Safety of Immersive Virtual Reality for the Management of Parkinson’s Disease
Abstract
1. Introduction
2. Methods
3. Results
3.1. Frequency and Type of Adverse Event, Setting, and Causality
3.1.1. Discomfort/Pain
3.1.2. Motor Fluctuations
3.1.3. Falls
3.2. Severity of Adverse Events
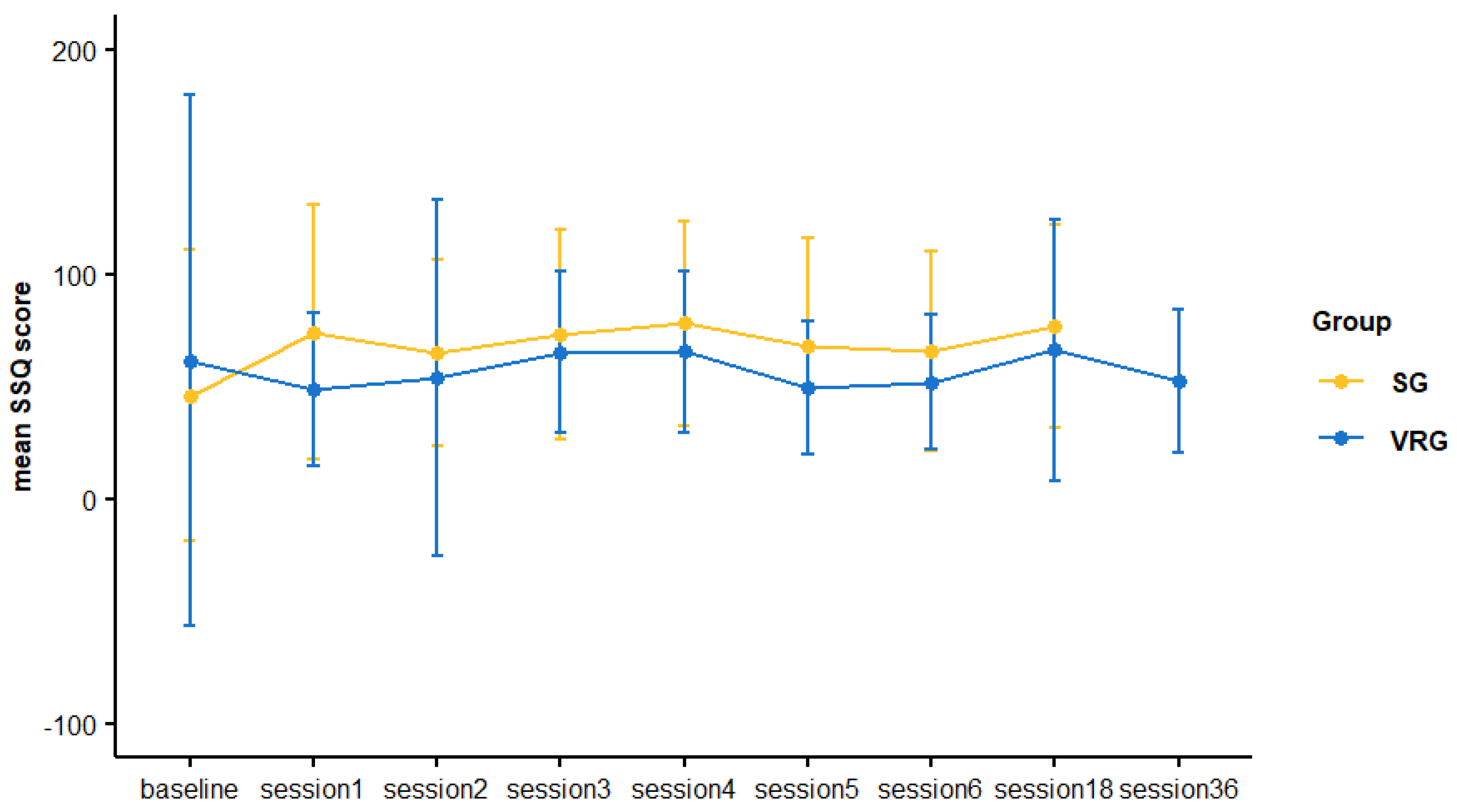
3.3. Cybersickness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Keus, S.; Munneke, M.; Graziano, M.; Paltamaa, J.; Pelosin, E.; Domingos, J.; Brühlmann, S.; Ramaswamy, B.; Prins, J.; Struiksma, C.; et al. European Physiotherapy Guideline for Parkinson’s disease. KNGF/Park. Neth. 2014, 191, 1–153. [Google Scholar]
- Ernst, M.; Folkerts, A.K.; Gollan, R.; Lieker, E.; Caro-Valenzuela, J.; Adams, A.; Cryns, N.; Monsef, I.; Dresen, A.; Roheger, M.; et al. Physical exercise for people with Parkinson’s disease: A systematic review and network meta-analysis. Cochrane Database Syst. Rev. 2023, 1, CD013856. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.E.; Sherrington, C.; Suriyarachchi, G.D.; Paul, S.S.; Song, J.; Canning, C.G. Exercise and motor training in people with Parkinson’s disease: A systematic review of participant characteristics, intervention delivery, retention rates, adherence, and adverse events in clinical trials. Park. Dis. 2012, 2012, 854328. [Google Scholar] [CrossRef]
- Perez-Lloret, S.; Rey, M.V.; Fabre, N.; Ory, F.; Spampinato, U.; Montastruc, J.L.; Rascol, O. Do Parkinson’s disease patients disclose their adverse events spontaneously? Eur. J. Clin. Pharmacol. 2012, 68, 857–865. [Google Scholar] [CrossRef]
- Caniça, V.; Bouça-Machado, R.; Rosa, M.M.; Ferreira, J.J.; Group, C.N.S.P.S. Adverse Events of Physiotherapy Interventions in Parkinsonian Patients. Mov. Disord. Clin. Pract. 2022, 9, 744–750. [Google Scholar] [CrossRef]
- Mirelman, A.; Rochester, L.; Maidan, I.; Del Din, S.; Alcock, L.; Nieuwhof, F.; Rikkert, M.O.; Bloem, B.R.; Pelosin, E.; Avanzino, L.; et al. Addition of a non-immersive virtual reality component to treadmill training to reduce fall risk in older adults (V-TIME): A randomised controlled trial. Lancet 2016, 388, 1170–1182. [Google Scholar] [CrossRef]
- van der Kolk, N.M.; de Vries, N.M.; Kessels, R.P.C.; Joosten, H.; Zwinderman, A.H.; Post, B.; Bloem, B.R. Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: A double-blind, randomised controlled trial. Lancet Neurol. 2019, 18, 998–1008. [Google Scholar] [CrossRef]
- Maggio, M.G.; De Cola, M.C.; Latella, D.; Maresca, G.; Finocchiaro, C.; La Rosa, G.; Cimino, V.; Sorbera, C.; Bramanti, P.; De Luca, R.; et al. What About the Role of Virtual Reality in Parkinson Disease’s Cognitive Rehabilitation? Preliminary Findings From a Randomized Clinical Trial. J. Geriatr. Psychiatry Neurol. 2018, 31, 312–318. [Google Scholar] [CrossRef]
- Santos, P.; Machado, T.; Santos, L.; Ribeiro, N.; Melo, A. Efficacy of the Nintendo Wii combination with Conventional Exercises in the rehabilitation of individuals with Parkinson’s disease: A randomized clinical trial. NeuroRehabilitation 2019, 45, 255–263. [Google Scholar] [CrossRef]
- Dockx, K.; Bekkers, E.M.; Van den Bergh, V.; Ginis, P.; Rochester, L.; Hausdorff, J.M.; Mirelman, A.; Nieuwboer, A. Virtual reality for rehabilitation in Parkinson’s disease. Cochrane Database Syst. Rev. 2016, 12, CD010760. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wu, J.; Lu, J.; Wei, X.; Zheng, K.; Liu, B.; Xiao, W.; Shi, Q.; Xiong, L.; Ren, Z. Efficacy of virtual reality training on motor performance, activity of daily living, and quality of life in patients with Parkinson’s disease: An umbrella review comprising meta-analyses of randomized controlled trials. J. Neuroeng. Rehabil. 2023, 20, 133. [Google Scholar] [CrossRef] [PubMed]
- Saredakis, D.; Szpak, A.; Birckhead, B.; Keage, H.A.D.; Rizzo, A.; Loetscher, T. Factors Associated With Virtual Reality Sickness in Head-Mounted Displays: A Systematic Review and Meta-Analysis. Front. Hum. Neurosci. 2020, 14, 96. [Google Scholar] [CrossRef] [PubMed]
- Rose, T.; Nam, C.S.; Chen, K.B. Immersion of virtual reality for rehabilitation—Review. Appl. Ergon. 2018, 69, 153–161. [Google Scholar] [CrossRef]
- Kim, A.; Darakjian, N.; Finley, J.M. Walking in fully immersive virtual environments: An evaluation of potential adverse effects in older adults and individuals with Parkinson’s disease. J. Neuroeng. Rehabil. 2017, 14, 16. [Google Scholar] [CrossRef]
- Brandín-De la Cruz, N.; Secorro, N.; Calvo, S.; Benyoucef, Y.; Herrero, P.; Bellosta-López, P. Immersive virtual reality and antigravity treadmill training for gait rehabilitation in Parkinson’s disease: A pilot and feasibility study. Rev. Neurol. 2020, 71, 447–454. [Google Scholar] [CrossRef]
- Bouca-Machado, R.; Duarte, G.S.; Patriarca, M.; Castro Caldas, A.; Alarcao, J.; Fernandes, R.M.; Mestre, T.A.; Matias, R.; Ferreira, J.J. Measurement Instruments to Assess Functional Mobility in Parkinson’s Disease: A Systematic Review. Mov. Disord. Clin. Pract. 2020, 7, 129–139. [Google Scholar] [CrossRef]
- Bloem, B.R.; Marinus, J.; Almeida, Q.; Dibble, L.; Nieuwboer, A.; Post, B.; Ruzicka, E.; Goetz, C.; Stebbins, G.; Martinez-Martin, P.; et al. Measurement instruments to assess posture, gait, and balance in Parkinson’s disease: Critique and recommendations. Mov. Disord. 2016, 31, 1342–1355. [Google Scholar] [CrossRef]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Clinical Safety Data Management: Definitions and Standards for Expedited Reporting E2A. Available online: https://database.ich.org/sites/default/files/E2A_Guideline.pdf (accessed on 11 November 2024).
- U.S. Department of Health and Human Services; National Institutes of Health; National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed on 11 November 2024).
- FDA. What Is a Serious Adverse Event? Available online: https://www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event (accessed on 28 October 2024).
- World Health Organization. The Use of the WHO-UMC System for Standardised Case Causality Assessment. 2013. Available online: https://www.who.int/publications/m/item/WHO-causality-assessment (accessed on 11 November 2024).
- Kennedy, R.S.; Lane, N.E.; Berbaum, K.S.; Lilienthal, M.G. Simulator Sickness Questionnaire: An Enhanced Method for Quantifying Simulator Sickness. Int. J. Aviat. Psychol. 1993, 3, 203–220. [Google Scholar] [CrossRef]
- Carvalho, M.; Costa, R.; Nardi, A. Simulator Sickness Questionnaire: Translation and cross-cultural adaptation. J. Bras. Psiquiatr. 2010, 60, 247–252. [Google Scholar] [CrossRef]
- Bimberg, P.; Weissker, T.; Kulik, A. On the Usage of the Simulator Sickness Questionnaire for Virtual Reality Research. In Proceedings of the 2020 IEEE Conference on Virtual Reality and 3D User Interfaces Abstracts and Workshops (VRW), Atlanta, GA, USA, 22–26 March 2020; pp. 464–467. [Google Scholar]
- Cikajlo, I.; Peterlin Potisk, K. Advantages of using 3D virtual reality based training in persons with Parkinson’s disease: A parallel study. J. Neuroeng. Rehabil. 2019, 16, 119. [Google Scholar] [CrossRef] [PubMed]
- de Melo, G.E.L.; Kleiner, A.F.R.; Lopes, J.B.P.; Dumont, A.J.L.; Lazzari, R.D.; Galli, M.; Oliveira, C.S. Effect of virtual reality training on walking distance and physical fitness in individuals with Parkinson’s disease. NeuroRehabilitation 2018, 42, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Hajebrahimi, F.; Velioglu, H.A.; Bayraktaroglu, Z.; Helvaci Yilmaz, N.; Hanoglu, L. Clinical evaluation and resting state fMRI analysis of virtual reality based training in Parkinson’s disease through a randomized controlled trial. Sci. Rep. 2022, 12, 8024. [Google Scholar] [CrossRef] [PubMed]
- Kashif, M.; Ahmad, A.; Bandpei, M.A.M.; Gilani, S.A.; Hanif, A.; Iram, H. Combined effects of virtual reality techniques and motor imagery on balance, motor function and activities of daily living in patients with Parkinson’s disease: A randomized controlled trial. BMC Geriatr. 2022, 22, 381. [Google Scholar] [CrossRef]
- Maranesi, E.; Casoni, E.; Baldoni, R.; Barboni, I.; Rinaldi, N.; Tramontana, B.; Amabili, G.; Benadduci, M.; Barbarossa, F.; Luzi, R.; et al. The Effect of Non-Immersive Virtual Reality Exergames versus Traditional Physiotherapy in Parkinson’s Disease Older Patients: Preliminary Results from a Randomized-Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 14818. [Google Scholar] [CrossRef]
- Kwon, S.H.; Park, J.K.; Koh, Y.H. A systematic review and meta-analysis on the effect of virtual reality-based rehabilitation for people with Parkinson’s disease. J. Neuroeng. Rehabil. 2023, 20, 94. [Google Scholar] [CrossRef]
- Rodríguez-Mansilla, J.; Bedmar-Vargas, C.; Garrido-Ardila, E.M.; Torres-Piles, S.T.; González-Sánchez, B.; Rodríguez-Domínguez, M.T.; Ramírez-Durán, M.V.; Jiménez-Palomares, M. Effects of Virtual Reality in the Rehabilitation of Parkinson’s Disease: A Systematic Review. J. Clin. Med. 2023, 12, 4896. [Google Scholar] [CrossRef]
- Ma, H.I.; Hwang, W.J.; Fang, J.J.; Kuo, J.K.; Wang, C.Y.; Leong, I.F.; Wang, T.Y. Effects of virtual reality training on functional reaching movements in people with Parkinson’s disease: A randomized controlled pilot trial. Clin. Rehabil. 2011, 25, 892–902. [Google Scholar] [CrossRef]
- Robles-García, V.; Corral-Bergantiños, Y.; Espinosa, N.; García-Sancho, C.; Sanmartín, G.; Flores, J.; Cudeiro, J.; Arias, P. Effects of movement imitation training in Parkinson’s disease: A virtual reality pilot study. Park. Relat. Disord. 2016, 26, 17–23. [Google Scholar] [CrossRef]
- Allen, N.E.; Song, J.; Paul, S.S.; Smith, S.; O’Duffy, J.; Schmidt, M.; Love, R.; Sherrington, C.; Canning, C.G. An interactive videogame for arm and hand exercise in people with Parkinson’s disease: A randomized controlled trial. Park. Relat. Disord. 2017, 41, 66–72. [Google Scholar] [CrossRef]
- Gandolfi, M.; Geroin, C.; Dimitrova, E.; Boldrini, P.; Waldner, A.; Bonadiman, S.; Picelli, A.; Regazzo, S.; Stirbu, E.; Primon, D.; et al. Virtual Reality Telerehabilitation for Postural Instability in Parkinson’s Disease: A Multicenter, Single-Blind, Randomized, Controlled Trial. BioMed Res. Int. 2017, 2017, 7962826. [Google Scholar] [CrossRef] [PubMed]
- Pompeu, J.E.; Mendes, F.A.; Silva, K.G.; Lobo, A.M.; Oliveira Tde, P.; Zomignani, A.P.; Piemonte, M.E. Effect of Nintendo Wii™-based motor and cognitive training on activities of daily living in patients with Parkinson’s disease: A randomised clinical trial. Physiotherapy 2012, 98, 196–204. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, M.R.; Kwakkel, G.; Beek, P.J.; Berendse, H.W.; Daffertshofer, A.; van Wegen, E.E. Effects of augmented visual feedback during balance training in Parkinson’s disease: A pilot randomized clinical trial. Park. Relat. Disord. 2014, 20, 1352–1358. [Google Scholar] [CrossRef]
- Cancela-Carral, J.M.; Campo-Prieto, P.; Rodriguez-Fuentes, G. The IntegraPark Study: An Opportunity to Facilitate High-Intensity Exercise with Immersive Virtual Reality in Parkinson’s Disease Patients. J. Funct. Morphol. Kinesiol. 2024, 9, 156. [Google Scholar] [CrossRef]
- Campo-Prieto, P.; Cancela-Carral, J.M.; Rodriguez-Fuentes, G. Wearable Immersive Virtual Reality Device for Promoting Physical Activity in Parkinson’s Disease Patients. Sensors 2022, 22, 3302. [Google Scholar] [CrossRef]
- Schenkman, M.; Moore, C.G.; Kohrt, W.M.; Hall, D.A.; Delitto, A.; Comella, C.L.; Josbeno, D.A.; Christiansen, C.L.; Berman, B.D.; Kluger, B.M.; et al. Effect of High-Intensity Treadmill Exercise on Motor Symptoms in Patients With De Novo Parkinson Disease: A Phase 2 Randomized Clinical Trial. JAMA Neurol. 2018, 75, 219–226. [Google Scholar] [CrossRef]
- Uc, E.Y.; Doerschug, K.C.; Magnotta, V.; Dawson, J.D.; Thomsen, T.R.; Kline, J.N.; Rizzo, M.; Newman, S.R.; Mehta, S.; Grabowski, T.J.; et al. Phase I/II randomized trial of aerobic exercise in Parkinson disease in a community setting. Neurology 2014, 83, 413–425. [Google Scholar] [CrossRef]
- Schenkman, M.; Hall, D.A.; Baron, A.E.; Schwartz, R.S.; Mettler, P.; Kohrt, W.M. Exercise for people in early- or mid-stage Parkinson disease: A 16-month randomized controlled trial. Phys. Ther. 2012, 92, 1395–1410. [Google Scholar] [CrossRef]
- Perez-Marcos, D. Virtual reality experiences, embodiment, videogames and their dimensions in neurorehabilitation. J. Neuroeng. Rehabil. 2018, 15, 113. [Google Scholar] [CrossRef]
- Mantri, S.; Lepore, M.; Edison, B.; Daeschler, M.; Kopil, C.M.; Marras, C.; Chahine, L.M. The Experience of OFF Periods in Parkinson’s Disease: Descriptions, Triggers, and Alleviating Factors. J. Patient Cent. Res. Rev. 2021, 8, 232–238. [Google Scholar] [CrossRef]
- Figura, M.; Mrozowicz, A.; Milanowski, Ł.; Szlufik, S.; Raćkowska, E.; Lypkan, H.; Friedman, A.; Koziorowski, D.; Giebułtowicz, J. Impact of Physical Exercise on Levodopa Therapy Across Parkinson’s Disease Stages. J. Parkinsons Dis. 2024, 14, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Lin, I.; Edison, B.; Mantri, S.; Albert, S.; Daeschler, M.; Kopil, C.; Marras, C.; Chahine, L.M. Triggers and alleviating factors for fatigue in Parkinson’s disease. PLoS ONE 2021, 16, e0245285. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Fuentes, G.; Campo-Prieto, P.; Cancela-Carral, J.M. Immersive Virtual Reality High-Intensity Aerobic Training to Slow Parkinson’s Disease: The ReViPark Program. Appl. Sci. 2024, 14, 4708. [Google Scholar] [CrossRef]

| VR Group (n = 15) | Sequential Group (n = 15) | |
|---|---|---|
| Age (years), median [IQR] | 61 [58, 66] | 68 [62, 72.5] |
| Gender (men), n (%) | 9 (60) | 8 (53.3) |
| Education, n (%) | ||
| Low | 5 (33.3) | 7 (46.7) |
| Medium | 4 (26.7) | 1 (0.07) |
| High | 6 (40) | 7 (46.7) |
| Disease duration (years), median [IQR] | 7 [6, 10] | 9 [5, 11.5] |
| First symptom, n (%) | ||
| Tremor | 7 (46.7) | 7 (46.7) |
| Bradykinesia-rigidity | 7 (46.7) | 7 (46.7) |
| Other | 1 (0.07) | 1 (0.07) |
| MDS-UPDRS part 3, median [IQR] | 31 [23, 35.5] | 23 [13, 24.5] |
| HY, median [IQR] | 2 [2, 2] | 2 [2, 2] |
| Adverse Event (AE) | N (%) | VR Group | Sequential Group | ||
|---|---|---|---|---|---|
| 0–6 Week | 6–12 Week | 0–6 Week | 6–12 Week | ||
| Discomfort/pain | 46 (2.7) | 15 (2.9) | 6 (1.2) | 5 (2.0) | 20 (4.3) |
| Motor fluctuations | 34 (2.0) | 16 (3.1) | 14 (2.8) | 1 (0.4) | 3 (0.7) |
| Falls | 11 (0.6) | 3 (0.6) | 0 | 2 (0.8) | 6 (1.3) |
| Fatigue | 9 (0.5) | 5 (1) | 0 | 1 (0.4) | 3 (0.7) |
| Near falls | 8 (0.5) | 4 (0.8) | 2 (0.4) | 0 | 2 (0.4) |
| Hypotension | 6 (0.3) | 1 (0.2) | 0 | 4 (1.6) | 1 (0.2) |
| Dizziness | 3 (0.2) | 0 | 1 (0.2) | 0 | 2 (0.4) |
| Hyperhidrosis | 3 (0.2) | 3 (0.6) | 0 | 0 | 0 |
| Dyskinesias | 3 (0.2) | 3 (0.6) | 0 | 0 | 0 |
| Irritability/frustration | 2 (0.1) | 0 | 1 (0.2) | 0 | 1 (0.2) |
| Gut disturbance | 2 (0.1) | 0 | 1 (0.2) | 0 | 1 (0.2) |
| Headache | 2 (0.1) | 1 (0.2) | 0 | 0 | 1 (0.2) |
| Impulse control disorder | 2 (0.1) | 0 | 2 (0.4) | 0 | 0 |
| Muscle soreness | 2 (0.1) | 2 (0.4) | 0 | 0 | 0 |
| Hypertension | 1 (0.06) | 1 (0.2) | 0 | 0 | 0 |
| Numbness/tingling | 1 (0.06) | 1 (0.2) | 0 | 0 | 0 |
| Syncope | 1 (0.06) | 1 (0.2) | 0 | 0 | 0 |
| Sleep disturbance | 1 (0.06) | 0 | 1 (0.2) | 0 | 0 |
| Dry mouth | 1 (0.06) | 0 | 0 | 1 (0.4) | 0 |
| Hematuria | 1 (0.06) | 0 | 0 | 1 (0.4) | 0 |
| Cramps | 1 (0.06) | 0 | 0 | 0 | 1 (0.2) |
| Gout | 1 (0.06) | 0 | 0 | 0 | 1 (0.2) |
| Chin rash | 1 (0.06) | 0 | 0 | 0 | 1 (0.2) |
| Complicated hernia | 1 (0.06) | 0 | 1 (0.2) | 0 | 0 |
| Flu-like symptoms | 1 (0.06) | 1 (0.2) | 0 | 0 | 0 |
| Total | 144 (8.4) | 57 (11.2) | 29 (5.8) | 15 (6.1) | 43 (9.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pimenta Silva, D.; Pona-Ferreira, F.; Santos, B.; Campo-Prieto, P.; Bouça-Machado, R.; Ferreira, J.J. Safety of Immersive Virtual Reality for the Management of Parkinson’s Disease. Sensors 2024, 24, 8188. https://doi.org/10.3390/s24248188
Pimenta Silva D, Pona-Ferreira F, Santos B, Campo-Prieto P, Bouça-Machado R, Ferreira JJ. Safety of Immersive Virtual Reality for the Management of Parkinson’s Disease. Sensors. 2024; 24(24):8188. https://doi.org/10.3390/s24248188
Chicago/Turabian StylePimenta Silva, Daniela, Filipa Pona-Ferreira, Beatriz Santos, Pablo Campo-Prieto, Raquel Bouça-Machado, and Joaquim J. Ferreira. 2024. "Safety of Immersive Virtual Reality for the Management of Parkinson’s Disease" Sensors 24, no. 24: 8188. https://doi.org/10.3390/s24248188
APA StylePimenta Silva, D., Pona-Ferreira, F., Santos, B., Campo-Prieto, P., Bouça-Machado, R., & Ferreira, J. J. (2024). Safety of Immersive Virtual Reality for the Management of Parkinson’s Disease. Sensors, 24(24), 8188. https://doi.org/10.3390/s24248188







