iTex Gloves: Design and In-Home Evaluation of an E-Textile Glove System for Tele-Assessment of Parkinson’s Disease
Abstract
1. Introduction
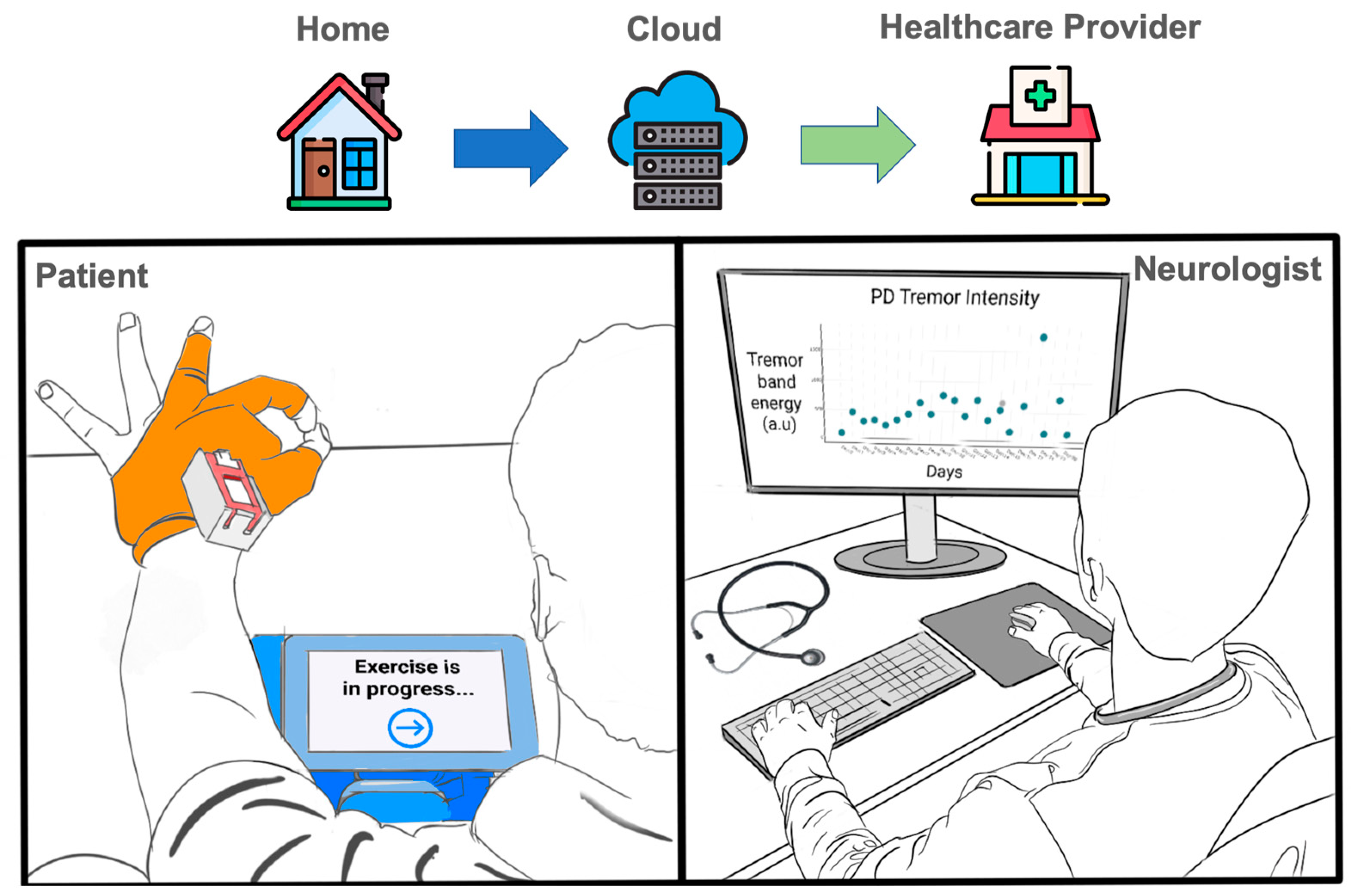
- Smart Textiles—iTex Gloves: Sensing gloves are specially engineered to acquire fine and gross motor symptoms through finger flexion and inertial signals for movement in daily living conditions. iTex gloves require no expensive infrastructure such as cameras. The inclusion of flexion sensing enables the assessment of upper body movement tasks that are found in the standard protocols such as UPDRS Part III. The gloves are designed with the consideration of ease of use for accommodating the movement impairments of PD.
- Wearable IoT System: The IoT system connects with the gloves and displays the patient interface. It ensures the orchestration of the motor assessment delivery that requires on-demand data acquisition of movement data using WiFi-based MQTT protocol.
- Patient-Centered Exercise Application: A patient-centered user interface was developed to guide PwPD through the movement screening tasks, perform data collection of the gloves, and deliver the daily questionnaires. Section 3 offers more details on the various components of the application from the user interface, flask server, IoT data acquisition, time-series database, and cloud export.
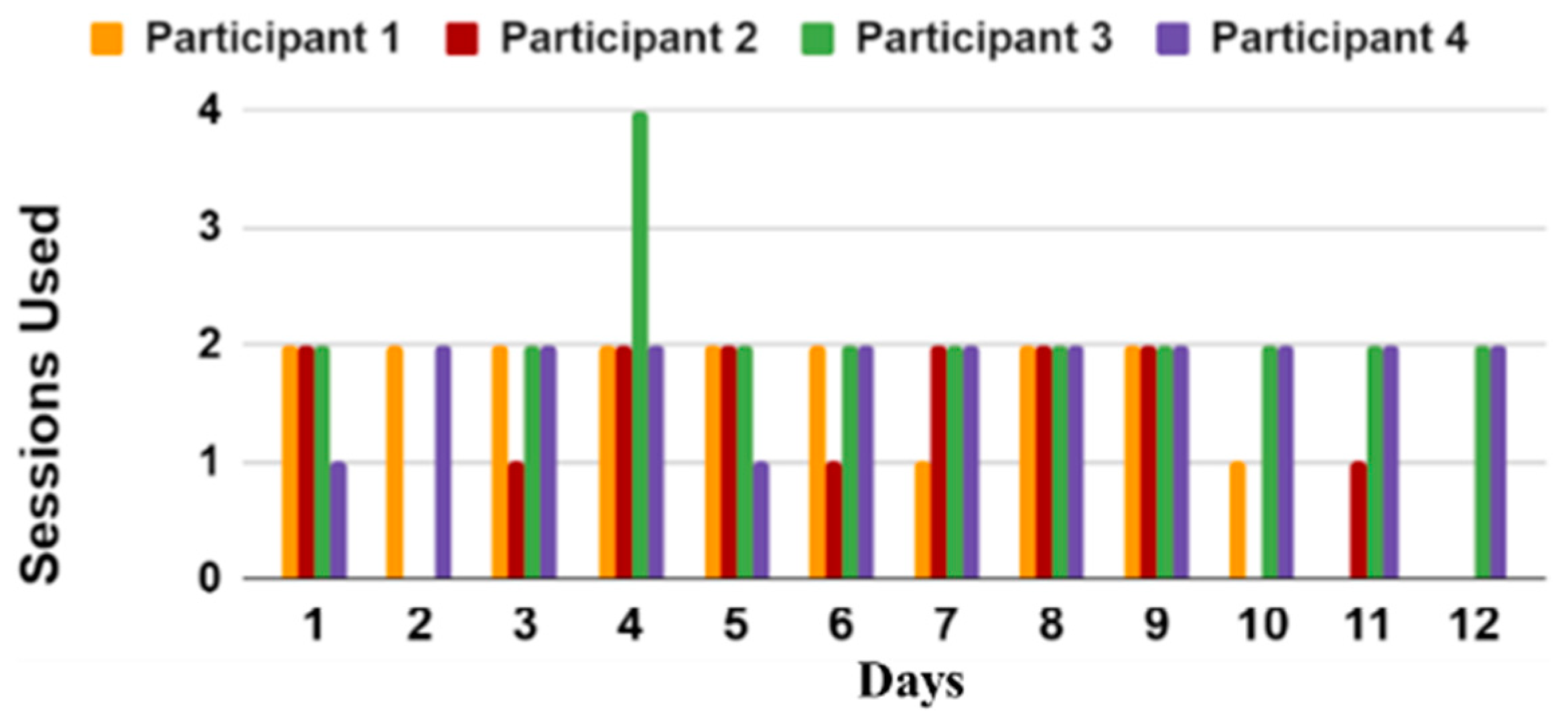
- In-Home Evaluation of the iTex Glove with PwPD: A feasibility study was conducted in real-world home environments. Four PwPDs were recruited to use the iTex system independently two times a day for a week. Real-world data throughput, adherence, and timing of different system operations are studied for the proposed system. To examine the usability constraints of the system with PwPD, a brief exit interview was conducted.
- Signal Processing, Feature Analysis, and Medication Status Classification: Features specific to kinetic movement tasks and stationary tasks were extracted from inertial (pitch, roll) and index flexion signals. Preliminary analysis of movement assessment activity data indicates variability in signal features based on medication usage states. Performance of different machine learning classification models for the feature dataset is reported along with SHAP-based feature importance scores.
2. Background
- Parkinson’s disease and its management
- B.
- Telemedicine and its role in PD symptom management
3. Materials
- Design and Development of iTex Gloves:
- B.
- IoT Data Collection Architecture:
- C.
- Design of Patient-Centered User Interface with Accessibility Factors:
- D.
- Data Logging:
- E.
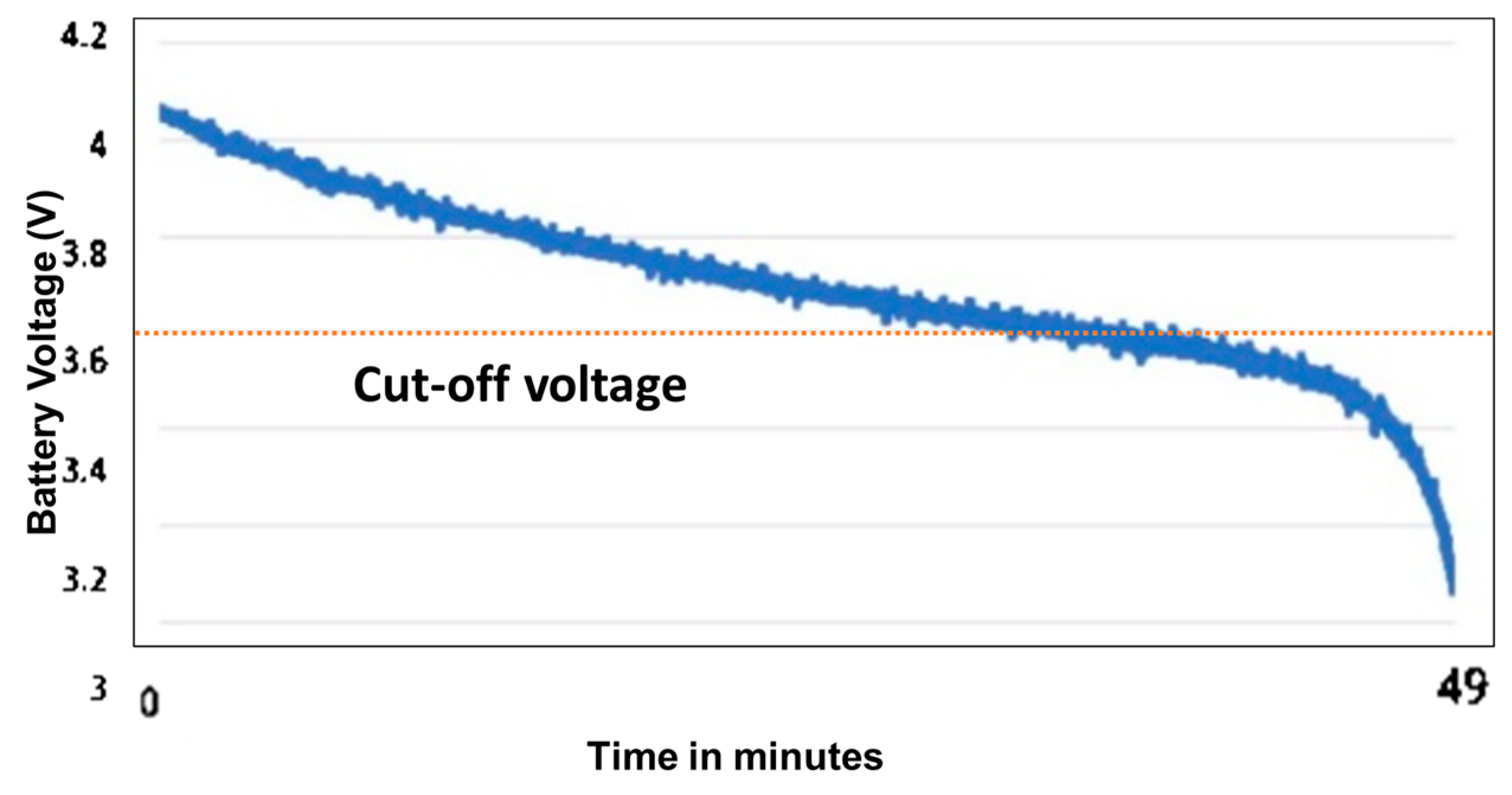
- Telemetry and Remote Logging:
4. Methods
- Glove Data Measures
- B.
- Glove Sensor Signal Processing
- C.
- Participants and Study Overview:
5. Results and Discussion
5.1. System Performance
5.2. Usability Results
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PD | Parkinson’s disease |
| PwPD | People with Parkinson’s disease |
| IMU | Inertial measurement unit |
| DOF | Degrees of freedom |
| MDS-UPDRS | Movement Disorder Society Unified Parkinson’s Disease Rating Scale |
| IoT | Internet of Things |
| PT | Physical therapy |
| ADC | Analog to digital converter |
| MQTT | Message Queuing Telemetry Transport |
| UTC | Universal Time Coordinated |
| PSD | Power spectral density |
| RMS | Root mean square |
| ZCR | Zero crossing rate |
| AU | Arbitrary units |
Appendix A
| Type of Task | Movement Task | Signal Type | Features Extracted |
|---|---|---|---|
| Kinetic Task (start, mid, end) | FT | Flexion |
|
| OC | Flexion | ||
| HF | Inertial | ||
| FN | Inertial | ||
| Stationary Task (start, end) | HH | Inertial |
|
| RH | Inertial |

References
- Parkinson’s Prevalence Statistics. Parkinson’s Foundation. Available online: https://www.parkinson.org/understanding-parkinsons/statistics (accessed on 8 June 2022).
- Magrinelli, F.; Picelli, A.; Tocco, P.; Federico, A.; Roncari, L.; Smania, N.; Zanette, G.; Tamburin, S. Pathophysiology of Motor Dysfunction in Parkinson’s Disease as the Rationale for Drug Treatment and Rehabilitation. Park. Dis. 2016, 2016, 9832839. [Google Scholar] [CrossRef] [PubMed]
- Antonini, A.; Martinez-Martin, P.; Chaudhuri, K.R.; Merello, M.; Hauser, R.; Katzenschlager, R.; Odin, P.; Stacy, M.; Stocchi, F.; Poewe, W.; et al. Wearing-off scales in Parkinson’s disease: Critique and recommendations. Mov. Disord. 2011, 26, 2169–2175. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Richards, M.; Marder, K.; Cote, L.; Mayeux, R. Interrater reliability of the unified Parkinson’s disease rating scale motor examination. Mov. Disord. 1994, 9, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, W.W.; Kim, S.K.; Park, H.; Jeon, H.S.; Kim, H.B.; Jeon, B.S.; Park, K.S. Tremor frequency characteristics in Parkinson’s disease under resting-state and stress-state conditions. J. Neurol. Sci. 2016, 362, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.; Yao, H.M.; Gupta, S.; Modi, N.B. Comparison of the pharmacokinetics of an oral extended-release capsule formulation of carbidopa-levodopa (ipx066) with immediate-release carbidopa-levodopa (sinemet®), sustained-release carbidopa-levodopa (sinemet® cr), and carbidopa-levodopa-entacapone (stalevo®). J. Clin. Pharmacol. 2015, 55, 995–1003. [Google Scholar] [PubMed]
- Zach, H.; Dirkx, M.F.; Pasman, J.W.; Bloem, B.R.; Helmich, R.C. Cognitive Stress Reduces the Effect of Levodopa on Parkinson’s Resting Tremor. CNS Neurosci. Ther. 2017, 23, 209–215. [Google Scholar] [CrossRef]
- Jankovic, J. Motor fluctuations and dyskinesias in Parkinson’s disease: Clinical manifestations. Mov. Disord. 2005, 20, S11–S16. [Google Scholar] [CrossRef]
- Hung, S.W.; Adeli, G.M.; Arenovich, T.; Fox, S.H.; Lang, A. Patient perception of dyskinesia in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2010, 81, 1112–1115. [Google Scholar] [CrossRef]
- Richardson, B.R.; Truter, P.; Blumke, R.; Russell, T.G. Physiotherapy assessment and diagnosis of musculoskeletal disorders of the knee via telerehabilitation. J. Telemed. Telecare 2016, 23, 88–95. [Google Scholar] [CrossRef]
- Vizcarra, J.A.; Sánchez-Ferro, A.; Maetzler, W.; Marsili, L.; Zavala, L.; Lang, A.E.; Martinez-Martin, P.; Mestre, T.A.; Reilmann, R.; Hausdorff, J.M.; et al. The parkinson’s disease e-diary: Developing a clinical and research tool for the digital age. Mov. Disord. 2019, 34, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.K.; Karlin, D.R.; Ho, B.K.; Thomas, K.C.; Parisi, F.; Vergara-Diaz, G.P.; Daneault, J.-F.; Wacnik, P.W.; Zhang, H.; Kangarloo, T.; et al. mHealth and wearable technology should replace motor diaries to track motor fluctuations in Parkinson’s disease. NPJ Digit. Med. 2020, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Buongiorno, D.; Bortone, I.; Cascarano, G.D.; Trotta, G.F.; Brunetti, A.; Bevilacqua, V. A low-cost vision system based on the analysis of motor features for recognition and severity rating of Parkinson’s Disease. BMC Med. Informatics Decis. Mak. 2019, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- López-Blanco, R.; Velasco, M.A.; Méndez-Guerrero, A.; Romero, J.P.; Del Castillo, M.D.; Serrano, J.I.; Rocon, E.; Benito-León, J. Smartwatch for the analysis of rest tremor in patients with parkinson’s disease. J. Neurol. Sci. 2019, 401, 37–42. [Google Scholar] [CrossRef]
- Niazmand, K.; Tonn, K.; Kalaras, A.; Fietzek, U.M.; Mehrkens, J.H.; Lueth, T.C. Quantitative evaluation of parkinson’s disease using sensor based smart glove. In Proceedings of the 2011 24th International Symposium on Computer-Based Medical Systems (CBMS), Bristol, UK, 27–30 June 2011; IEEE: New, York, NY, USA, 2011; pp. 1–8. [Google Scholar]
- m5stack. m5stack/m5stickc-plus. Available online: https://github.com/m5stack/M5StickC-Plus (accessed on 8 June 2022).
- FlexPoint. Single Direction Flex Sensor. Available online: https://media.digikey.com/pdf/Data%20Sheets/FlexPoint%20PDF’s/BendSensorFLXT.pdf (accessed on 8 June 2022).
- Simone, L.K.; Kamper, D.G. Design considerations for a wearable monitor to measure finger posture. J. Neuroeng. Rehabil. 2005, 2, 5. [Google Scholar] [CrossRef]
- RaspAP. Raspap/Raspap-Webgui. Available online: https://github.com/RaspAP/raspap-webgui (accessed on 8 June 2022).
- Light, R.A. Mosquitto: Server and client implementation of the MQTT protocol. J. Open Source Softw. 2017, 1–3. [Google Scholar] [CrossRef]
- Nunes, F.; Silva, P.A.; Cevada, J.; Barros, A.C.; Teixeira, L. User interface design guidelines for smartphone applications for people with Parkinson’s disease. Univers. Access Inf. Soc. 2015, 15, 659–679. [Google Scholar] [CrossRef]
- Naqvi, S.N.Z.; Yfantidou, S.; Zimányi, E. Time series databases and influxdb. Stud. Univ. Libre De Brux. 2017, 12–15. Available online: https://cs.ulb.ac.be/public/_media/teaching/influxdb_2017.pdf (accessed on 8 June 2022).
- N.C.-Rclone, W. rclone. Available online: https://github.com/rclone/rclone (accessed on 8 June 2022).
- Tom Bäckström. Voice Activity Detection (vad). 2019. Available online: https://wiki.aalto.fi/pages/viewpage.action?pageId=151500905 (accessed on 11 April 2022).
- Tsipouras, M.G.; Tzallas, A.T.; Rigas, G.; Tsouli, S.; Fotiadis, D.I.; Konitsiotis, S. An automated methodology for levodopa-induced dyskinesia: Assessment based on gyroscope and accelerometer signals. Artif. Intell. Med. 2012, 55, 127–135. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The montreal cognitive assessment, moca: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Shawen, N.; O’Brien, M.K.; Venkatesan, S.; Lonini, L.; Simuni, T.; Hamilton, J.L.; Ghaffari, R.; Rogers, J.A.; Jayaraman, A. Role of data measurement characteristics in the accurate detection of Parkinson’s disease symptoms using wearable sensors. J. Neuroeng. Rehabilitation 2020, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]










| Movement Task | Task Instruction |
|---|---|
| Finger Tapping (FT) | Tap the index finger on the thumb 10 times as quickly AND as big as possible. |
| Open and Close Hand (OC) | Open the hand 10 times as fully AND as quickly as possible. |
| Hand Flip (HF) | Extend the arm out in front of the body with the palms down, and then turn the palm up and down alternately 10 times as fast and as fully as possible. |
| Both Hands Out (HH) | Stretch the arms out in front of the body with palms down for 10 s. |
| Finger to Nose (FN) | Point your left index finger towards the screen, then bring your index finger to your nose and point it out again 10 times as quickly and steadily as possible. |
| Resting Hands (RH) | Sit in a chair and rest your arms on your armrests or on your thighs for 10 s. |
| Data Measures | Channel | Sensor | Sampling Rate |
|---|---|---|---|
| Flexion sensing | Index, thumb, ring | Flexpoint (resistive) | 128 Hz |
| Inertial sensing | Wrist acceleration, gyroscope [AHRS computed] | MPU6886 (MEMS IMU) | 128 Hz |
| Power usage | Battery discharge voltage, current | AXP192 | 16 Hz |
| Model | Precision | Recall | F1-Score | Accuracy |
|---|---|---|---|---|
| K-nearest neighbors | 0.69 | 0.65 | 0.63 | 0.65 |
| Random forest | 0.72 | 0.71 | 0.71 | 0.71 |
| Naïve-Bayes | 0.67 | 0.59 | 0.53 | 0.58 |
| Multilayer perceptron | 0.71 | 0.68 | 0.67 | 0.68 |
| Support vector machine | 0.69 | 0.65 | 0.63 | 0.66 |
| Participant | Left Glove (Hz) | Right Glove (Hz) |
|---|---|---|
| 1 | 82 | 84 |
| 2 | 84 | 84 |
| 3 | 82 | 83 |
| 4 | 87 | 86 |
| Operation | Payload Parsing | JSON Formatting | InfluxDB Write | Inter Payload Interval |
|---|---|---|---|---|
| Time taken (ms) | 0.296 ± 0.03 | 1.71 ± 0.4 | 13 ± 15 | 364 ± 23 |
| Question | Participant 1 | Participant 2 | Participant 3 | Participant 4 |
|---|---|---|---|---|
| Ease of use | Very Easy | Easy | Very Easy | Very Easy |
| Clarity of instruction | Easy | Very Easy | Very Easy | Very Easy |
| Preferred time to use application | Morning and afternoon | Morning | Morning | Morning |
| Application usability and medication use | Did not affect | Did not affect | Hard to select questionnaire responses when medicated | Did not affect |
| Time spent per session | 7 min | 5 min | 8–15 min | 10–15 min |
| Question | Participant 1 | Participant 2 | Participant 3 | Participant 4 |
|---|---|---|---|---|
| Ease of wearing [1 = Difficult, 10 = Effortless] | 8 | 9 | 4 | 7 |
| Fit of gloves [1 = Improper fit, 10 = Perfect fit] | 10 | 8 | 4 | 7 |
| Ease of charging [1 = Difficult, 10 = Effortless] | 10 | 10 | 10 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravichandran, V.; Sadhu, S.; Convey, D.; Guerrier, S.; Chomal, S.; Dupre, A.-M.; Akbar, U.; Solanki, D.; Mankodiya, K. iTex Gloves: Design and In-Home Evaluation of an E-Textile Glove System for Tele-Assessment of Parkinson’s Disease. Sensors 2023, 23, 2877. https://doi.org/10.3390/s23062877
Ravichandran V, Sadhu S, Convey D, Guerrier S, Chomal S, Dupre A-M, Akbar U, Solanki D, Mankodiya K. iTex Gloves: Design and In-Home Evaluation of an E-Textile Glove System for Tele-Assessment of Parkinson’s Disease. Sensors. 2023; 23(6):2877. https://doi.org/10.3390/s23062877
Chicago/Turabian StyleRavichandran, Vignesh, Shehjar Sadhu, Daniel Convey, Sebastien Guerrier, Shubham Chomal, Anne-Marie Dupre, Umer Akbar, Dhaval Solanki, and Kunal Mankodiya. 2023. "iTex Gloves: Design and In-Home Evaluation of an E-Textile Glove System for Tele-Assessment of Parkinson’s Disease" Sensors 23, no. 6: 2877. https://doi.org/10.3390/s23062877
APA StyleRavichandran, V., Sadhu, S., Convey, D., Guerrier, S., Chomal, S., Dupre, A.-M., Akbar, U., Solanki, D., & Mankodiya, K. (2023). iTex Gloves: Design and In-Home Evaluation of an E-Textile Glove System for Tele-Assessment of Parkinson’s Disease. Sensors, 23(6), 2877. https://doi.org/10.3390/s23062877







