Abstract
Modern chemical production processes often emit complex mixtures of gases, including hazardous pollutants such as NO2. Although widely used, gas sensors based on metal oxide semiconductors such as WO3 respond to a wide range of interfering gases other than NO2. Consequently, developing WO3 gas sensors with high NO2 selectivity is challenging. In this study, a simple one-step hydrothermal method was used to prepare WO3 nanorods modified with black phosphorus (BP) flakes as sensitive materials for NO2 sensing, and BP-WO3-based micro-electromechanical system gas sensors were fabricated. The characterization of the as-prepared BP-WO3 composite through X-ray diffraction scanning electron microscopy and X-ray photoelectron spectroscopy confirmed the successful formation of the sandwich-like nanostructures. The result of gas-sensing tests with 2–14 ppm NO2 indicated that the sensor response was 1.25–2.21 with response–recovery times of 36 and 36 s, respectively, at 190 °C. In contrast to pure WO3, which exhibited a response of 1.07–2.2 to 0.3–5 ppm H2S at 160 °C, BP-WO3 showed almost no response to H2S. Thus, compared with pure WO3, BP-WO3 exhibited significantly improved NO2 selectivity. Overall, the BP-WO3 composite with sandwich-like nanostructures is a promising material for developing highly selective NO2 sensors for practical applications.
1. Introduction
With the rapid development of modern industry, various health-threatening atmospheric pollutants have emerged. A representative example is the inorganic compound NO2, a toxic gas with an irritating odor [1]. NO2 inhalation can lead to interstitial lung lesions, respiratory infections, and lung fibrosis [2,3]. Anthropogenic NO2 mainly originates from high-temperature combustion processes and is released through fuel combustion, motor vehicle exhaust, and boiler exhaust emissions [4]. In practice, NO2 is often accompanied by interfering gases such as H2S, CO, and SO2. Therefore, sensors with high selectivity and response must be developed to meet NO2 detection requirements.
In recent years, resistive gas sensors based on metal oxide semiconductors (MOS) have been widely used for NO2 detection because of their low cost, environmental friendliness, and excellent performance. Hydrothermal methods for oxide synthesis are being widely utilized today. Jamnani et al. synthesized Sm2O3 nanorods through a straightforward hydrothermal process, employing cetyltrimethylammonium bromide (CTAB) as an organic surfactant for detecting volatile organic compounds; they achieved a notable response of 3.41–1 ppm for acetone [5]. Furthermore, flower-like MoS2/WS2 composites were prepared via a simple one-pot hydrothermal method, enhancing the detection of NO2 at room temperature [6]. MOS materials such as WO3 [7], SnO2 [8], In2O3 [9], ZnO [10], and CuO [11] are promising for meeting the low-limit, high-response requirements of NO2 detection. Additionally, Ag2Te, an n-type semiconductor, exhibited commendable NO2 sensing performance. The resistance of the Ag2Te sensor remained stable in air and at 1 ppm NO2 over a humidity range of 10–90%, demonstrating its excellent humidity-resistant properties [12].
WO3 is a typical n-type MOS with an adjustable bandgap of 2.5–2.8 eV [13]. Owing to its crystal structure and surface properties, WO3 can adopt various microscopic morphologies including nanowires [14], nanospheres [15], nanosheets [16], and urchin-like nanostructures [17]. The microscopic morphology of WO3 can be adjusted to strengthen the interactions with gases and increase the possibility of gas adsorption. However, similar to sensors based on other metal oxides, WO3 nanomaterial gas sensors exhibit poor selectivity and cannot accurately detect NO2 in complex environments in which multiple gases are mixed [18].
MOSs have been doped with different catalysts to improve sensor selectivity and increase the reaction rate between the target gas and sensing layer. Based on electronic sensitization and chemical sensitization mechanisms [19,20], doping with an appropriate amount of a noble metal can improve the gas response of MOS materials. For example, compared with pure ZnO, Pd-modified ZnO nanowires improved response and significantly higher NO2 selectivity [21]. As an alternative approach, gas sensors have been prepared using composites of MOSs and reduced graphene oxide (rGO), which has a large surface area and exhibits high electron mobility and high conductivity at low temperatures [22]. Notably, SnO2 nanofibers sensitized with rGO nanosheets achieved high response and selectivity for acetone and H2S [23]. In addition, recently, phosphorene has garnered research interest owing to its honeycomb structure, high carrier mobility, and tunable bandgap, which ranges from 0.3 eV in bulk to approximately 1.9 eV in monolayers [24]. Black phosphorus (BP), a novel single-element two-dimensional layered material, has been extensively utilized in gas sensing [25]. Han fabricated a BP gas sensor featuring high-quality, few-layered BP micro-ribbons. Energy-dispersive spectroscopy mapping revealed that BP underwent only slight oxidation, achieving low-limit detection and high NO2 selectivity under both N2 and air conditions [26]. Zhou fabricated a two-dimensional SnO2 nanosheet micro-electromechanical system (MEMS) gas sensor decorated with black phosphorus (BP) to detect H2S [27]. The BP-SnO2 sensor had a lower optimal operating temperature and faster response–recovery times and exhibited higher selectivity than the pure SnO2 sensor. When BP, which is a p-type semiconductor, is added to WO3, which is an n-type semiconductor, more p-n heterojunctions can be formed. Moreover, the excellent conductivity of BP can increase the carrier mobility of WO3. Therefore, BP-WO3 composites are of interest for gas-sensing applications.
We fabricated a highly selective BP-WO3 gas sensor for NO2 detection in this study. BP crystals were prepared via the chemical vapor-phase transport method, and BP-WO3 sandwich-like nanostructures were formed by a simple one-step hydrothermal method. The successful preparation of the BP-WO3 composite was confirmed through its characterization via scanning electron microscopy (SEM), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS). Subsequently, a gas-sensing test platform was constructed and the selectivity, response, operating temperature, and concentration range of the BP-WO3 sensor were demonstrated to be superior to those of pure WO3. This improved sensing performance could be attributed to the enhanced electron-trapping ability of BP-WO3.
2. Materials and Methods
2.1. Preparation of Sensing Materials
BP crystals were prepared via the chemical vapor-phase transport method. First, 0.5 g of red phosphorus, 0.07 g of Sn powder, and 0.03 g of I2 were placed in a quartz glass tube with an outer diameter of 18 mm. The quartz tube was then pumped to a vacuum and sealed by fusing with a cylindrical quartz block. The end of the quartz tube containing the raw materials (hot end) was placed in the center of the high-temperature zone of a tube furnace. The heating stage was heated to 600 °C at a rate of 20 °C/h and held for 2 h, cooled to 500 °C at a rate of 10 °C/h and held for 2 h, and finally cooled to 25 °C at a rate of 15 °C/h. The BP crystals formed at the cold end of the quartz tube. The BP crystals were then ground into a fine powder and a 10 mg/mL aqueous solution was prepared.
WO3 was prepared via a hydrothermal method. First, 2.05 g of Na2WO4∙2H2O, 0.25 g of Na2SO4, and 0.3 g of K2SO4 were added to 40 mL of deionized water. After stirring for 20 min, the pH was adjusted to 1.6 with 2 M HCl. The precursor solution was transferred to a 100 mL Teflon-lined autoclave and heated at 180 °C for 24 h. After cooling to 25 °C, the precipitate was centrifuged at 4000 rpm for 15 min and washed with water and anhydrous ethanol (6 times). Finally, the WO3 sample was obtained by vacuum drying at 60 °C for 8 h. The WO3 synthesis process is outlined in Figure 1. The BP-WO3 composite was synthesized using the same method, except that 1 mL of the aqueous BP solution was added after adjusting the pH.
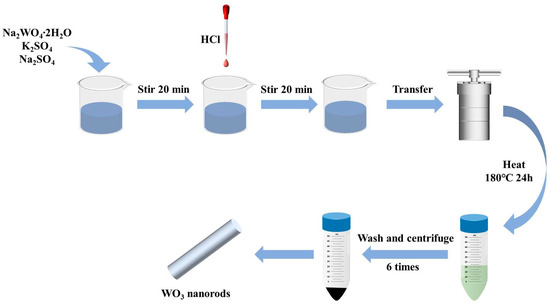
Figure 1.
Schematic of the preparation of WO3 nanorods.
2.2. Characterization of Sensing Materials
The crystal structures of WO3 and BP-WO3 were analyzed using an X-ray diffractometer (SmartLab 3 kW, Rigaku Corporation, Tokyo, Japan) using Cu Kα radiation in the scanning range of 20°–70°. The valence states of the elements were analyzed using an X-ray photoelectron spectrometer (ESCALAB 250Xi, Thermo Fisher Scientific, Waltham, MA, USA). The morphologies of the samples were observed through field-emission SEM (ZEISS Gemini SEM 300, Carl Zeiss AG, Oberkochen, Germany) at acceleration voltages of 3–15 kV.
2.3. Test Platform for Gas Sensing
Sensors were fabricated by coating the sensing material on a MEMS substrate, consisting of an interdigitated electrode, an insulating layer, a heating electrode, a supporting layer, and a silicon substrate (Figure 2a,b). Specifically, the sensing material was ground in an agate mortar to 200 mesh and sieved. A slurry was prepared by uniformly mixing this sample with terpineol. The slurry was coated on the sensing layer of the MEMS substrate, which was then vacuum dried at 65 °C for 5 h and aged at the operating temperature for 1 week. Figure 2c,d show the physical images of the sensor. The test platform comprised a 7.5 L closed air chamber, fan, DC power supply, data-acquisition unit, and PC (Figure 2e). A syringe was used to inject a specific volume of gas into the gas chamber. The fan diffused the gas evenly and quickly into the gas chamber, and the DC power supply provided the working voltage of the sensor. Gas adsorption onto the gas-sensitive material caused a change in the sensor resistance, which was recorded by the data-acquisition unit and then uploaded to the PC.
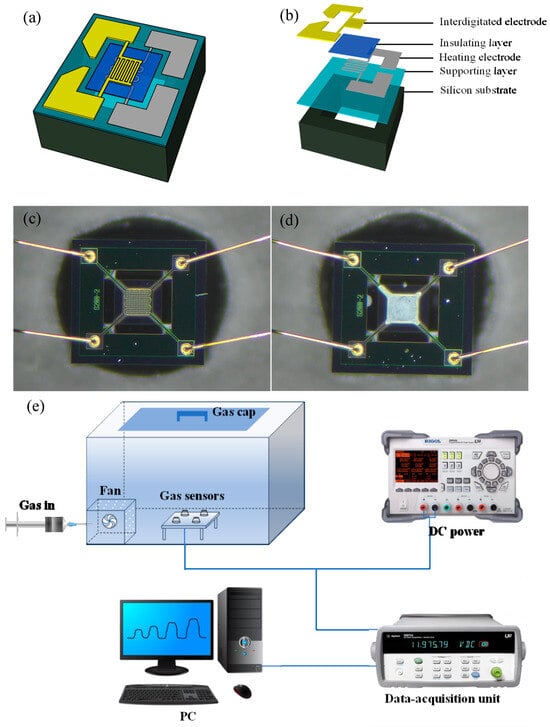
Figure 2.
(a,b) Schematics of micro-electromechanical system (MEMS) structure; images of the MEMS substrate (c) before and (d) after coating with the sensing material; (e) test platform for gas sensing.
3. Results and Discussion
3.1. Material Characterization
Figure 3 shows the XRD patterns of the pure WO3 and BP-WO3 samples at 25 °C. Both materials are relatively well crystallized and match well with the standard JCPDS card for hexagonal WO3 (PDF#97-008-0634), with lattice parameters of a = b = 7.3244 Å and c = 7.6628 Å and diffraction peaks at 13.95°, 23.20°, 24.28°, 28.11°, 33.83°, 36.76°, and 49.75° corresponding to the (100), (002), (110), (200), (112), (202), and (220) crystal planes, respectively. However, because of the extremely low BP content of BP-WO3, the XRD pattern of this sample does not contain any diffraction peaks corresponding to phosphorus. Therefore, the samples were further characterized using other methods.
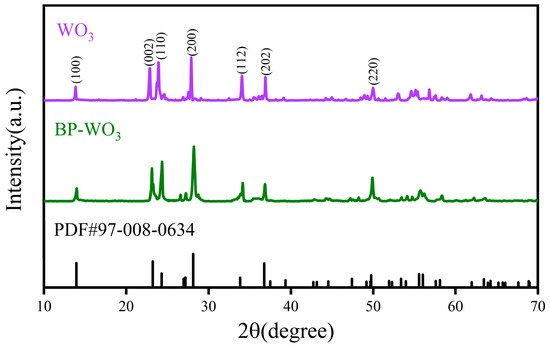
Figure 3.
X-ray diffraction (XRD) patterns of pure WO3 and BP-WO3 at 25 °C.
Figure 4a–c show the SEM images of pure WO3. The microscopic morphology of pure WO3 consists of rod-like structures with diameters of 50 nm and lengths ranging from 100 nm to 1 μm, resulting in a rectangular cross-section. Most nanorods are stacked in parallel rather than dispersed, indicating the presence of strong interaction forces between them. Remarkably, the rod-like structures consist of many fine nanorods (5–10 nm in diameter) assembled into bundles. Energy-dispersive X-ray spectroscopy (EDS) results extracted from the region in Figure 4b show that W and O were uniformly distributed on the nanorods (Figure 4d,e). Based on the characteristic W and O peaks (Figure 4f), the mass fractions of W and O were calculated to be 80.95% and 19.05%, respectively.
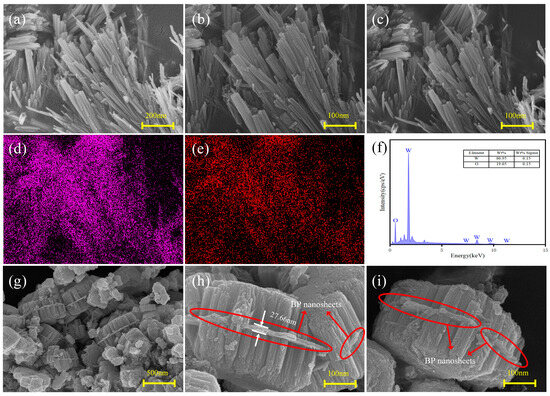
Figure 4.
(a–c) SEM images of pure WO3; EDS elemental mapping profiles of (d) W and (e) O in pure WO3; (f) EDS spectrum of pure WO3; (g–i) SEM images of BP-WO3.
Figure 4g–i show the SEM images of BP-WO3. The BP-WO3 composite was found to possess a novel sandwich-like nanostructure assembled from WO3 nanorods and multilayer BP nanosheets. As shown in Figure 4g, the BP nanosheets provide attachment sites for nanowire growth, and both sides of each BP nanosheet are uniformly and densely loaded with nanorods. Notably, the surrounding free WO3 nanorods and BP nanosheets do not bind together. According to a previous study, monolayer BP nanosheets have a thickness of 3–5 nm after ultrasonic liquid-phase stripping. In the BP-WO3 composite, the nanosheet in the middle position has a thickness of 27.66 nm (Figure 4h), which suggests that this structure consists of 5–9 layers of monolayer BP nanosheets. The magnified SEM images in Figure 4h,j reveal nearly perpendicular nanorod growth on both sides of the BP nanosheets. Moreover, these nanorods are of the same length and cover the nanosheets almost completely. This structure explains why no P was detected through XRD or EDS.
XPS was performed to further investigate the chemical binding states of the samples. Figure 5a shows the XPS profiles of pure WO3 and BP-WO3. In the WO3 sample, the atomic ratio of W to O is 24.02%:75.98%, which corresponds well with the expected atomic ratio (1:3). However, because the XPS beam can only penetrate the sample to a depth of 3–10 nm, the analysis is limited to the sample surface and P was not detected. Figure 5b and c show the W 4f and O 1s XPS spectra of the BP-WO3 sample. The W 4f spectrum exhibits two major peaks centered at 37.9 and 35.7 eV [28], corresponding to W6+ 4f5/2 and 4f7/2, respectively. The O 1s spectrum exhibits a major peak at 530.5 eV [29], corresponding to the lattice oxygen (O2−) of WO3, and a small peak at 532.1 eV [30], ascribed to the chemisorbed oxygen or weakly bonded oxygen species on the WO3 nanorods.
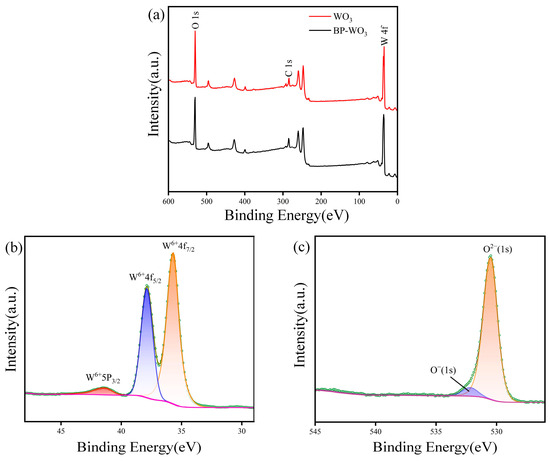
Figure 5.
(a) XPS survey spectra of pure WO3 and BP-WO3; (b) W 4f and (c) O 1s XPS spectra of BP-WO3.
3.2. Gas-Sensing Properties
The operating temperature is a crucial sensor metric for actual measurement. To explore the effect of the operating temperature on the sensor performance, we performed static measurements using the BP-WO3 sensor at 10 ppm NO2. Figure 6a shows the variation in the resistance of the BP-WO3 sensor over time at temperatures between 190 and 270 °C. The initial resistance of the sensor reaches tens of megahertz and decreases with increasing temperature. The resistance increases significantly after the target gas is introduced and slowly returns to the initial level after releasing the gas. The lowest operating temperature point was selected at 190 °C to avoid large measurement errors caused by high sensor resistance. Figure 6b illustrates the response (Ra/Rg, where Ra is the resistance of the sensor in air and Rg is the resistance of the sensor in the target gas) at different temperatures. Increasing the temperature induces a significant decrease in response; however, the response–recovery times of the sensor become faster. The response of the sensor is only 1.14 at 270 °C but increases to 1.73 at 190 °C. The response–recovery times (defined as the time required for a change of the resistance of 90%) of the sensor are 72 and 92 s, respectively, at 190 °C, but 36 and 36 s, respectively, at 270 °C. The improved response–recovery times of the sensor at 270 °C are favorable for commercial applications. However, as visualization of the sensor response is critical during gas sensitivity tests, we performed further tests at 190 °C.
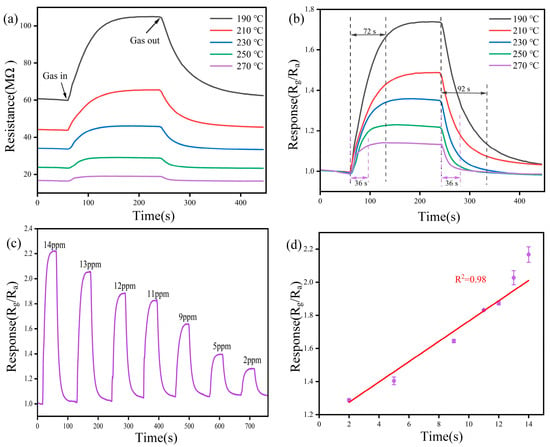
Figure 6.
(a) Resistance and (b) response of the BP-WO3 sensor to 10 ppm NO2 at various operating temperatures; (c) response of the BP-WO3 sensor to various NO2 concentrations at 190 °C; (d) linear fitting of the BP-WO3 sensor response as a function of NO2 concentration.
Figure 6c shows the response of the BP-WO3 sensor to NO2 concentrations in the range of 2–14 ppm. The sensor response decreases significantly as the gas concentration decreases, indicating an excellent resolving power. In addition, the sensor exhibits fast response–recovery times for NO2. Figure 6d shows the relationship between the response of the BP-WO3 sensor and NO2 concentration in the 2–14 ppm range. The results show an excellent linear relationship (R2 = 0.98) with an equation of y = 1.1522 + 0.0613x, implying that the BP-WO3 sensor has good calibration capability. Based on the linear fit results, the theoretical BP-WO3 response to 10 ppb NO2 can be calculated as 1.15.
To verify the NO2 selectivity of the sensor, we performed gas sensitivity tests with the BP-WO3 and pure WO3 sensors using H2S as a representative interfering gas. Figure 7a shows the response (Ra/Rg) of the pure WO3 sensor to 4 ppm H2S at different operating temperatures. The sensor response decreases drastically with increasing temperature, but the response–recovery times become significantly shorter. Although the response is higher at 160 °C than at 240 °C, the response–recovery times decrease significantly (from 60 and 100 s, respectively, at 160 °C, to 13 and 15 s, respectively, at 240 °C), consistent with the trend observed for BP-WO3. The investigation of the response of the pure WO3 sensor to H2S at the concentration of 0.3–5 ppm (Figure 7b) reveals good resolving power in the range of 1–5 ppm. However, the difference in response is negligible at concentrations of 0.3 and 0.5 ppm (1.024 and 1.064, respectively), suggesting that the detection limit has been reached. Figure 7c shows the relationship between the response of the pure WO3 sensor and H2S concentration in the range of 0.3–5 ppm. The results show a good linear relationship (R2 = 0.97) with an equation of y = 0.9617 + 0.2225x, indicating the good calibratable capability of the pure WO3 sensor. Based on the linear fit results, the theoretical pure WO3 response to 10 ppb H2S can be calculated as 0.96. In contrast, the BP-WO3 sensor exhibited no response to various H2S concentrations (Figure 7d), demonstrating that the BP-WO3 sensor can effectively avoid the influence of the interfering gas, H2S, unlike pure WO3, resulting in improved selectivity.
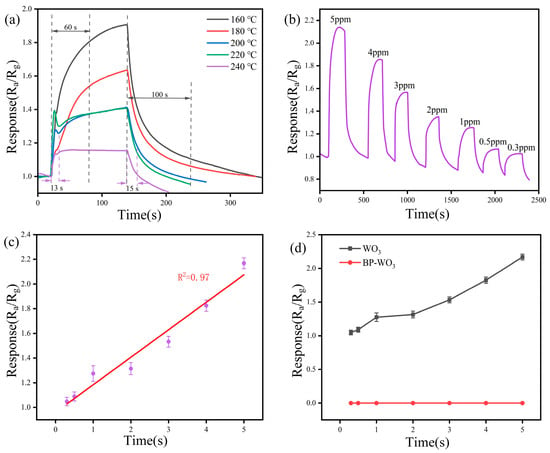
Figure 7.
(a) Response of the WO3 sensor to 4 ppm H2S at various operating temperatures; (b) response of WO3 sensor to various H2S concentrations at 160 °C; (c) linear fitting of the WO3 sensor response as a function of H2S concentration; (d) response of the WO3 and BP-WO3 sensors as a function of H2S concentration.
Figure 8a,b show the response curves of BP-WO3 and pure WO3 over seven cycles at 12 ppm NO2 and 3.5 ppm H2S, respectively. The response values and response–recovery times of the sensor during each cycle are similar, indicating that the BP-WO3 and pure WO3 sensors have excellent repeatability. In addition, we tested the responses of the BP-WO3 and pure WO3 sensors to 1 ppm H2S, NO2, C2H6O, CO2, and CO (Figure 8b). The pure WO3 sensor responded well to both H2S and NO2, whereas the BP-WO3 sensor exhibited almost no response to H2S, confirming that the BP-WO3 sensor exhibits better NO2 selectivity than the pure WO3 sensor. The response values of the BP-WO3 sensor to NO2 and H2S over 15 days are shown in Figure 8c. Over this period, the response of the sensor to NO2 only decreases slightly, with the retention of more than 95% of the initial performance. Moreover, the sensor remains unresponsive to H2S for 15 days, demonstrating the long-term stability of BP-WO3. In addition, the gas-sensing properties of previously reported WO3-based sensors and the as-prepared system are summarized in Table 1. It can be seen that the BP-WO3 sensor demonstrates similar performance to or outperforms other sensors.
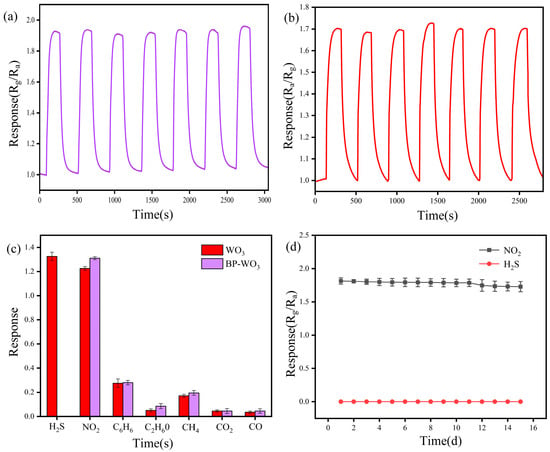
Figure 8.
(a) Repeatability of the BP-WO3 sensor response to 12 ppm NO2; (b) repeatability of the WO3 sensor response to 3.5 ppm H2S; (c) selectivities of the BP-WO3 and WO3 sensors for various gases; (d) response of the BP-WO3 sensor to 12 ppm NO2 and 4 ppm H2S over 15 days.

Table 1.
Gas-sensing properties of various WO3-based NO2 gas sensors.
3.3. Gas-Sensing Mechanism
The gas-sensing mechanism of WO3 is similar to that of other MOSs, where charge transfer to the gas during adsorption leads to a change in resistance. When the sensor is exposed to air, oxygen molecules adsorb on the surface of the WO3 nanorods and capture free electrons from the WO3 conduction band. Thus, the oxygen molecules are converted to the chemisorbed oxygen species ( and ) [37], which form an electron-withdrawing layer near the surface of the WO3 nanorods. Consequently, the carrier concentration of WO3, which is an n-type semiconductor, decreases, leading to an increase in resistance. Upon introducing NO2, NO2 molecules adsorb on the WO3 surface and react with the adsorbed oxygen ions [38]. This process can be described by Equations (1)–(5).
Doping with BP, a p-type semiconductor, causes most carriers to diffuse on the surfaces of BP and WO3, forming numerous p-n heterojunctions. Owing to the difference in the work functions of WO3 and BP in the air (5.15 and 3.9 eV [39,40], respectively), electrons are transferred from WO3 to the BP surface, while holes move in the opposite direction until a unified Fermi level is formed. An atomic structure model of the BP-WO3 heterojunction is shown in Figure 9. When the sensor is exposed to air, the adsorption of oxygen leads to a decrease in the electron density on the surface of WO3 and an increase in the hole density of BP, resulting in the formation of an electron depletion layer on the WO3 side and a hole accumulation layer on the BP side. As more electrons can be trapped in the material in the presence of NO2, the potential barrier of the p-n junctions increases, resulting in a decrease in the conductivity of the material. Although the sandwich-like nanostructure of BP-WO3 may contribute to its enhanced selectivity, BP-WO3 has a smaller specific surface area than the pure WO3 nanorods and thus does not provide enough adsorption sites for the interfering gases. Therefore, the stronger ability of BP-WO3 to trap electrons from NO2 is likely the main reason for the high selectivity of this material.
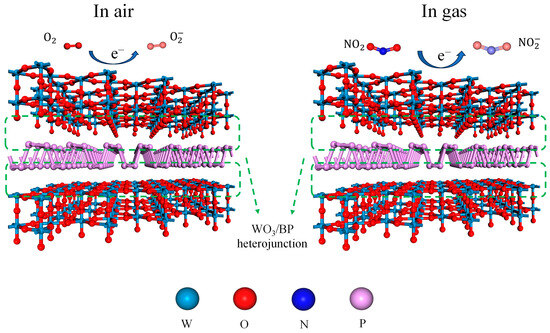
Figure 9.
Schematic diagrams of the gas-sensing mechanism of BP-WO3.
Regarding the exceptional selectivity of BP-WO3, particularly toward interfering gas H2S, several reasons can be posited. First, NO2, a nitrogen-based gas, exhibits higher adsorption energy and adhesion coefficient than H2S. Previous studies indicate that the adhesion coefficient of H2S to phosphorene at 300 K is 0.81, whereas that of NO2 is 1.0. Second, the adsorption interaction between NO2 and phosphorene induces a more significant alteration in the electronic structure than that achieved with other gases [41]. Finally, the unique BP-WO3 sandwich-like nanostructure contributes to its selectivity; the BP nanosheets hinder electron migration in the WO3 nanorods, unlike the interaction with whole nanorods, which is less favorable for H2S and WO3 interaction. However, NO2 can extract electrons from both WO3 and BP, enhancing its detection.
4. Conclusions
BP-WO3 sandwich-like nanostructures were fabricated for the first time using a simple one-step hydrothermal method. The applicability of the corresponding BP-WO3-based MEMS sensor was applied to highly selective NO2 sensing. Unlike pure WO3, BP-WO3 was largely insensitive to interfering gases, especially H2S. In addition, BP-WO3 achieved a response of 1.73 for 10 ppm NO2 at an operating temperature of 190 °C, response–recovery times of only 40 and 44 s, respectively, at 270 °C, and excellent resolving power and repeatability. The high selectivity of BP-WO3 was attributed to the surface oxygen ions having a much higher reaction rate with NO2 with than with the interfering gases. The use of the as-prepared BP-WO3 composite will allow for the enhancement of the selectivity of gas sensors.
Author Contributions
Methodology, C.Z.; Writing—original draft, C.Z.; Writing—review and editing, C.Z.; Formal analysis, B.T.; Validation B.T.; Data Curation, J.Z.; Supervision, Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant no. 62271176) and the China National Basic Enhancement Program (2022-JCJQ-JJ-0438).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Xie, T.; Sullivan, N.; Steffens, K.; Wen, B.M.; Liu, G.N.; Debnath, R.; Davydov, A.; Gomez, R.; Motayed, A. UV-assisted room-temperature chemiresistive NO2 sensor based on TiO2 thin film. J. Alloys Compd. 2015, 653, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.J.; Lin, H.C.; Yang, Y.F.; Chen, C.Y.; Ling, M.P.; Chen, S.C.; Chen, W.Y.; You, S.H.; Lu, T.H.; Liao, C.M. Association Between Ambient Air Pollution and Elevated Risk of Tuberculosis Development. Infect. Drug Resist. 2019, 12, 3835–3847. [Google Scholar] [CrossRef]
- Maji, S.; Ghosh, S.; Ahmed, S. Association of air quality with respiratory and cardiovascular morbidity rate in Delhi, India. Int. J. Environ. Health Res. 2018, 28, 471–490. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.X.; Sun, X.Y.; Diao, W.Q.; Shen, N.; He, B. Correlation of Clinical Symptoms and Sputum Inflammatory Markers with Air Pollutants in Stable COPD Patients in Beijing Area. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 1507–1517. [Google Scholar] [CrossRef]
- Jamnani, S.R.; Moghaddam, H.M.; Leonardi, S.G.; Neri, G.; Ferlazzo, A. VOCs sensing properties of samarium oxide nanorods. Ceram. Int. 2024, 50, 403–411. [Google Scholar] [CrossRef]
- Liang, Z.; Wang, M.; Liu, S.; Hassan, M.; Zhang, X.; Lei, S.; Qiao, G.; Liu, G. One-pot hydrothermal synthesis of self-assembled MoS2/WS2 nanoflowers for chemiresistive room-temperature NO2 sensors. Sens. Actuators B Chem. 2024, 403, 135215. [Google Scholar] [CrossRef]
- Mane, A.T.; Kulkarni, S.B.; Navale, S.T.; Ghanwat, A.A.; Shinde, N.M.; Kim, J.; Patil, V.B. NO2 sensing properties of nanostructured tungsten oxide thin films. Ceram. Int. 2014, 40, 16495–16502. [Google Scholar] [CrossRef]
- Du, W.J.; Wu, N.N.; Wang, Z.; Liu, J.R.; Xu, D.M.; Liu, W. High response and selectivity of platinum modified tin oxide porous spheres for nitrogen dioxide gas sensing at low temperature. Sens. Actuators B Chem. 2018, 257, 427–435. [Google Scholar] [CrossRef]
- Ueda, T.; Boehme, I.; Hyodo, T.; Shimizu, Y.; Weimar, U.; Barsan, N. Effects of Gas Adsorption Properties of an Au-Loaded Porous In2O3 Sensor on NO2-Sensing Properties. ACS Sens. 2021, 6, 4019–4028. [Google Scholar] [CrossRef]
- Dang, V.T.; Nguyen, T.T.O.; Truong, T.H.; Le, A.T.; Nguyen, T.D. Facile synthesis of different ZnO nanostructures for detecting sub-ppm NO2 gas. Mater. Today Commun. 2020, 22, 100826. [Google Scholar] [CrossRef]
- Bai, H.N.; Guo, H.; Wang, J.; Dong, Y.; Liu, B.; Xie, Z.L.; Guo, F.Q.; Chen, D.J.; Zhang, R.; Zheng, Y.D. A room-temperature NO2 gas sensor based on CuO nanoflakes modified with rGO nanosheets. Sens. Actuators B Chem. 2021, 337, 129783. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhao, Q.; Duan, Z.; Xie, C.; Duan, X.; Li, S.; Ye, Z.; Jiang, Y.; Tai, H. Ag2Te nanowires for humidity-resistant trace-level NO2 detection at room temperature. Sens. Actuators B Chem. 2022, 363, 131790. [Google Scholar] [CrossRef]
- Salama, T.M.; Morsy, M.; Abou Shahba, R.M.; Mohamed, S.H.; Mohamed, M.M. Synthesis of Graphene Oxide Interspersed in Hexagonal WO3 Nanorods for High-Efficiency Visible-Light Driven Photocatalysis and NH3 Gas Sensing. Front. Chem. 2019, 7, 722. [Google Scholar] [CrossRef]
- Punginsang, M.; Zappa, D.; Comini, E.; Wisitsoraat, A.; Sberveglieri, G.; Ponzoni, A.; Liewhiran, C. Selective H2S gas sensors based on ohmic hetero-interface of Au-functionalized WO3 nanowires. Appl. Surf. Sci. 2022, 571, 151262. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.Q.; Sun, X.Y.; Sun, Y.F.; Liu, F.M.; Yan, X.; Wang, C.G.; Sun, P.; Lu, G.Y. Preparation of Pd/PdO loaded WO3 microspheres for H2S detection. Sens. Actuators B Chem. 2020, 321, 128629. [Google Scholar] [CrossRef]
- Huang, D.; Yuan, W.J.; Fan, S.R.; Tian, C.; Hua, Z.Q.; Tian, X.M.; Wu, Y.; Li, E.P. Hydrogen sensing mechanism of Ru-loaded WO3 nanosheets. Sens. Actuators B Chem. 2020, 304, 127339. [Google Scholar] [CrossRef]
- Zhou, R.; Lin, X.P.; Xue, D.Y.; Zong, F.Y.; Zhang, J.M.; Duan, X.C.; Li, Q.H.; Wang, T.H. Enhanced H2 gas sensing properties by Pd-loaded urchin-like W18O49 hierarchical nanostructures. Sens. Actuators B Chem. 2018, 260, 900–907. [Google Scholar] [CrossRef]
- Kim, M.H.; Jang, J.S.; Koo, W.T.; Choi, S.J.; Kim, S.J.; Kim, D.H.; Kim, I.D. Bimodally Porous WO3 Microbelts Functionalized with Pt Catalysts for Selective H2S Sensors. ACS Appl. Mater. Interfaces 2018, 10, 20643–20651. [Google Scholar] [CrossRef]
- Yang, X.H.; Fu, H.T.; Tian, Y.; Xie, Q.; Xiong, S.X.; Han, D.Z.; Zhang, H.; An, X.Z. Au decorated In2O3 hollow nanospheres: A novel sensing material toward amine. Sens. Actuators B Chem. 2019, 296, 126696. [Google Scholar] [CrossRef]
- Kim, T.H.; Hasani, A.; Quyet, L.V.; Kim, Y.; Park, S.Y.; Lee, M.G.; Sohn, W.; Nguyen, T.P.; Choi, K.S.; Kim, S.Y.; et al. NO2 sensing properties of porous Au-incorporated tungsten oxide thin films prepared by solution process. Sens. Actuators B Chem. 2019, 286, 512–520. [Google Scholar] [CrossRef]
- Chen, X.X.; Shen, Y.B.; Zhou, P.F.; Zhao, S.K.; Zhong, X.X.; Li, T.T.; Han, C.; Wei, D.Z.; Meng, D. NO2 sensing properties of one-pot-synthesized ZnO nanowires with Pd functionalization. Sens. Actuators B Chem. 2019, 280, 151–161. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-J.; Jang, B.-H.; Lee, S.-J.; Min, B.K.; Rothschild, A.; Kim, I.-D. Selective detection of acetone and hydrogen sulfide for the diagnosis of diabetes and halitosis using SnO2 nanofibers functionalized with reduced graphene oxide nanosheets. ACS Appl. Mater. Interfaces 2014, 6, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.N.; Zhang, H.; Lu, S.B.; Wang, Z.T.; Tang, S.Y.; Shao, J.D.; Sun, Z.B.; Xie, H.H.; Wang, H.Y.; Yu, X.F.; et al. From Black Phosphorus to Phosphorene: Basic Solvent Exfoliation, Evolution of Raman Scattering, and Applications to Ultrafast Photonics. Adv. Funct. Mater. 2015, 25, 6996–7002. [Google Scholar] [CrossRef]
- Abbas, A.N.; Liu, B.; Chen, L.; Ma, Y.; Cong, S.; Aroonyadet, N.; Kopf, M.; Nilges, T.; Zhou, C. Black phosphorus gas sensors. ACS Nano 2015, 9, 5618–5624. [Google Scholar] [CrossRef]
- Han, D.; Han, X.; Liu, L.; Li, D.; Liu, Y.; Liu, Z.; Liu, D.; Chen, Y.; Zhuo, K.; Sang, S. Sub-ppb-level detection of nitrogen dioxide based on high-quality black phosphorus. ACS Appl. Mater. Interfaces 2022, 14, 13942–13951. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, Z.H.; Zhao, H.C.; Wang, Y.J.; Li, J.; Zou, C. Two-dimensional black phosphorus/tin oxide heterojunctions for high-performance chemiresistive H2S sensing. Anal. Chim. Acta 2023, 1245, 340825. [Google Scholar] [CrossRef]
- Katrib, A.; Hemming, F.; Wehrer, P.; Hilaire, L.; Maire, G. The multi-surface structure and catalytic properties of partially reduced WO3, WO2 and WC+ O2 or W+ O2 as characterized by XPS. J. Electron Spectrosc. Relat. Phenom. 1995, 76, 195–200. [Google Scholar] [CrossRef]
- Colton, R.J.; Rabalais, J.W. Electronic structure to tungsten and some of its borides, carbides, nitrides, and oxides by X-ray electron spectroscopy. Inorg. Chem. 1976, 15, 236–238. [Google Scholar] [CrossRef]
- Pashutski, A.; Folman, M. Low temperature XPS studies of NO and N2O adsorption on Al (100). Surf. Sci. 1989, 216, 395–408. [Google Scholar] [CrossRef]
- Su, P.-G.; Pan, T.-T. Fabrication of a room-temperature NO2 gas sensor based on WO3 films and WO3/MWCNT nanocomposite films by combining polyol process with metal organic decomposition method. Mater. Chem. Phys. 2011, 125, 351–357. [Google Scholar] [CrossRef]
- Yaqoob, U.; Uddin, A.I.; Chung, G.-S. A high-performance flexible NO2 sensor based on WO3 NPs decorated on MWCNTs and RGO hybrids on PI/PET substrates. Sens. Actuators B Chem. 2016, 224, 738–746. [Google Scholar] [CrossRef]
- Barbosa, M.S.; Barbosa, D.N.; da Silva, R.A.; Orlandi, M.O. NO2-sensing proprieties of WS2/WO3 heterostructures obtained by hydrothermal treatment of tungsten oxide seed materials. Chem. Phys. Lett. 2023, 812, 140269. [Google Scholar] [CrossRef]
- Tesfamichael, T.; Piloto, C.; Arita, M.; Bell, J. Fabrication of Fe-doped WO3 films for NO2 sensing at lower operating temperature. Sens. Actuators B Chem. 2015, 221, 393–400. [Google Scholar] [CrossRef]
- Mane, A.; Navale, S.; Patil, V. Room temperature NO2 gas sensing properties of DBSA doped PPy–WO3 hybrid nanocomposite sensor. Org. Electron. 2015, 19, 15–25. [Google Scholar] [CrossRef]
- Behera, B.; Chandra, S. Synthesis of WO3 nanorods by thermal oxidation technique for NO2 gas sensing application. Mater. Sci. Semicond. Process. 2018, 86, 79–84. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, Z.; Wang, W.; Li, P.; Li, G.; Zhang, W.; Chen, L.; Zhuivkov, S.; Hu, J. Selective gas detection using Mn3O4/WO3 composites as a sensing layer. Beilstein J. Nanotechnol. 2019, 10, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.Y.; Song, J.G.; Kim, Y.; Choi, T.; Shin, S.; Lee, C.W.; Lee, K.; Koo, J.; Lee, H.; Kim, J.; et al. Improvement of Gas-Sensing Performance of Large-Area Tungsten Disulfide Nanosheets by Surface Functionalization. ACS Nano 2016, 10, 9287–9296. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Z.; Luo, X.; Liu, Z.; Zhang, Y. Sol-gel processed tungsten trioxide nanocrystals layer for efficient hole-injection in quantum dot light-emitting diodes. Thin Solid Film. 2021, 730, 138722. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Li, J.; Zhang, R.; Zhao, H.; Wang, Y. Ag decoration-enabled sensitization enhancement of black phosphorus nanosheets for trace NO2 detection at room temperature. J. Hazard. Mater. 2022, 435, 129086. [Google Scholar] [CrossRef]
- Cui, S.; Pu, H.; Wells, S.A.; Wen, Z.; Mao, S.; Chang, J.; Hersam, M.C.; Chen, J. Ultrahigh sensitivity and layer-dependent sensing performance of phosphorene-based gas sensors. Nat. Commun. 2015, 6, 8632. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).