Sensitivity Analysis of an Optical Interferometric Surface Stress Ethanol Gas Sensor with a Freestanding Nanosheet
Abstract
:1. Introduction
2. Structure and Detection Principle
2.1. Structure and Operational Principle of the EtOH Gas Sensor
2.2. Effect of Shape Parameters on Surface Stress Sensitivity
3. Fabrication and Problem
3.1. Fabrication Process of the EtOH Gas Sensor
3.1.1. Microcavity Formation (Figure 3a)
3.1.2. SPM Cleaning
3.1.3. Silane Coupling Treatment
3.1.4. Parylene C Deposition (Figure 3b)
3.1.5. Bottom Parylene C Etching (Figure 3c)
3.1.6. Freestanding Membrane Preparation (Figure 3d–g)
3.1.7. Freestanding Membrane Transfer (Figure 3h)
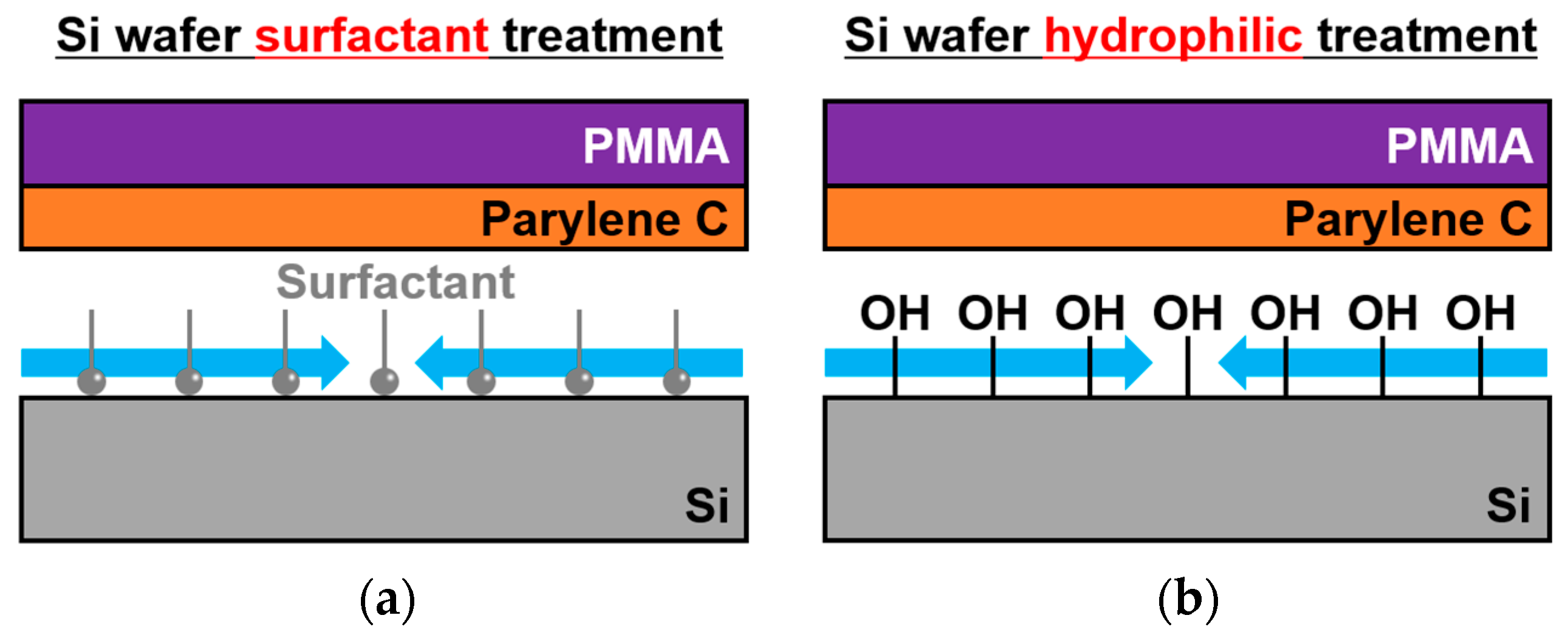
3.2. Nanosheet Release Using Surfactants
3.3. New Fabrication Process Using Surfactant-Free Parylene C Release
4. Results and Discussion
4.1. Experimental System for Detecting EtOH Gas
4.2. Time Response to EtOH Gas
4.3. Thickness Dependence of Parylene C Membrane
4.4. Thickness Dependence of PMMA Membrane
4.5. Lower Detection Limit for EtOH Gas Sensor
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aleksanyan, M.; Sayunts, A.; Shahkhatuni, G.; Simonyan, Z.; Shahnazaryan, G.; Aroutiounian, V. Gas Sensor Based on ZnO Nanostructured Film for the Detection of Ethanol Vapor. Chemosensors 2022, 10, 245. [Google Scholar] [CrossRef]
- Benvenho, A.R.V.; Li, R.W.C.; Gruber, J. Polymeric Electronic Gas Sensor for Determining Alcohol Content in Automotive Fuels. Sens. Actuators B Chem. 2009, 136, 173–176. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, D.; Zhang, L.; Lu, G. Non-Invasive Blood Glucose Monitoring for Diabetics by Means of Breath Signal Analysis. Sens. Actuators B Chem. 2012, 173, 106–113. [Google Scholar] [CrossRef]
- Ma, M.; Yang, X.; Ying, X.; Shi, C.; Jia, Z.; Jia, B. Applications of Gas Sensing in Food Quality Detection: A Review. Foods 2023, 12, 3966. [Google Scholar] [CrossRef] [PubMed]
- Galstyan, V.; Bhandari, M.P.; Sberveglieri, V.; Sberveglieri, G.; Comini, E. Metal Oxide Nanostructures in Food Applications: Quality Control and Packaging. Chemosensors 2018, 6, 16. [Google Scholar] [CrossRef]
- Irshad, M.; Mujahid, A.; Afzal, A.; Bajwa, S.Z.; Hussain, T.; Zaman, W.; Latif, U.; Athar, M.M. A Miniaturized Electronic Sensor for Instant Monitoring of Ethanol in Gasohol Fuel Blends. RSC Adv. 2018, 8, 22952–22962. [Google Scholar] [CrossRef]
- Längkvist, M.; Coradeschi, S.; Loutfi, A.; Rayappan, J.B.B. Fast Classification of Meat Spoilage Markers Using Nanostructured ZnO Thin Films and Unsupervised Feature Learning. Sensors 2013, 13, 1578–1592. [Google Scholar] [CrossRef]
- Senapati, M.; Sahu, P.P. Meat Quality Assessment Using Au Patch Electrode Ag-SnO2/SiO2/Si MIS Capacitive Gas Sensor at Room Temperature. Food Chem. 2020, 324, 126893. [Google Scholar] [CrossRef] [PubMed]
- Mayr, D.; Margesin, R.; Klingsbichel, E.; Hartungen, E.; Jenewein, D.; Schinner, F.; Märk, T.D. Rapid Detection of Meat Spoilage by Measuring Volatile Organic Compounds by Using Proton Transfer Reaction Mass Spectrometry. Appl. Environ. Microbiol. 2003, 69, 4697–4705. [Google Scholar] [CrossRef]
- Thomas, G.W.; Sousan, S.; Tatum, M.; Liu, X.; Zuidema, C.; Fitzpatrick, M.; Koehler, K.A.; Peters, T.M. Low-Cost, Distributed Environmental Monitors for Factory Worker Health. Sensors 2018, 18, 1411. [Google Scholar] [CrossRef]
- Tang, J.; Fang, J.; Liang, Y.; Zhang, B.; Luo, Y.; Liu, X.; Li, Z.; Cai, X.; Xian, J.; Lin, H.; et al. All-Fiber-Optic VOC Gas Sensor Based on Side-Polished Fiber Wavelength Selectively Coupled with Cholesteric Liquid Crystal Film. Sens. Actuators B Chem. 2018, 273, 1816–1826. [Google Scholar] [CrossRef]
- Toshiyoshi, H. MEMS Vibrational Energy Harvester for IoT Wireless Sensors. In Proceedings of the 2020 IEEE International Electron Devices Meeting (IEDM), San Francisco, CA, USA, 12–18 December 2020; pp. 37.3.1–37.3.4. [Google Scholar] [CrossRef]
- Honma, H.; Mitsuya, H.; Hashiguchi, G.; Fujita, H.; Toshiyoshi, H. Power Generation Demonstration of Electrostatic Vibrational Energy Harvester with Comb Electrodes and Suspensions Located in Upper and Lower Decks. Sens. Mater. 2022, 34, 1527. [Google Scholar] [CrossRef]
- Ponzoni, A. Morphological Effects in SnO2 Chemiresistors for Ethanol Detection: A Review in Terms of Central Performances and Outliers. Sensors 2021, 21, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; An, G.; He, X. Highly Porous SnO2 Fibers by Electrospinning and Oxygen Plasma Etching and Its Ethanol-Sensing Properties. Sens. Actuators B Chem. 2010, 144, 43–48. [Google Scholar] [CrossRef]
- Ivanov, P.; Llobet, E.; Vilanova, X.; Brezmes, J.; Hubalek, J.; Correig, X. Development of High Sensitivity Ethanol Gas Sensors Based on Pt-Doped SnO2 Surfaces. Sens. Actuators B Chem. 2004, 99, 201–206. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured Materials for Room-Temperature Gas Sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef]
- Liu, J.; Lin, X.; Sun, M.; Du, B.; Li, L.; Bai, J.; Zhou, M. Thiourea-Assistant Growth of In2O3 Porous Pompon Assembled from 2D Nanosheets for Enhanced Ethanol Sensing Performance. Talanta 2020, 219, 121323. [Google Scholar] [CrossRef]
- Xiao, J.; Che, Y.; Lv, B.; Benedicte, M.-C.; Feng, G.; Sun, T.; Song, C. Synthesis of WO3 Nanorods and Their Excellent Ethanol Gas-Sensing Performance. Mater. Res. 2021, 24, e20200434. [Google Scholar] [CrossRef]
- Shailja; Singh, K.J.; Singh, R.C. Highly Sensitive and Selective Ethanol Gas Sensor Based on Ga-Doped NiO Nanoparticles. J. Mater. Sci. Mater. Electron. 2021, 32, 11274–11290. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, J. Highly Sensitive Ethanol Gas Sensors Based on Co-Doped SnO2 Nanobelts and Pure SnO2 Nanobelts. Phys. E Low-Dimens. Syst. Nanostructures 2023, 147, 115604. [Google Scholar] [CrossRef]
- Guo, L.; Liang, H.; An, D.; Yang, H. One-Step Hydrothermal Synthesis of Uniform Ag-Doped SnO2 Nanoparticles for Highly Sensitive Ethanol Sensing. Phys. E Low-Dimens. Syst. Nanostructures 2023, 151, 115717. [Google Scholar] [CrossRef]
- Wang, L.; Kang, Y.; Liu, X.; Zhang, S.; Huang, W.; Wang, S. ZnO Nanorod Gas Sensor for Ethanol Detection. Sens. Actuators B Chem. 2012, 162, 237–243. [Google Scholar] [CrossRef]
- Li, H.; Yang, Z.; Ling, W.; Zhu, D.; Pu, Y. UV Excited Gas Sensing SnO2-ZnO Aerogels to Ppb-Level Ethanol Detection. Sens. Actuators B Chem. 2021, 337, 129815. [Google Scholar] [CrossRef]
- Jiang, G.; Goledzinowski, M.; Comeau, F.J.E.; Zarrin, H.; Lui, G.; Lenos, J.; Veileux, A.; Liu, G.; Zhang, J.; Hemmati, S.; et al. Free-Standing Functionalized Graphene Oxide Solid Electrolytes in Electrochemical Gas Sensors. Adv. Funct. Mater. 2016, 26, 1729–1736. [Google Scholar] [CrossRef]
- Dhall, S.; Mehta, B.R.; Tyagi, A.K.; Sood, K. A Review on Environmental Gas Sensors: Materials and Technologies. Sens. Int. 2021, 2, 100116. [Google Scholar] [CrossRef]
- Constantinoiu, I.; Viespe, C. Detection of Volatile Organic Compounds Using Surface Acoustic Wave Sensor Based on Nanoparticles Incorporated in Polymer. Coatings 2019, 9, 373. [Google Scholar] [CrossRef]
- Shooshtari, M.; Sacco, L.N.; van Ginkel, J.; Vollebregt, S.; Salehi, A. Enhancement of Room Temperature Ethanol Sensing by Optimizing the Density of Vertically Aligned Carbon Nanofibers Decorated with Gold Nanoparticles. Materials 2022, 15, 1383. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, R.J.S.; Nguyen, F.; Yoshikawa, G. Real-Time Gas Identification on Mobile Platforms Using a Nanomechanical Membrane-Type Surface Stress Sensor. EPJ Tech. Instrum. 2014, 1, 9. [Google Scholar] [CrossRef]
- Vasagiri, S.; Burra, R.K.; Vankara, J.; Kumar Patnaik, M.S.P. A Survey of MEMS Cantilever Applications in Determining Volatile Organic Compounds. AIP Adv. 2022, 12, 030701. [Google Scholar] [CrossRef]
- Yoshikawa, G.; Akiyama, T.; Gautsch, S.; Vettiger, P.; Rohrer, H. Nanomechanical Membrane-Type Surface Stress Sensor. Nano Lett. 2011, 11, 1044–1048. [Google Scholar] [CrossRef]
- Takahashi, T.; Choi, Y.-J.; Sawada, K.; Takahashi, K. A Ppm Ethanol Sensor Based on Fabry–Perot Interferometric Surface Stress Transducer at Room Temperature. Sensors 2020, 20, 6868. [Google Scholar] [CrossRef]
- Yao, T.-J.; Yang, X.; Tai, Y.-C. BrF3 Dry Release Technology for Large Freestanding Parylene Microstructures and Electrostatic Actuators. Sens. Actuators Phys. 2002, 97–98, 771–775. [Google Scholar] [CrossRef]
- Kim, H.; Najafi, K. Characterization of Aligned Wafer-Level Transfer of Thin and Flexible Parylene Membranes. J. Microelectromechanical Syst. 2007, 16, 1386–1396. [Google Scholar] [CrossRef]
- Shiba, K.; Tamura, R.; Imamura, G.; Yoshikawa, G. Data-Driven Nanomechanical Sensing: Specific Information Extraction from a Complex System. Sci. Rep. 2017, 7, 3661. [Google Scholar] [CrossRef]
- Yoshikawa, G. Mechanical Analysis and Optimization of a Microcantilever Sensor Coated with a Solid Receptor Film. Appl. Phys. Lett. 2011, 98, 173502. [Google Scholar] [CrossRef]













| Material | Temperature (°C) | LOD (ppm) |
|---|---|---|
| Highly porous SnO2 fibers [15] | 340 | 0.001 |
| Pt-doped SnO2 [16] | 300 | 0.001 |
| In2O3 porous pompon [18] | 260 | 0.4 |
| WO3 nanorods [19] | 160 | 20 |
| Ga-doped NiO nanoparticles [20] | 250 | 10 |
| Co-doped SnO2 nanobelts [21] | 300 | 50 |
| Ag-doped SnO2 nanoparticles [22] | 180 | 1.0 |
| ZnO nanorod [23] | 220 | 1.0 |
| ZnO nanostructured membrane [1] | 250 | 0.7 |
| SnO2-ZnO aerogels [24] | 300 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sogame, R.; Choi, Y.-J.; Noda, T.; Sawada, K.; Takahashi, K. Sensitivity Analysis of an Optical Interferometric Surface Stress Ethanol Gas Sensor with a Freestanding Nanosheet. Sensors 2024, 24, 8055. https://doi.org/10.3390/s24248055
Sogame R, Choi Y-J, Noda T, Sawada K, Takahashi K. Sensitivity Analysis of an Optical Interferometric Surface Stress Ethanol Gas Sensor with a Freestanding Nanosheet. Sensors. 2024; 24(24):8055. https://doi.org/10.3390/s24248055
Chicago/Turabian StyleSogame, Ryusei, Yong-Joon Choi, Toshihiko Noda, Kazuaki Sawada, and Kazuhiro Takahashi. 2024. "Sensitivity Analysis of an Optical Interferometric Surface Stress Ethanol Gas Sensor with a Freestanding Nanosheet" Sensors 24, no. 24: 8055. https://doi.org/10.3390/s24248055
APA StyleSogame, R., Choi, Y.-J., Noda, T., Sawada, K., & Takahashi, K. (2024). Sensitivity Analysis of an Optical Interferometric Surface Stress Ethanol Gas Sensor with a Freestanding Nanosheet. Sensors, 24(24), 8055. https://doi.org/10.3390/s24248055







