology of the PPG signal, particularly amplitude, induced by elasticity changes to simulate arterial stiffness. The vessel manufacturing process can be improved by altering the dip-coating stage to enable the production of longer custom vessels. Other materials, such as PlatSil Gel-25, can also be explored to extend the stiffness of the custom vessels. Further analysis of the PPG waveform is necessary, by extracting a range of features from phantoms utilising non-Newtonian fluids, to unravel the link between PPG and arterial stiffness.