A Telechelic Fluorescent Indicator Based on Polymer Conformational Change for Free Copper(II) Ions
Abstract
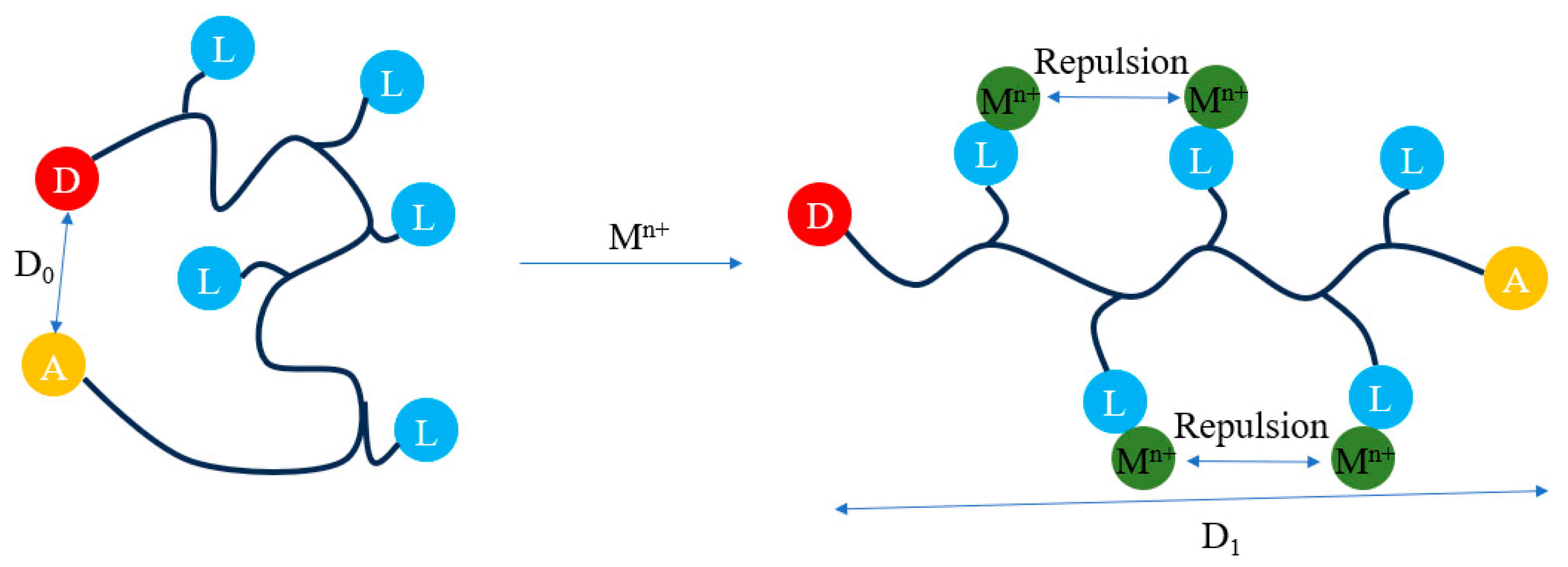
:1. Introduction
2. Materials and Methods
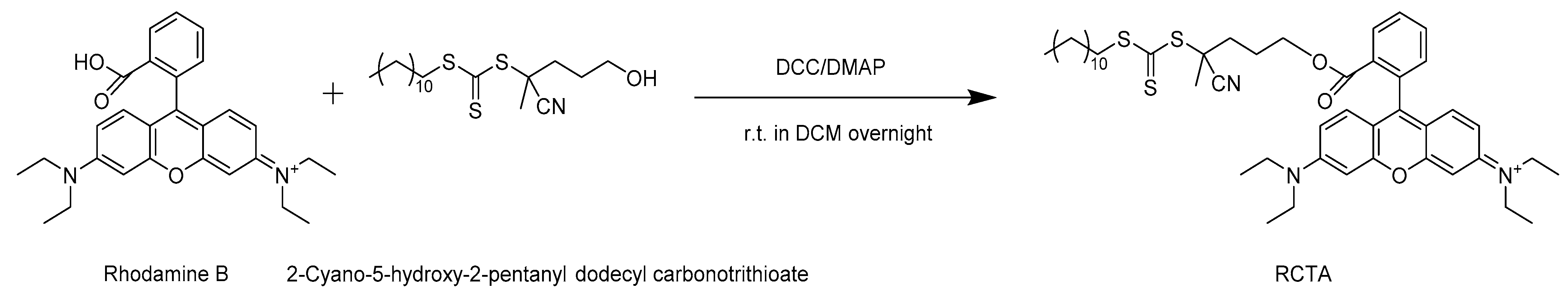
2.1. Preparation of the Rhodamine-Tagged Chain Transfer Agent (RCTA)
2.2. RAFT Polymerization
2.3. Preparation of the Telechelic Polymer Indicator
2.4. Instrumentation
2.4.1. Fluorescent Measurement
2.4.2. Nuclear Magnetic Resonance (NMR) Measurement
3. Results and Discussion
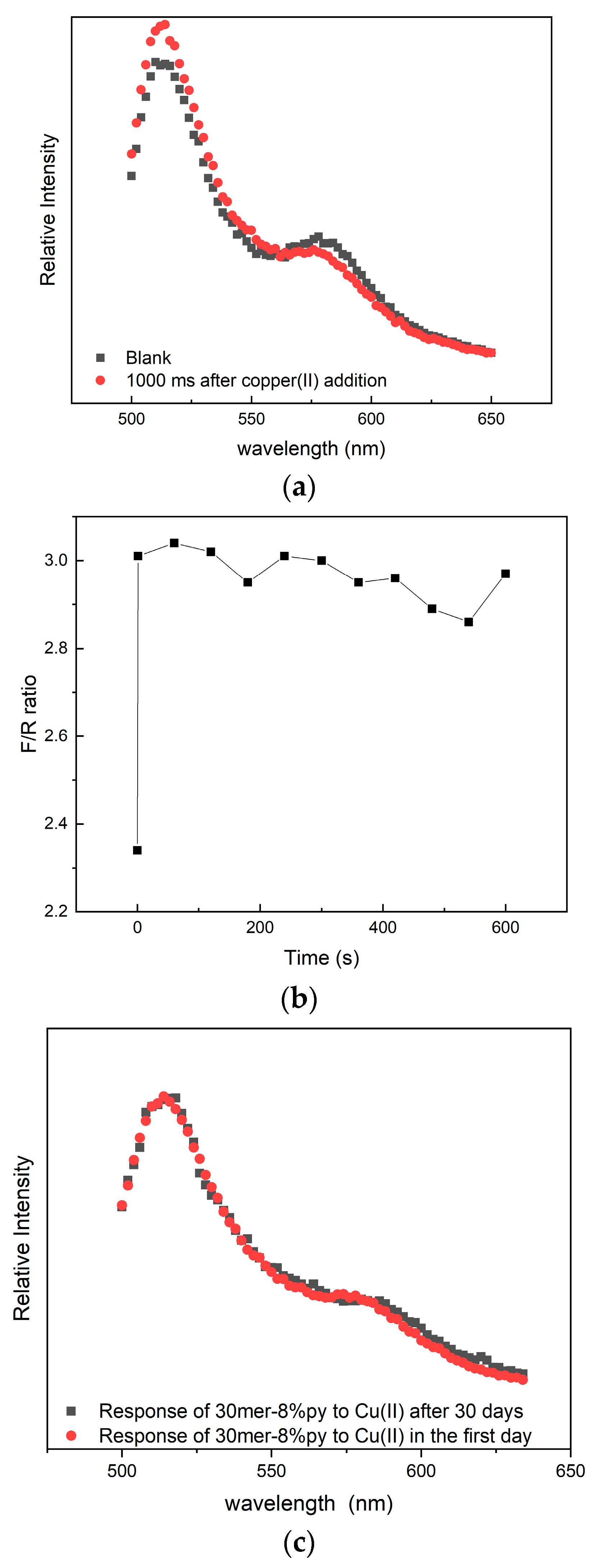
3.1. Fluorescent Response of FRET-Based Copper Ion Indicator
3.2. FRET Response with Time
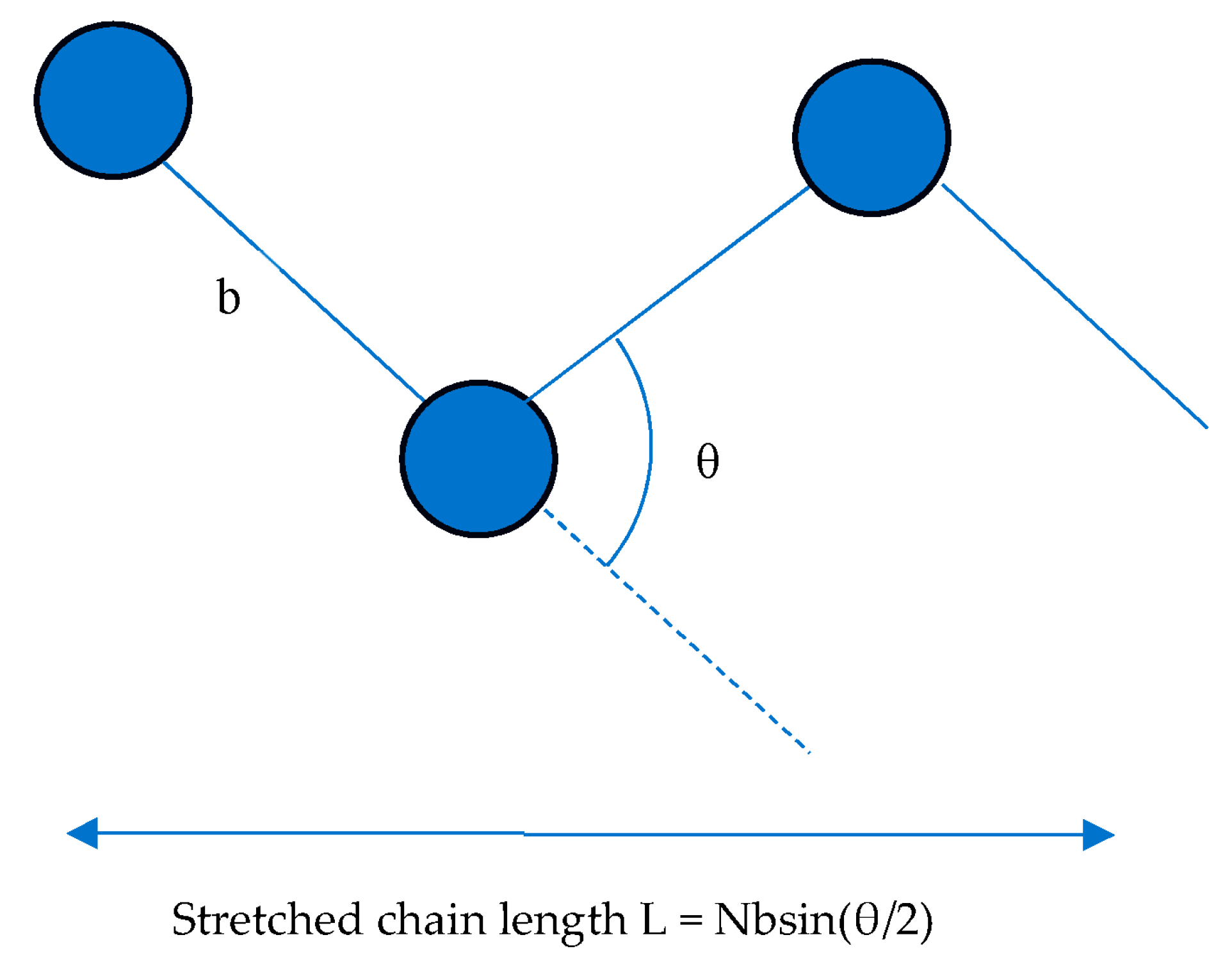
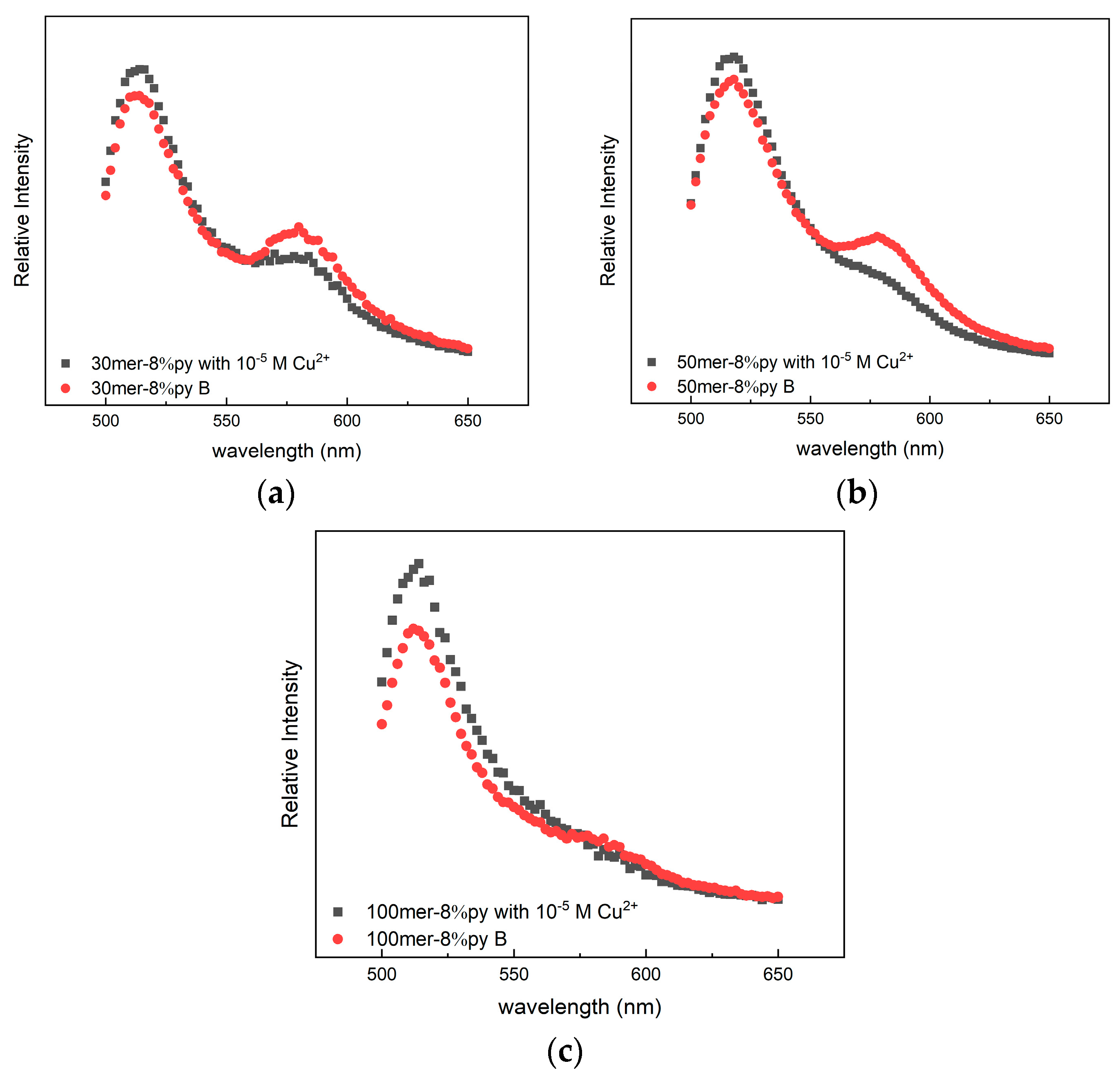
3.3. Change in the FRET Signal with the Polymer Chain Length
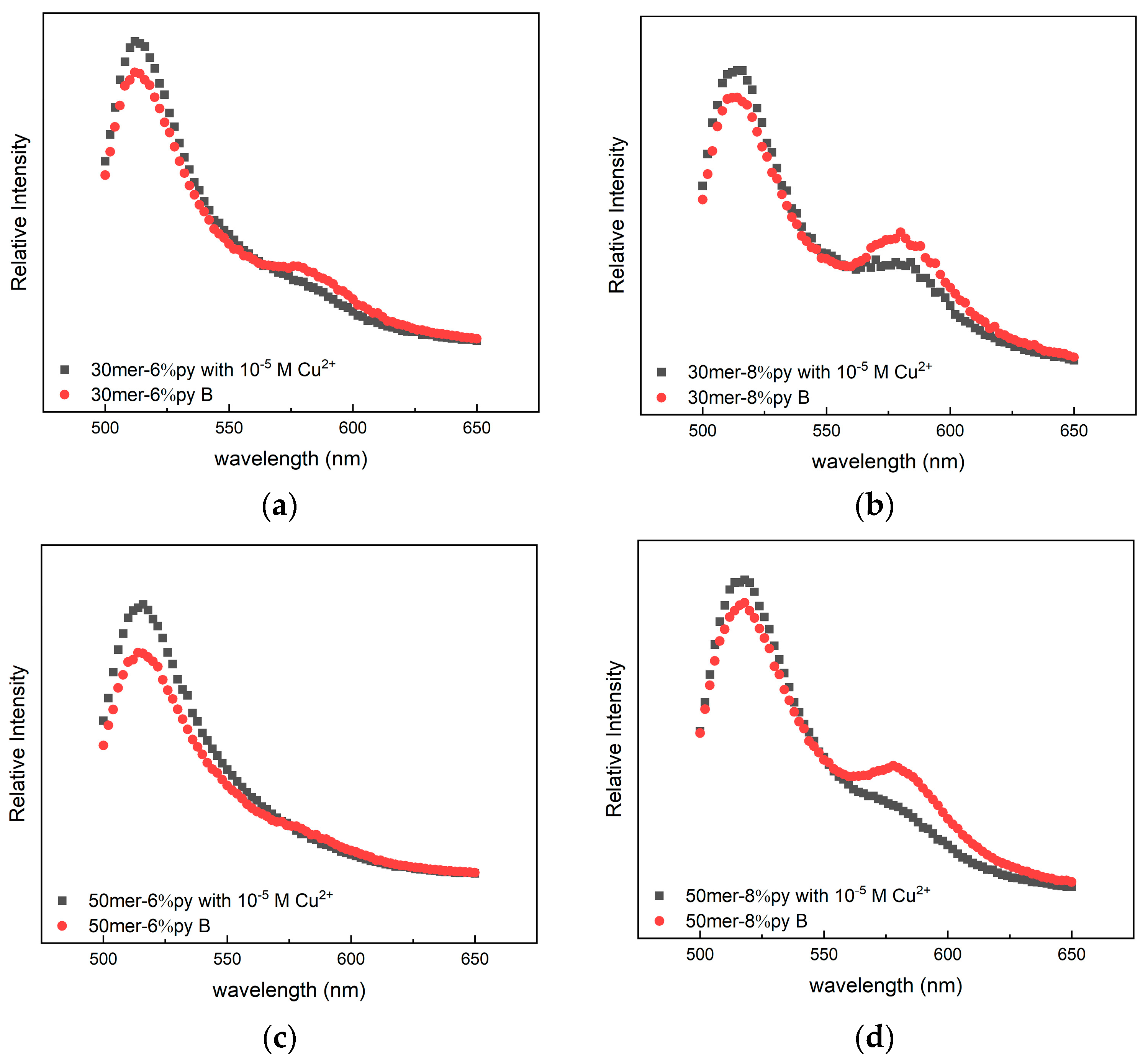
3.4. Change in the FRET Signal with the Amount of Bipyridine on the Chain
3.5. Copper ion FRET Calibration Curve for 30mer-8%py
3.6. Selectivity for other Transition Metals by 50mer-8%py
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, C.-M.; Campbell, P.G.C.; Wilkinson, K.J.; Zhao, C.-M.; Campbell, P.G.C.; Wilkinson, K.J. When are metal complexes bioavailable? Environ. Chem. 2016, 13, 425–433. [Google Scholar] [CrossRef]
- Parker, D.R.; Pedler, J.F. Reevaluating the free-ion activity model of trace metal availability to higher plants. Plant Soil 1997, 196, 223–228. [Google Scholar] [CrossRef]
- de Paiva Magalhães, D.; da Costa Marques, M.R.; Baptista, D.F.; Buss, D.F. Metal bioavailability and toxicity in freshwaters. Environ. Chem. Lett. 2015, 13, 69–87. [Google Scholar] [CrossRef]
- Flemming, C.A.; Trevors, J.T. Copper toxicity and chemistry in the environment: A review. Water Air Soil Pollut. 1989, 44, 143–158. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.J. The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 2009, 14, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Tiberg, C.; Smolders, E.; Fröberg, M.; Gustafsson, J.P.; Kleja, D.B. Combining a Standardized Batch Test with the Biotic Ligand Model to Predict Copper and Zinc Ecotoxicity in Soils. Environ. Toxicol. Chem. 2022, 41, 1540–1554. [Google Scholar] [CrossRef] [PubMed]
- Paquin, P.R.; Gorsuch, J.W.; Apte, S.; Batley, G.E.; Bowles, K.C.; Campbell, P.G.C.; Delos, C.G.; Di Toro, D.M.; Dwyer, R.L.; Galvez, F.; et al. The biotic ligand model: A historical overview. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 133, 3–35. [Google Scholar] [CrossRef]
- Nor, Y.M. Ecotoxicity of copper to aquatic biota: A review. Environ. Res. 1987, 43, 274–282. [Google Scholar] [CrossRef]
- de Polo, A.; Scrimshaw, M.D. Challenges for the development of a biotic ligand model predicting copper toxicity in estuaries and seas. Environ. Toxicol. Chem. 2012, 31, 230–238. [Google Scholar] [CrossRef]
- Faraji, M.; Yamini, Y.; Saleh, A.; Rezaee, M.; Ghambarian, M.; Hassani, R. A nanoparticle-based solid-phase extraction procedure followed by flow injection inductively coupled plasma-optical emission spectrometry to determine some heavy metal ions in water samples. Anal. Chim. Acta 2010, 659, 172–177. [Google Scholar] [CrossRef]
- Puthongkham, P.; Venton, B.J. Recent advances in fast-scan cyclic voltammetry. Cite This Anal. 2020, 145, 1087. [Google Scholar] [CrossRef]
- Staub, C.; Buffle, J.; Haerdi, W. Measurement of Complexation Properties of Metal Ions in Natural Conditions by Ultrafiltration: Influence of Various Factors on the Retention of Metals and Ligands by Neutral and Negatively Charged Membranes Characteristic Properties of the Membranes. Anal. Chem. 1984, 56, 2843–2849. [Google Scholar] [CrossRef]
- Temminghoff, E.J.M.; Plette, A.C.C.; Van Eck, R.; Van Riemsdijk, W.H. Determination of the chemical speciation of trace metals in aqueous systems by the Wageningen Donnan Membrane Technique. Anal. Chim. Acta 2000, 417, 149–157. [Google Scholar] [CrossRef]
- Vasylyeva, H.; Mironyuk, I.; Strilchuk, M.; Maliuk, I.; Savka, K.; Vasyliev, O. Adsorption and possibility of separation of heavy metal cations by strong cation exchange resin. Chem. Phys. Impact 2021, 3, 100056. [Google Scholar] [CrossRef]
- Que, E.L.; Domaille, D.W.; Chang, C.J. Metals in neurobiology: Probing their chemistry and biology with molecular imaging. Chem. Rev. 2008, 108, 1517–1549. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, H. Förster resonance energy transfer—A spectroscopic nanoruler: Principle and applications. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 20–30. [Google Scholar] [CrossRef]
- Hochreiter, B.; Garcia, A.P.; Schmid, J.A. Fluorescent Proteins as Genetically Encoded FRET Biosensors in Life Sciences. Sensors 2015, 15, 26281–26314. [Google Scholar] [CrossRef]
- Aoki, K.; Komatsu, N.; Hirata, E.; Kamioka, Y.; Matsuda, M. Stable expression of FRET biosensors: A new light in cancer research. Cancer Sci. 2012, 103, 614–619. [Google Scholar] [CrossRef]
- Clegg, R.M. Chapter 1 Förster resonance energy transfer—FRET what is it, why do it, and how it’s done. Lab. Tech. Biochem. Mol. Biol. 2009, 33, 1–57. [Google Scholar] [CrossRef]
- Deniz, A.A.; Laurence, T.A.; Beligere, G.S.; Dahan, M.; Martin, A.B.; Chemla, D.S.; Dawson, P.E.; Schultz, P.G.; Weiss, S. Single-molecule protein folding: Diffusion fluorescence resonance energy transfer studies of the denaturation of chymotrypsin inhibitor 2. Proc. Natl. Acad. Sci. USA 2000, 97, 5179–5184. [Google Scholar] [CrossRef]
- He, J.; Wink, S.; de Bont, H.; Le Dévédec, S.; Zhang, Y.; van de Water, B. FRET biosensor-based kinase inhibitor screen for ERK and AKT activity reveals differential kinase dependencies for proliferation in TNBC cells. Biochem. Pharmacol. 2019, 169, 113640. [Google Scholar] [CrossRef]
- Yao, S.; Jones, A.M.; Du, J.; Jackson, R.K.; Massing, J.O.; Kennedy, D.P.; Bencivenga, N.E.; Planalp, R.P.; Burdette, S.C.; Seitz, W.R. Intermolecular approach to metal ion indicators based on polymer phase transitions coupled to fluorescence resonance energy transfer. Analyst 2012, 137, 4734–4741. [Google Scholar] [CrossRef] [PubMed]
- Osambo, J.; Rudolf Seitz, W.; Kennedy, D.P.; Planalp, R.P.; Jones, A.M.; Jackson, R.K.; Burdette, S. Fluorescent Ratiometric Indicators Based on Cu(II)-Induced Changes in Poly(NIPAM) Microparticle Volume. Sensors 2013, 13, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Schild, H.G. Poly(N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Perrier, S. 50th Anniversary Perspective: RAFT Polymerization—A User Guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Tsukahara, Y.; Adachi, K. Telechelic Polymer: Preparation and Application. In Encyclopedia Polymeric Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2015; pp. 2491–2498. [Google Scholar] [CrossRef]
- Gu, C.; Du, Z.; Ouyang, X.; Xiang, H.; Zhu, M.; Luo, J.; Liu, G. Pinching Dynamics of Telechelic Associating and Coupling Polymers. Macromolecules 2022, 55, 7059–7070. [Google Scholar] [CrossRef]
- Li, W.; Li, S. Molecular imprinting: A versatile tool for separation, sensors and catalysis. Adv. Polym. Sci. 2007, 206, 191–210. [Google Scholar] [CrossRef]
- Pilvenyte, G.; Ratautaite, V.; Boguzaite, R.; Ramanavicius, A.; Viter, R.; Ramanavicius, S. Molecularly Imprinted Polymers for the Determination of Cancer Biomarkers. Int. J. Mol. Sci. 2023, 24, 4105. [Google Scholar] [CrossRef]
- Kobayashi, H.; Winkler, R.G. Universal conformational properties of polymers in ionic nanogels. Sci. Rep. 2016, 6, 19836. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, T.; Bramlitt, S.; Seitz, W.R. Silica covered stannic oxide nanoparticles—An easily prepared robust substrate for optical sensors. Nanotechnology 2022, 33, 105704. [Google Scholar] [CrossRef]
- Du, J.; Yao, S.; Seitz, W.R.; Bencivenga, N.E.; Massing, J.O.; Planalp, R.P.; Jackson, R.K.; Kennedy, D.P.; Burdette, S.C. A ratiometric fluorescent metal ion indicator based on dansyl labeled poly(N-isopropylacrylamide) responds to a quenching metal ion. Analyst 2011, 136, 5006–5011. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, Q.; Henschke, L.; Weber, G.; Weil, T. An efficient and versatile approach for the preparation of a rhodamine B ester bioprobe library. Dye. Pigment. 2012, 94, 296–303. [Google Scholar] [CrossRef]
- Wallyn, S.; Zhang, Z.; Driessen, F.; Pietrasik, J.; De Geest, B.G.; Hoogenboom, R.; Du Prez, F.E. Straightforward RAFT Procedure for the Synthesis of Heterotelechelic Poly(acrylamide)s. Macromol. Rapid Commun. 2014, 35, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.; Gathergood, N.; Heise, A. Synthesis of Polypeptide Block Copolymer Hybrids by the Combination of N-Carboxyanhydride Polymerization and RAFT. Macromol. Rapid Commun. 2013, 34, 1325–1329. [Google Scholar] [CrossRef] [PubMed]
- Scales, C.W.; Convertine, A.J.; McCormick, C.L. Fluorescent labelling of RAFT-generated poly(N-isopropylacrylamide) via a facile maleimide-thiol coupling reaction. Biomacromolecules 2006, 7, 1389–1392. [Google Scholar] [CrossRef]
- Harrison, J.S.; Higgins, C.D.; O’Meara, M.J.; Koellhoffer, J.F.; Kuhlman, B.A.; Lai, J.R. Role of Electrostatic Repulsion in Controlling pH-Dependent Conformational Changes of Viral Fusion Proteins. Structure 2013, 21, 1085–1096. [Google Scholar] [CrossRef]
- Saha, J.; Datta Roy, A.; Dey, D.; Chakraborty, S.; Bhattacharjee, D.; Paul, P.; Arshad Hussain, S. Investigation of Fluorescence Resonance Energy Transfer between Fluorescein and Rhodamine 6G. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 143–149. [Google Scholar] [CrossRef]
- Abbott, L.J.; Tucker, A.K.; Stevens, M.J. Single Chain structure of a poly(N -isopropylacrylamide) surfactant in water. J. Phys. Chem. B 2015, 119, 3837–3845. [Google Scholar] [CrossRef]
- Dietz, J.D.; Nan, K.; Hoy, R.S. Unexpected Ductility in Semiflexible Polymer Glasses with Entanglement Length Equal to Their Kuhn Length. Phys. Rev. Lett. 2022, 129, 127801. [Google Scholar] [CrossRef]
- Dinic, J.; Sharma, V. Flexibility, Extensibility, and Ratio of Kuhn Length to Packing Length Govern the Pinching Dynamics, Coil-Stretch Transition, and Rheology of Polymer Solutions. Macromolecules 2020, 53, 4821–4835. [Google Scholar] [CrossRef]
- Heyduk, T. Measuring protein conformational changes by FRET/LRET. Curr. Opin. Biotechnol. 2002, 13, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Skolnick, J.; Fixman, M. Electrostatic Persistence Length of a Wormlike Polyelectrolyte. Macromolecules 2023, 19, 2. [Google Scholar] [CrossRef]
- Odijk, T. Polyelectrolytes near the rod limit. J. Polym. Sci. Polym. Phys. Ed. 1977, 15, 477–483. [Google Scholar] [CrossRef]
- Dobrynin, A.V. Electrostatic persistence length of semiflexible and flexible polyelectrolytes. Macromolecules 2005, 38, 9304–9314. [Google Scholar] [CrossRef]
- Martell, A.E.; Smith, R.M. Critical Stability Constants. In Critical Stability Constants; Plenum Press: New York, NY, USA, 1982. [Google Scholar] [CrossRef]



| RCTA | NIPAM | AIBN | Bpy | |
|---|---|---|---|---|
| 100mer-8%py | 5.12 mg (0.00602 mmol) | 140 mg (1.24 mmol) | 0.102 mg (0.000621 mmol) | 30.0 mg (0.102 mmol) |
| 100mer-6%py | 5.12 mg (0.00602 mmol) | 140 mg (1.24 mmol) | 0.102 mg (0.000621 mmol) | 22.5 mg (0.0773 mmol) |
| 50mer-8%py | 6.14 mg (0.00721 mmol) | 140 mg (1.24 mmol) | 0.122 mg (0.000682 mmol) | 30.0 mg (0.102 mmol) |
| 50mer-6%py | 6.14 mg (0.00721 mmol) | 140 mg (1.24 mmol) | 0.122 mg (0.000682 mmol) | 22.5 mg (0.0773 mmol) |
| 30mer-8%py | 10.2 mg (0.0119 mmol) | 140 mg (1.24 mmol) | 0.203 mg (0.00124 mmol) | 30.0 mg (0.102 mmol) |
| 30mer-6%py | 10.2 mg (0.0119 mmol) | 140 mg (1.24 mmol) | 0.203 mg (0.00124 mmol) | 22.5 mg (0.0773 mmol) |
| Target Composition (RCTA:Bipyridine-Ligand:NIPAM) | Actual Composition (RCTA:Bipyridine-Ligand:NIPAM | |
|---|---|---|
| 100mer-8%py | 1:8:100 | 1:7.0:117 |
| 100mer-6%py | 1:6:100 | 1:6.0:105 |
| 50mer-8%py | 1:4:50 | 1:4.3:64 |
| 50mer-6%py | 1:3:50 | 1:2.5:52 |
| 30mer-8%py | 1:2.4:30 | 1:2.4:25 |
| 30mer-6%py | 1:1.8:30 | 1:1.6:26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Si, B.; Cote, N.; Planalp, R.P.; Seitz, R. A Telechelic Fluorescent Indicator Based on Polymer Conformational Change for Free Copper(II) Ions. Sensors 2023, 23, 9476. https://doi.org/10.3390/s23239476
Chen Y, Si B, Cote N, Planalp RP, Seitz R. A Telechelic Fluorescent Indicator Based on Polymer Conformational Change for Free Copper(II) Ions. Sensors. 2023; 23(23):9476. https://doi.org/10.3390/s23239476
Chicago/Turabian StyleChen, Yuan, Bo Si, Noah Cote, Roy P. Planalp, and Rudi Seitz. 2023. "A Telechelic Fluorescent Indicator Based on Polymer Conformational Change for Free Copper(II) Ions" Sensors 23, no. 23: 9476. https://doi.org/10.3390/s23239476
APA StyleChen, Y., Si, B., Cote, N., Planalp, R. P., & Seitz, R. (2023). A Telechelic Fluorescent Indicator Based on Polymer Conformational Change for Free Copper(II) Ions. Sensors, 23(23), 9476. https://doi.org/10.3390/s23239476







