1. Introduction
Trace gases in the air determine many hazardous situations such as chemical accidents or affect our health not only passively but also actively via markers in medical diagnostics. To date, the identification of trace substances in the air has mainly been achieved using mass spectrometry (MS), in which the necessary information is determined via the mass/charge ratio of the ions. Ideally, the retention time is also used for identification in advance by means of gas chromatographic pre-separation (GCGC-MS). Ion mobility spectrometry (IMS), in which the drift time of the ions in the air is determined, can also be used for very low concentrations of the target substances. It has the advantage that the device does not require a high vacuum, but it also has the disadvantage that not all substances can be detected and there is no referencing. In addition, chemical reactions cannot be ruled out with ionization at atmospheric pressure. Photon ionization is also a method that has been used in IMS in recent years. Direct ionization steps have been used by means of vacuum UV sources, but indirect ionization steps, which are excited by two-photon processes using lasers, have also been used. The latter laser–IMS (LIMS) devices [
1] can very selectively detect substances (usually aromatics) at very low concentrations in complex mixtures. LIMS or MS systems are relatively cost-intensive and almost not portable. However, there are hand-held IMS-based devices on the market, which are still larger in size than the solution sought here due to the required drift field.
Small hand-held measuring devices equipped with a photoionization detector (PID) and other micro gas sensors (e.g., metal oxide sensors [
2] or electrochemical cells) have been available on the market for years. The latter micro gas sensors detect the presence of gas groups with considerable sensitivity but lack any referenceable gas identification. In the former PID, substances in the air are ionized using high-energy UV light, and the ion current to be measured is then proportional to the concentration of the substances. As a rule, gas discharge lamps filled with krypton are used, which deliver a photon energy of 10.6 eV. This means that a PID alone is also not suitable for identifying substances, as the detector only provides information about the presence of substances with an ionization potential (IP) of less than or equal to 10.6 eV. Pollutants such as carbon monoxide (14.01 eV), hydrogen cyanide (13.65 eV), chloropicrin (11.42 eV), 1,2-dichloroethane (11.12 eV), and formaldehyde (10.87 eV) cannot be detected with a PID. The only way to detect some of these substances is to use a different UV source with a higher energy lamp, such as the 11.7 eV argon discharge lamp [
3]. The problem with that lamp is the hygroscopic UV window made of lithium fluoride, which only lasts around 6 weeks. Ideally, a UV lamp would be suitable, in which the wavelength could be varied to enable the identification of the substance via its ionization potential. This is currently only possible on a technically large scale using the synchrotron radiation of a ring accelerator.
Since the 2000s, attempts have been made to transfer the principle of the miniaturized photoionization detector (PID) to electron impact ionization in free air and to use this to create an ionizer with tunable energy [
4,
5]. The idea is reminiscent of the Franck–Hertz experiment [
6] from 1914, which is now part of many laboratory practicals in university courses. However, the resonance curves measured there are so heavily smeared in terms of energy with half-value widths (FWHM) that no identification of approx. 3000 relevant substances/molecules, which are listed in the NIST database [
7] with a typical 6–7 meV energy difference, could ever have been realized from the measurement data.
The main reason for the low line sharpness in the Franck–Hertz experiment is the use of thermal electrons and possible collisions of electrons with the gas molecules during acceleration, even though the collisions should be elastic below the resonance or ionization limits. Based on these considerations and previous work [
8], the external photoelectric effect was chosen to generate free electrons in air. Here, it is possible to obtain higher energy levels with UV-LED wavelengths that are selected to match the emitter materials’ work function
Φ. Compared with the year 2000, UV LEDs are now available with wavelengths of down to 250 nm (4.96 eV), typically have a spectral width of 10…20 nm, and reach an electrical power of 6 watts. As far as emitter materials are concerned, metallic materials are initially preferable as they have a high electron density at the filled valence band edge. In addition, due to the availability of LEDs, materials with the lowest possible work function were also a priority in the past, which is why experiments were carried out with lanthanum hexaboride (LaB
6) and later samarium (Sm) and ytterbium (Yb). Unfortunately, all these materials react with air and air humidity. Sm reaches a lifetime of a few days, Yb loses its emissivity to air within hours [
9].
LaB
6 remains emissive but loses its low work function of ideally 2.31 eV to significantly higher values [
10]. Subsequently, elements that naturally occur in the solid state were investigated and alloys thereof. Under different combinations of Ag, As, Ce, In, and Sm, As
xCe
ySm
z showed a comparatively high emissivity at a work function
Φ of 1.87 eV and a stability of >14 months [
5]. Unfortunately, the reproducible fabrication of alloys of high- and low-melting materials poses technical difficulties, so a simple silver layer was used for further experiments. This loses a large part of its emissivity within months due to the formation of sulfide layers but can be reconstituted by Ar-sputtering.
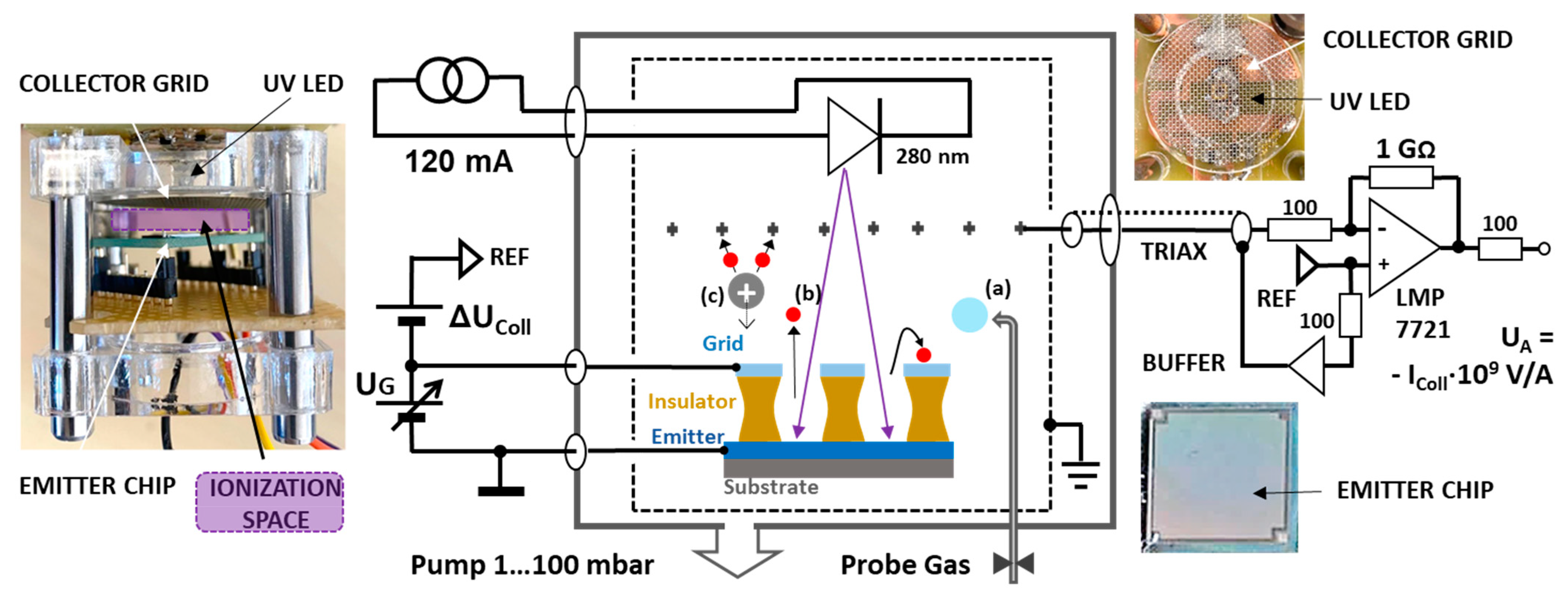
The acceleration structure was designed as a planar electrode on spin-on-glass or SU8 resist insulators and is shown in
Figure 1d. The insulator thickness of 700 nm is approximately ten times the mean free path length of nitrogen–nitrogen collisions, 70 nm at normal conditions. At 100 hPa and below, however, the regime of nano-vacuum technology is already reached, i.e., collisions between accelerating electrons and air molecules are already unlikely. Molecules to be detected, such as hydrocarbons, have an even smaller impact cross-section due to the Ramsauer minimum and even slightly increase the mean free path [
11].
During photoionization, an atom or molecule in a bound state /
i> absorbs a photon of energy
hν, exceeding the ionization energy
Eion. If
hν > Eion and an electron is lifted over the vacuum edge: the excess energy
hν −
Eion is thereby transformed into the kinetic energy of the ionization fragments. In electron impact ionization, in addition to this energy consideration, the momentum in the transition to the multibody system (electron plus molecule -> two electrons plus ion) must also be taken into account. In 1953, Wannier developed the basic theoretical description of the processes at the ionization threshold [
12] and established the relationship
for the ionization cross-section σ, where
Eel is the energy of the incident electron. For energies well above the ionization threshold, the ionization cross section increases rapidly and is better described by other models [
13,
14,
15,
16]. These are well summarized in the Lotz approximation [
17] in agreement with experimental data. Whether resonances occur in the vicinity of the ionization threshold is still being discussed [
18,
19] and what this means for the idea of two electrons at rest next to an ion, especially in ambient air, is not clear.
In the following, we report on the first measurements with PEIS on various substances. In addition, we consider comparative measurements of PEIS with PID for an initial determination of the sensitivity of the method and test whether electron impact ionization can also be used to measure beyond the 10.6 eV limit. This is followed by PEIS measurements in spectrometer mode with a comparison of broad and sharper energy distributions—also with regard to previously reported, preliminary resonance-like results [
20].
4. Discussion
With regard to the comparison measurements with the LaB6 electron emitter/10.6 eV lamp and the PID sensor, the general equivalence is not surprising, as most of the substances could also be photoionized. However, the measurement with methanol (10.85 eV) and chloroform (11.5 eV) deserves attention. Methanol is seen relatively weakly by the PEIS and also by the PID with a very small deflection: this should not actually be present, as the 10.6 eV lamps emit at a wavelength range of 116.2–117.2 nm, which corresponds to a photon energy of 10.67–10.58 eV. However, the following is conceivable: As the PEIS and PID were arranged close to each other in the measurement and the PEIS structure was open, ions or electrons from the PEIS structure could have been captured by the PID, which could have led to a very small signal. The same applies to the tiny deflections at 1 ppm chloroform. The low concentration was chosen because the precipitation of this gas on the surfaces of the PEIS led to high surface currents at the insulators and falsified the measurement (in order to be able to measure 30 nA at 30 volts UG, an insulation resistance of 1 GΩ is required, which represents a technological challenge with 10 M emitter holes of 700 nm depth). Also, the test with oleic acid could be questioned, as oleic acid has a very low vapor pressure. Since only a technical grade material was used here, volatile impurities could have caused the signal.
The detection accuracy of only 470 meV determined in the first spectrometry tests may be due to the fact that the 10.6 eV lamps not only emit the 116.7 nm wavelength but also emit a larger 123.8 nm line (10.0 eV). This results in a systematic energy uncertainty of 600 meV in the ionization measurements, which matches the resolution limit. The extent to which the residual energy of the 10.6 eV photons is also sharply reflected in the kinetic electron energy after overcoming the work function, or whether the momentum cone of the Fermi sphere in question causes an additional energy broadening, still needs to be clarified.
In general, there are several possible explanations for the observed flickering of the PEIS measurements at the ionization thresholds. Firstly, it was necessary to check whether the multiplication of charge carriers in the ionization space (a cube of 1 cm in length) could possibly lead to space charge effects, as was the case with earlier electron tubes. According to a rough estimate, these are very unlikely due to the low excitation current of approx. 100 pA. In purely mathematical terms, at an acceleration voltage of 10 V, the electrons are traveling in the collision-free case at 1700 km/s, which is so fast that only about three electrons will be traveling at the same time. The space charge clouds in electron tubes are found at charge densities millions of times higher than this.
It also had to be investigated whether the measurement fluctuations in the experimental setup could actually achieve large fluctuations in the vicinity of the ionization thresholds. In principle, there are three simultaneously occurring reaction paths in the very narrow energy range around the ionization thresholds: the further travel of an electron without ionization, the loss of all electron energy at the threshold, and the continued movement of two electrons in the collector field if the threshold was clearly exceeded and kinetic residual energy is split. The energetic conditions are simulated in
Figure 6. The LED spectrum with 280 +/− 6 nm is transferred to 4.43 eV center energy and a sigma ranging from 4.34 to 4.52 eV. All energies below the work function of polycrystalline silver are cut off from the overall spectrum due to the external photoelectric effect (left partial image). As the silver work function varies in the literature from 4.23 eV for photoemission from freshly prepared films [
21] to 4.3 eV for contact potential measurements [
22], a value of 4.26 eV is widely used and was taken here for calculations. Applying the grid voltage
UG results in an electron energy distribution as shown in the right-hand partial image for
UG = 10.03 V. One can see that the energy distribution is shifted to the right by
The interaction cross-section of the ionization starts at 10.17 eV in the Wannier and Lotz functionals for the IPA simulation example.
The convolution of the energy distribution function with the Lotz model provides a measure of the PEIS sensitivity to fluctuation in the measurement setup based on its relative increase (relative slope of the convolution divided by the input signal variable). The dotted curve in
Figure 7 shows the development of this sensitivity, which would be obtained by varying
UG in each case. Its maximum lies at 10.25 eV. The above considerations would result in an associated grid voltage of
UG = 10.25 V
− 0.17 V = 10.08 volts. Experimentally, the maximum of the noise was observed at 10.11 volts. The current resolution limit of the PEIS determination of the ionization energies would therefore be an achievable error of 30 meV and would be partly due to systematic reasons. The latter points to optimization possibilities. Firstly, however, the reliability of such flicker measurements would have to be increased.
In principle, there is a third consideration. If the ionization energy were hit sharply, two electrons with E = 0 would be obtained, i.e., they would remain next to their original ion. Readsorption would in turn lead to a frustrated Auger emission of an electron at rest. Slow drifts in the collector field would therefore have to be taken into account as well as possible resonances, as finally described by Franck–Hertz and later taken up again by Fano and Klar. The more precise quantum mechanical analysis of the processes, especially in the atmosphere, has so far eluded a simple conception and description.