A Cell State Monitoring System with Integrated In Situ Imaging and pH Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biochip Design and Manufacturing
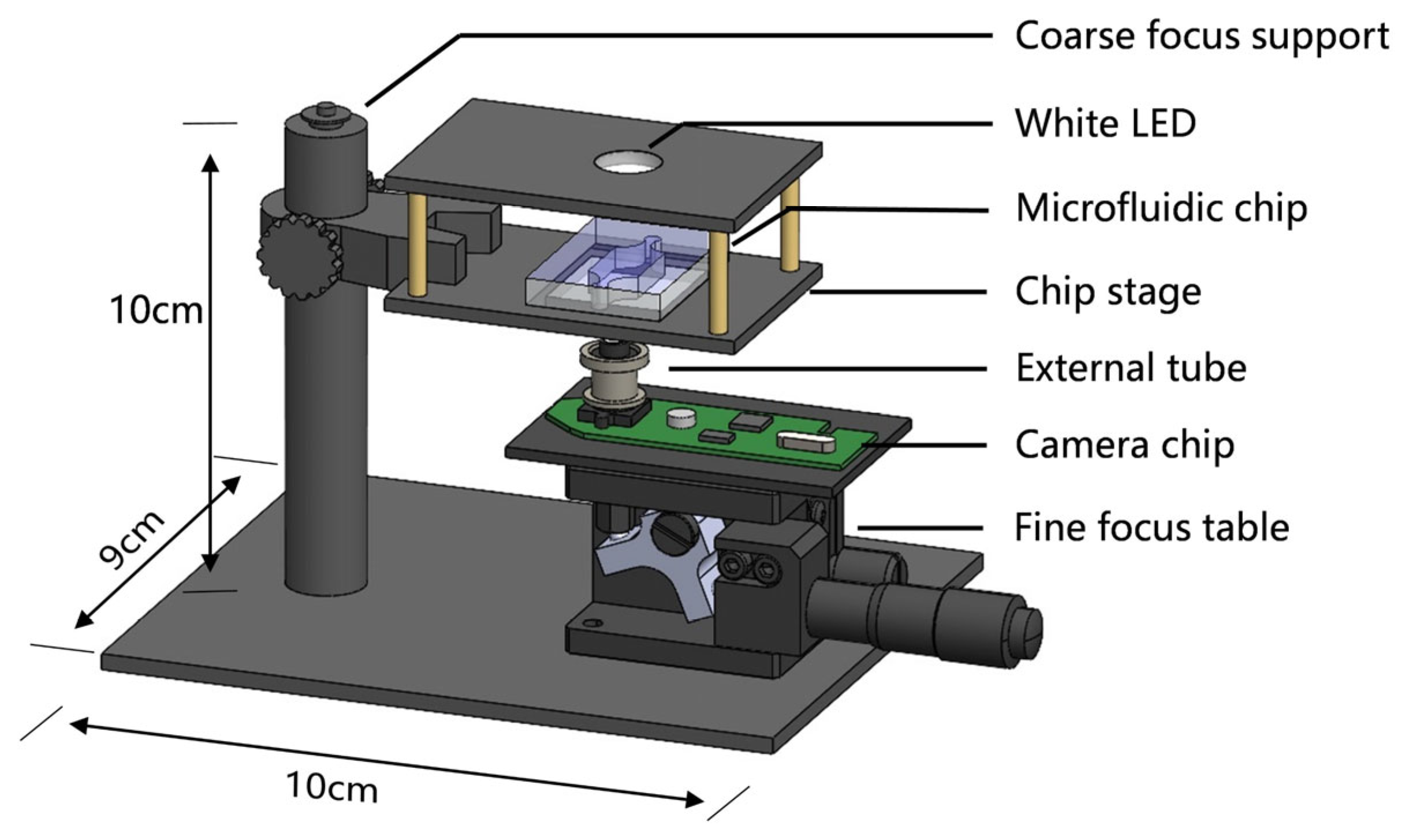
2.2. Mini-Microscope Design and Construction
2.3. Fabrication of pH Sensor
2.3.1. System Principle and Design
2.3.2. Detection Chip Design and Fabrication
2.4. Computer Program Design for Control of the Monitoring System
2.5. In-Chip Cell Culture
3. Results
3.1. Characterization of the Mini-Microscope
3.2. Calibration of pH Sensor
3.3. 24 h Continuous In Situ Observation of Cells in Biochip
3.4. pH Test of Cell Culture Medium in Chip
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitchell, P. Microfluidics—Downsizing large-scale biology. Nat. Biotechnol. 2001, 19, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Manz, A.; Harrison, D.J.; Verpoorte, E.M.J.; Fettinger, J.C.; Paulus, A.; Lüdi, H.; Widmer, H.M. Planar chips technology for miniaturization and integration of separation techniques into monitoring systems. J. Chromatogr. A 1992, 593, 253–258. [Google Scholar] [CrossRef]
- Chang, W.J.; Akin, D.; Sedlak, M.; Ladisch, M.R.; Bashir, R. Poly(dimethylsiloxane) (PDMS) and silicon hybrid biochip for bacterial culture. Biomed. Microdevices 2003, 5, 281–290. [Google Scholar] [CrossRef]
- Muguruma, M.; Teraoka, S.; Miyahara, K.; Ueda, A.; Asaoka, M.; Okazaki, M.; Kawate, T.; Kuroda, M.; Miyagi, Y.; Ishikawa, T. Differences in drug sensitivity between two-dimensional and three-dimensional culture systems in triple-negative breast cancer cell lines. Biochem. Biophys. Res. Commun. 2020, 533, 268–274. [Google Scholar] [CrossRef]
- Tutty, M.A.; Holmes, S.; Prina-Mello, A. Cancer Cell Culture: The Basics and Two-Dimensional Cultures. Methods Mol. Biol. 2023, 2645, 3–40. [Google Scholar] [CrossRef]
- Halldorsson, S.; Lucumi, E.; Gomez-Sjoberg, R.; Fleming, R.M.T. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens. Bioelectron. 2015, 63, 218–231. [Google Scholar] [CrossRef]
- Bricks, T.; Paullier, P.; Legendre, A.; Fleury, M.-J.; Zeller, P.; Merlier, F.; Anton, P.M.; Leclerc, E. Development of a new microfluidic platform integrating co-cultures of intestinal and liver cell lines. Toxicol. Vitr. 2014, 28, 885–895. [Google Scholar] [CrossRef]
- Cho, C.F.; Wolfe, J.M.; Fadzen, C.M.; Calligaris, D.; Hornburg, K.; Chiocca, E.A.; Agar, N.Y.R.; Pentelute, B.L.; Lawler, S.E. Blood-brain-barrier spheroids as an in vitro screening platform for brain-penetrating agents. Nat. Commun. 2017, 8, 15623. [Google Scholar] [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef]
- Huh, D.; Leslie, D.C.; Matthews, B.D.; Fraser, J.P.; Jurek, S.; Hamilton, G.A.; Thorneloe, K.S.; McAlexander, M.A.; Ingber, D.E. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci. Transl. Med. 2012, 4, 159ra147. [Google Scholar] [CrossRef]
- Hell, S.W.; Wichmann, J. Breaking the diffraction resolution limit by stimulated emission: Stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 1994, 19, 780–782. [Google Scholar] [CrossRef] [PubMed]
- Klar, T.A.; Hell, S.W. Subdiffraction resolution in far-field fluorescence microscopy. Opt. Lett. 1999, 24, 954–956. [Google Scholar] [CrossRef]
- Jan, R.; Chaudhry, G.E. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef]
- Stout, A.J.; Mirliani, A.B.; Rittenberg, M.L.; Shub, M.; White, E.C.; Yuen, J.S.K., Jr.; Kaplan, D.L. Simple and effective serum-free medium for sustained expansion of bovine satellite cells for cell cultured meat. Commun. Biol. 2022, 5, 466. [Google Scholar] [CrossRef] [PubMed]
- Jue, C.; Min, Z.; Zhisheng, Z.; Lin, C.; Yayun, Q.; Xuanyi, W.; Feng, J.; Haibo, W.; Youyang, S.; Tadashi, H.; et al. COE inhibits vasculogenic mimicry in hepatocellular carcinoma via suppressing Notch1 signaling. J. Ethnopharmacol. 2017, 208, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Stewart, H.; Bartlett, C.; Ross-Thriepland, D.; Shaw, J.; Griffin, S.; Harris, M. A novel method for the measurement of hepatitis C virus infectious titres using the IncuCyte ZOOM and its application to antiviral screening. J. Virol. Methods 2015, 218, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Brehove, M.; Rogers, C.; Menon, R.; Minor, P.; Allington, J.; Lam, A.; Vielmetter, J.; Menon, N. Cell monitoring with optical coherence tomography. Cytotherapy 2023, 25, 120–124. [Google Scholar] [CrossRef]
- Fu, R.; Su, Y.; Wang, R.; Lin, X.; Jin, X.; Yang, H.; Du, W.; Shan, X.; Lv, W.; Huang, G. Single cell capture, isolation, and long-term in-situ imaging using quantitative self-interference spectroscopy. Cytom. A 2021, 99, 601–609. [Google Scholar] [CrossRef]
- Greenbaum, A.; Zhang, Y.; Feizi, A.; Chung, P.-L.; Luo, W.; Kandukuri, S.R.; Ozcan, A. Wide-field computational imaging of pathology slides using lens-free on-chip microscopy. Sci. Transl. Med. 2014, 6, 267ra175. [Google Scholar] [CrossRef]
- Wei, Q.; Luo, W.; Chiang, S.; Kappel, T.; Mejia, C.; Tseng, D.; Chan, R.Y.; Yan, E.; Qi, H.; Shabbir, F.; et al. Imaging and sizing of single DNA molecules on a mobile phone. ACS Nano 2014, 8, 12725–12733. [Google Scholar] [CrossRef]
- Mir, S.M.; Chen, J.; Pinezich, M.R.; O’Neill, J.D.; Huang, S.X.L.; Vunjak-Novakovic, G.; Kim, J. Imaging-guided bioreactor for de-epithelialization and long-term cultivation of ex vivo rat trachea. Lab. A Chip 2022, 22, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Ribas, J.; Nadhman, A.; Aleman, J.; Selimovic, S.; Lesher-Perez, S.C.; Wang, T.; Manoharan, V.; Shin, S.R.; Damilano, A.; et al. A cost-effective fluorescence mini-microscope for biomedical applications. Lab. Chip 2015, 15, 3661–3669. [Google Scholar] [CrossRef] [PubMed]
- Andreazza, N.L.; Abreu, I.N.; Sawaya, A.C.; Eberlin, M.N.; Mazzafera, P. Production of imidazole alkaloids in cell cultures of jaborandi as affected by the medium pH. Biotechnol. Lett. 2009, 31, 607–614. [Google Scholar] [CrossRef]
- Neri, D.; Supuran, C.T. Interfering with pH regulation in tumours as a therapeutic strategy. Nat. Rev. Drug Discov. 2011, 10, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Messenger, A.G. Extracellular-Matrix and the Hair-Growth Cycle. J. Investig. Dermatol. 1991, 96, S75. [Google Scholar] [CrossRef] [PubMed]
- McAdams, T.A.; Miller, W.M.; Papoutsakis, E.T. pH is a potent modulator of erythroid differentiation. Br. J. Haematol. 1998, 103, 317–325. [Google Scholar] [CrossRef]
- Teo, A.; Mantalaris, A.; Lim, M. Influence of culture pH on proliferation and cardiac differentiation of murine embryonic stem cells. Biochem. Eng. J. 2014, 90, 8–15. [Google Scholar] [CrossRef]
- Refresh cell culture. Nat. Biomed. Eng. 2021, 5, 783–784. [CrossRef]
- Lewinska, A.; Wnuk, M.; Slota, E.; Bartosz, G. Total anti-oxidant capacity of cell culture media. Clin. Exp. Pharmacol. Physiol. 2007, 34, 781–786. [Google Scholar] [CrossRef]
- Kieninger, J.; Weltin, A.; Flamm, H.; Urban, G.A. Microsensor systems for cell metabolism—from 2D culture to organ-on-chip. Lab. Chip 2018, 18, 1274–1291. [Google Scholar] [CrossRef]
- Li, Y.; Huang, A.; Zhang, T.; Wen, L.; Shi, Z.; Shi, L. A pH Monitoring Algorithm for Orifice Plate Culture Medium. Appl. Sci. 2022, 12, 7560. [Google Scholar] [CrossRef]
- Müller, B.; Sulzer, P.; Walch, M.; Zirath, H.; Buryška, T.; Rothbauer, M.; Ertl, P.; Mayr, T. Measurement of respiration and acidification rates of mammalian cells in thermoplastic microfluidic devices. Sens. Actuators B Chem. 2021, 334, 129664. [Google Scholar] [CrossRef]
- Fuchs, S.; van Helden, R.W.J.; Wiendels, M.; de Graaf, M.N.S.; Orlova, V.V.; Mummery, C.L.; van Meer, B.J.; Mayr, T. On-chip analysis of glycolysis and mitochondrial respiration in human induced pluripotent stem cells. Mater. Today Bio 2022, 17, 100475. [Google Scholar] [CrossRef]
- Mousavi Shaegh, S.A.; De Ferrari, F.; Zhang, Y.S.; Nabavinia, M.; Binth Mohammad, N.; Ryan, J.; Pourmand, A.; Laukaitis, E.; Banan Sadeghian, R.; Nadhman, A.; et al. A microfluidic optical platform for real-time monitoring of pH and oxygen in microfluidic bioreactors and organ-on-chip devices. Biomicrofluidics 2016, 10, 044111. [Google Scholar] [CrossRef]
- Yin, H.; Tong, Z.; Shen, C.; Xu, X.; Ma, H.; Wu, Z.; Qi, Y.; Mao, H. Micro-PCR chip-based multifunctional ultrafast SARS-CoV-2 detection platform. Lab. A Chip 2022, 22, 2671–2681. [Google Scholar] [CrossRef]
- Prokopovic, V.Z.; Vikulina, A.S.; Sustr, D.; Shchukina, E.M.; Shchukin, D.G.; Volodkin, D.V. Binding Mechanism of the Model Charged Dye Carboxyfluorescein to Hyaluronan/Polylysine Multilayers. ACS Appl. Mater. Interfaces 2017, 9, 38908–38918. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhang, R.; Xu, F.; Yang, J.; Zhou, L.; Mao, H. A Cell State Monitoring System with Integrated In Situ Imaging and pH Detection. Sensors 2023, 23, 9340. https://doi.org/10.3390/s23239340
Li Z, Zhang R, Xu F, Yang J, Zhou L, Mao H. A Cell State Monitoring System with Integrated In Situ Imaging and pH Detection. Sensors. 2023; 23(23):9340. https://doi.org/10.3390/s23239340
Chicago/Turabian StyleLi, Zening, Rongtao Zhang, Fangliang Xu, Jian Yang, Lin Zhou, and Hongju Mao. 2023. "A Cell State Monitoring System with Integrated In Situ Imaging and pH Detection" Sensors 23, no. 23: 9340. https://doi.org/10.3390/s23239340
APA StyleLi, Z., Zhang, R., Xu, F., Yang, J., Zhou, L., & Mao, H. (2023). A Cell State Monitoring System with Integrated In Situ Imaging and pH Detection. Sensors, 23(23), 9340. https://doi.org/10.3390/s23239340






