Non-Destructive Direct Pericarp Thickness Measurement of Sorghum Kernels Using Extended-Focus Optical Coherence Microscopy
Abstract
1. Introduction
2. Materials and Methods
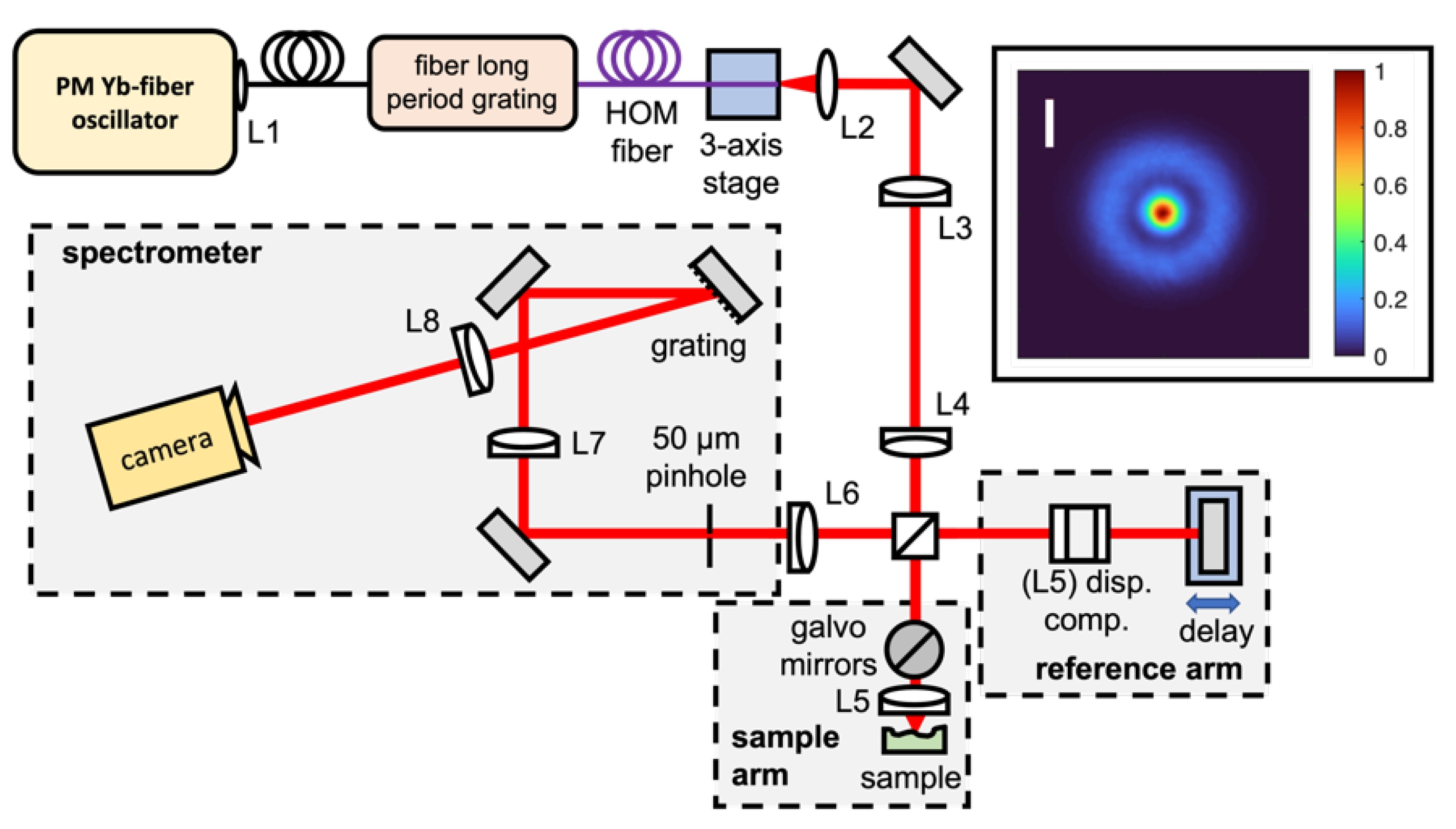
2.1. Bessel-like Beam High-Resolution Fourier Domain OCM Setup
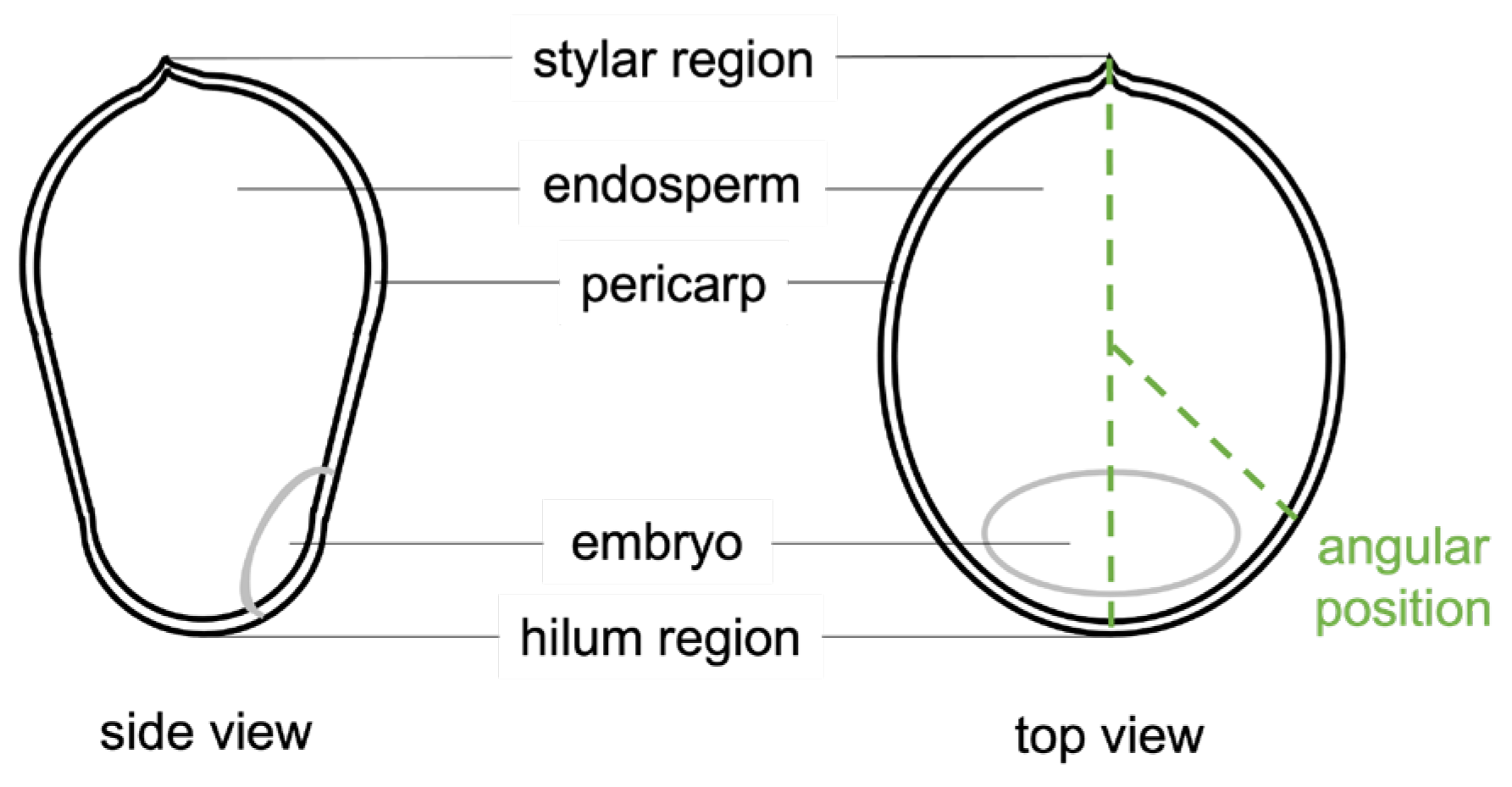
2.2. Seed Material
2.3. Validation Measurements
2.4. FD-OCM Imaging and Data-Processing
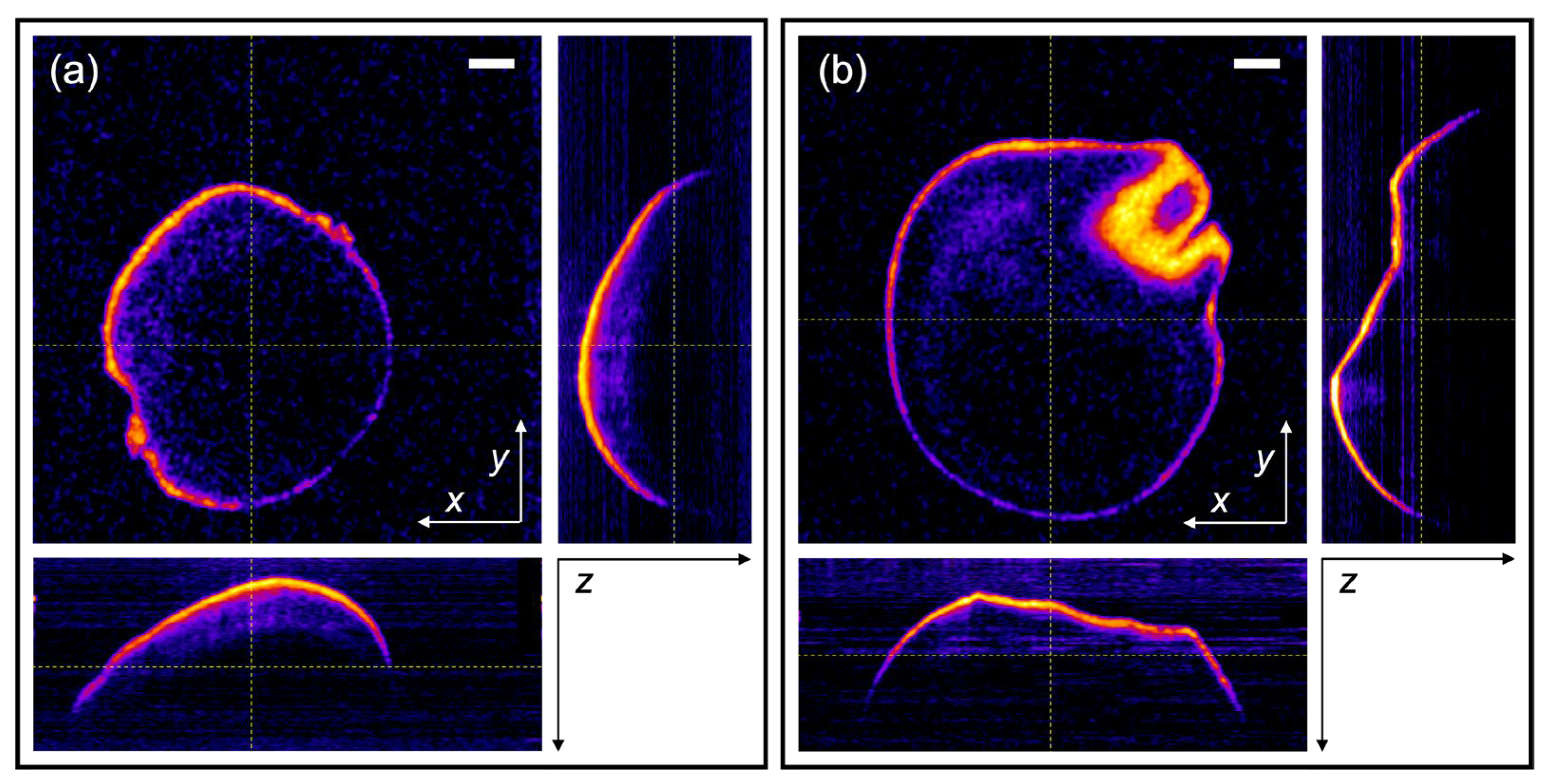
2.5. Data Analysis and Pericarp Thickness Measurements
2.6. Statistics
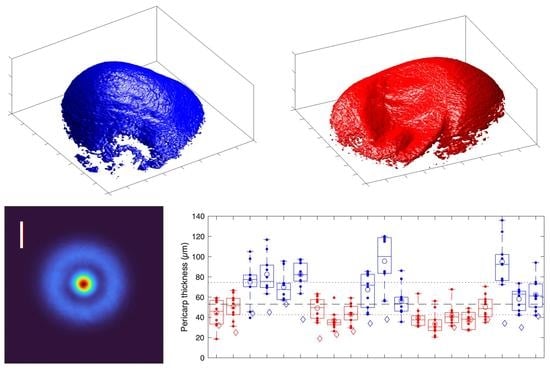
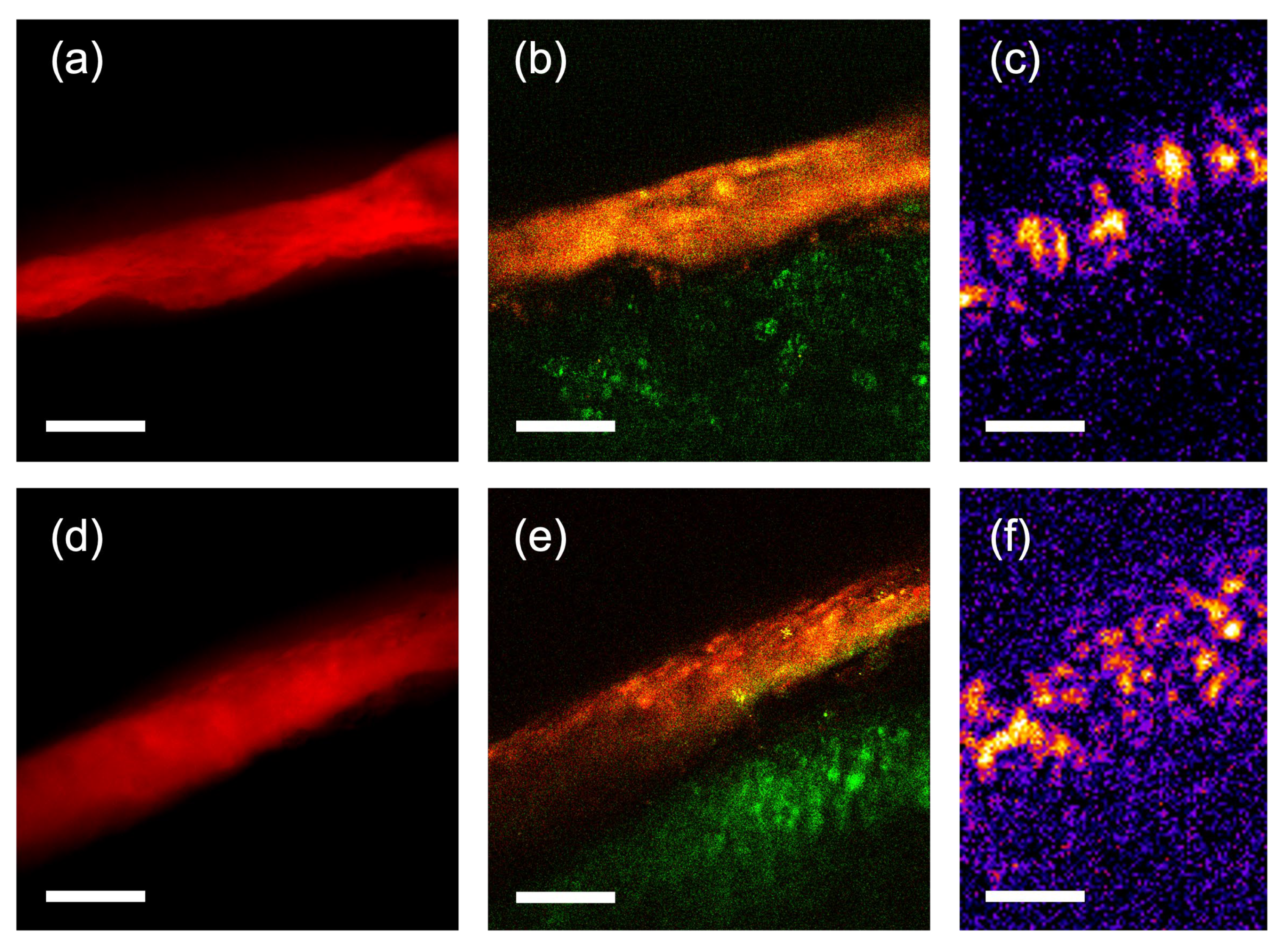
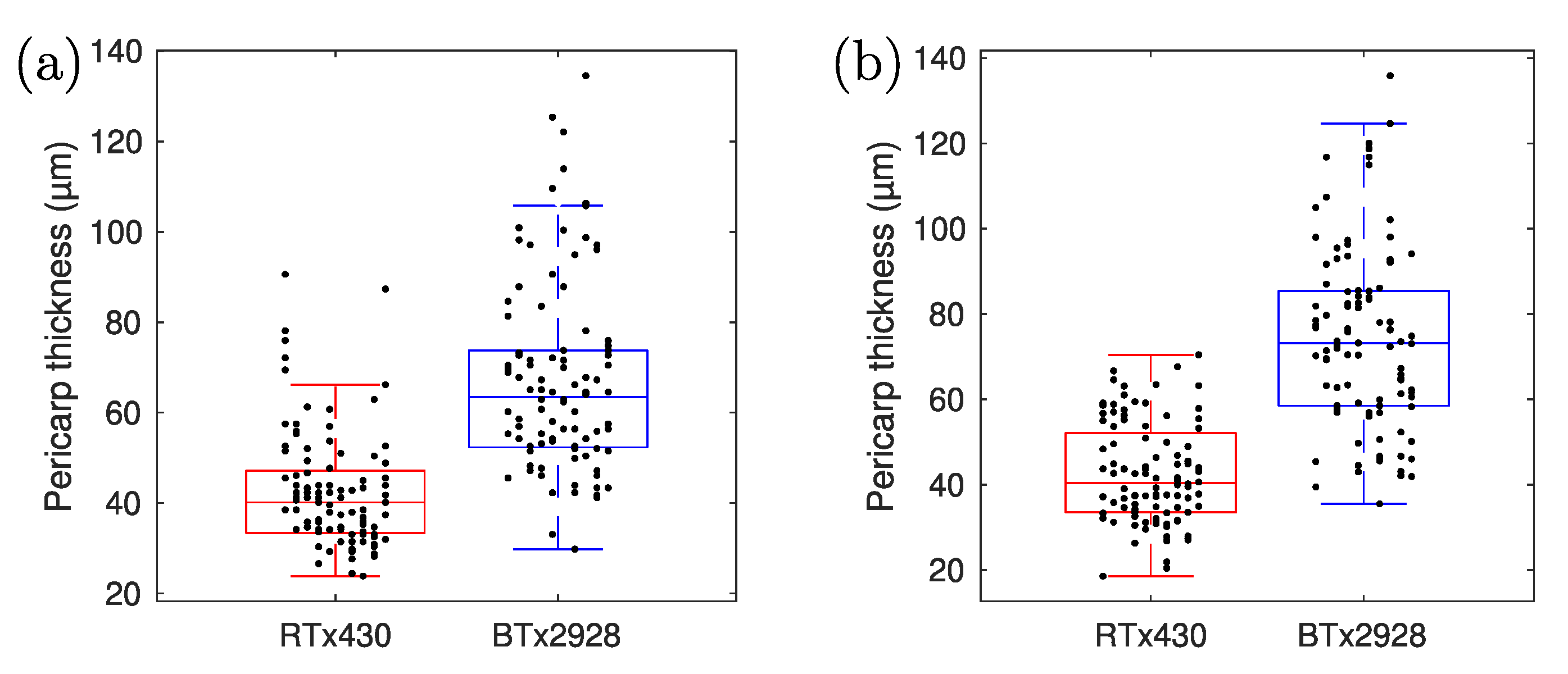
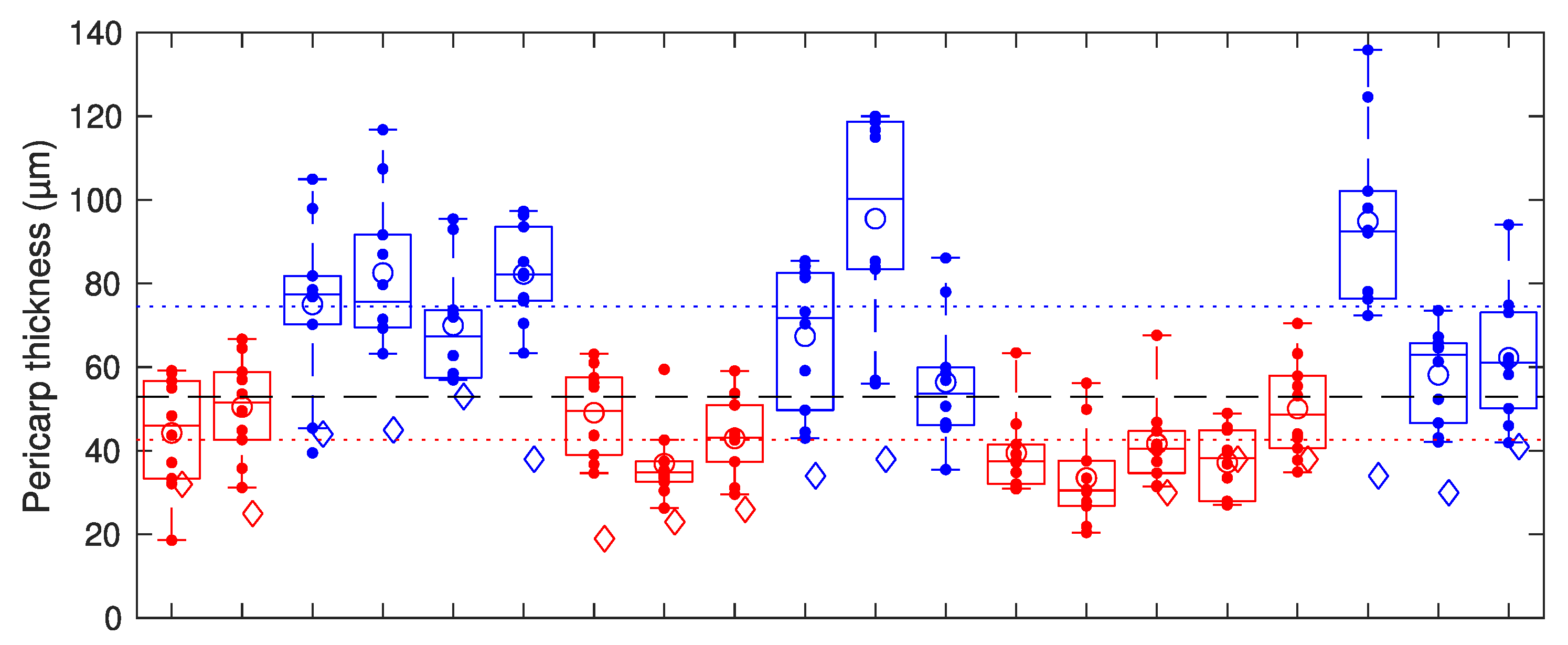
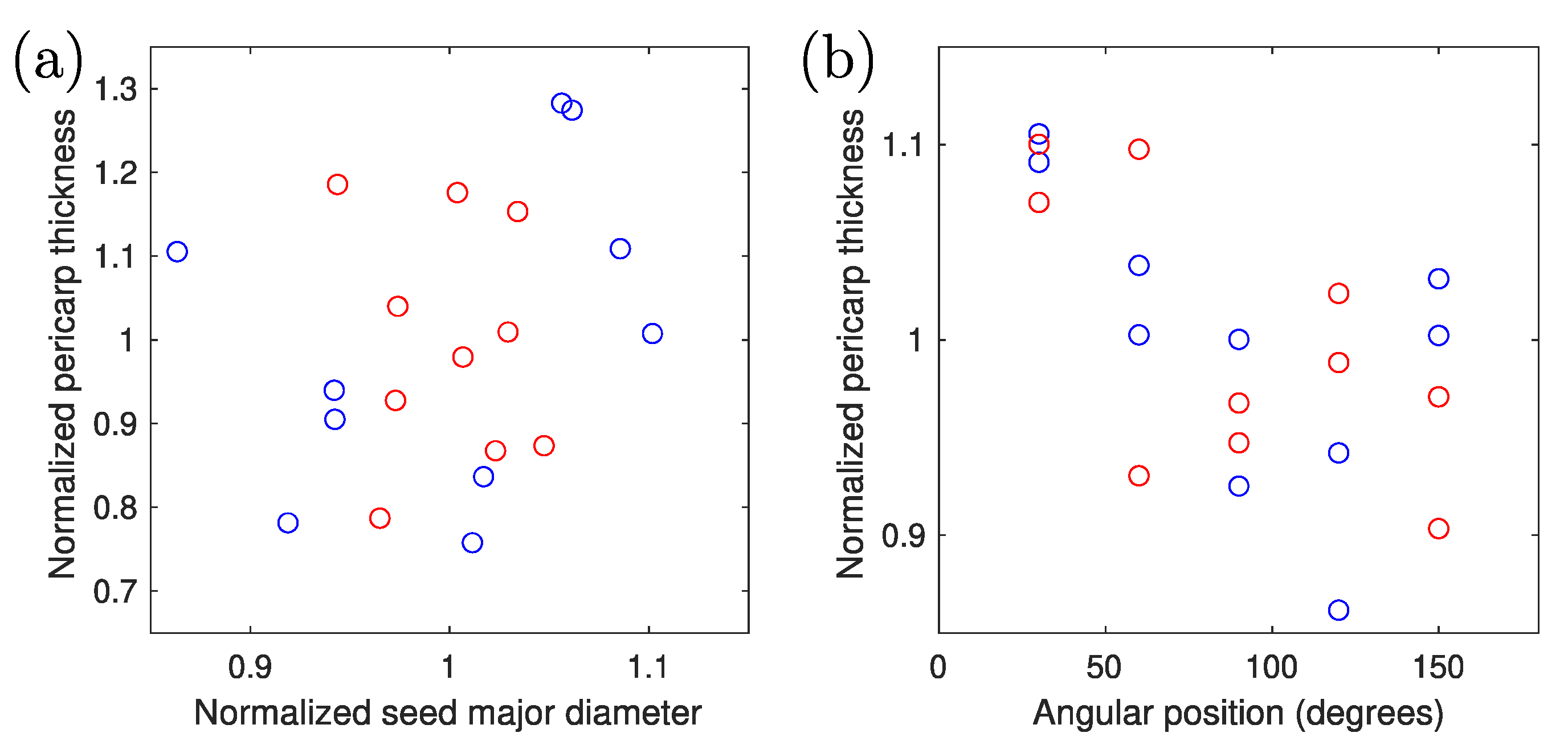
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Smith, C.W.; Frederiksen, R.A. Sorghum: Origin, History, Technology, and Production; John Wiley & Sons: New York, NY, USA, 2000; Volume 2. [Google Scholar]
- FAOSTAT. Food and Agriculure Organization of United Nations. Available online: https://www.fao.org/faostat/en/#home (accessed on 22 December 2022).
- Anglani, C. Sorghum for human food–A review. Plant Foods Hum. Nutr. 1998, 52, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Hoseney, R.C.; Davis, A.B.; Harbers, L.H. Pericarp and endosperm structure of sorghum shown by scanning electron microscopy. Cereal Chem. 1974, 51, 552–558. [Google Scholar]
- Earp, C.F.; McDonough, C.M.; Rooney, L.W. Microscopy of pericarp development in the caryopsis of Sorghum bicolor (L.) Moench. J. Cereal Sci. 2004, 39, 21–27. [Google Scholar] [CrossRef]
- Nooden, L.D.; Blakley, K.A.; Grzybowski, J.M. Control of seed coat thickness and permeability in soybean: A possible adaptation to stress. Plant Physiol. 1985, 79, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Scheuring, J.F.; Sidiibi, S.; Rooney, L.W.; Earp, C.F. Sorghum Pericarp Thickness and Its Relation to Decortication in a Wooden Mortar and Pestle. Cereal Chem. 1983, 60, 86–89. [Google Scholar]
- Glueck, J.A.; Rooney, L.W. Chemistry and structure of grain in relation to mold resistance. In Proceedings of the International Workshop on Sorghum Disease, Hyderabad, India, 11–15 December 1978; pp. 119–140. [Google Scholar]
- Mohamed, A.A.; Ashman, R.B.; Kirleis, A.W. Pericarp thickness and other kernel physical characteristics relate to microwave popping quality of popcorn. J. Food Sci. 1993, 58, 342–346. [Google Scholar] [CrossRef]
- Gomez, M.I.; Obilana, A.B.; Martin, D.F.; Madzvamuse, M.; Monyo, E.S. Manual of Laboratory Procedures for Quality Evaluation of Sorghum and Pearl Millet. Technical Manual no.2.; International Crops Research Institute for the Semi-Arid Tropics: Andhra Pradesh, India, 1997. [Google Scholar]
- Guindo, D.; Davrieux, F.; Teme, N.; Vaksmann, M.; Doumbia, M.; Fliedel, G.; Bastianelli, D.; Verdeil, J.L.; Mestres, C.; Kouressy, M.; et al. Pericarp thickness of sorghum whole grain is accurately predicted by NIRS and can affect the prediction of other grain quality parameters. J. Cereal Sci. 2016, 69, 218–227. [Google Scholar] [CrossRef]
- Earp, C.F.; Rooney, L.W. Scanning Electron Microscopy of the Pericarp and Testa of Several Sorghum Varieties. Food Structure 1982, 1, 125–134. [Google Scholar]
- Crozier, D.; Riera-Lizarazu, O.; Rooney, W.L. Application of X-ray computed tomography to analyze the structure of sorghum grain. Plant Methods 2022, 18, 3. [Google Scholar] [CrossRef]
- Drexler, W.; Fujimoto, J.G. (Eds.) Optical Coherence Tomography, Technology and Applications; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Fercher, A.F.; Hitzenberger, C.K.; Kamp, G.; El-Zaiat, S.Y. Measurement of intraocular distances by backscattering spectral interferometry. Opt. Commun. 1995, 117, 43–48. [Google Scholar] [CrossRef]
- Clements, J.C.; Zvyagin, A.V.; Silva, K.K.M.B.D.; Wanner, T.; Sampson, D.D.; Cowling, W.A. Optical coherence tomography as a novel tool for non-destructive measurement of the hull thickness of lupin seeds. Plant Breed. 2004, 123, 266–270. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Li, X.; Zhao, Z.; Zhang, Z.; Lin, H.; Kang, D.; Shen, Y. Nondestructive in situ monitoring of pea seeds germination using optical coherence tomography. Plant Direct 2022, 6, e428. [Google Scholar] [CrossRef]
- Lee, C.; Lee, S.Y.; Kim, J.Y.; Jung, H.Y.; Kim, J. Optical sensing method for screening disease in melon seeds by using optical coherence tomography. Sensors 2011, 11, 9467–9477. [Google Scholar] [CrossRef]
- Lee, S.; Lee, C.; Kim, J.; Jung, H.Y. Application of optical coherence tomography to detect Cucumber green mottle mosaic virus (CGMMV) infected cucumber seed. Hortic. Environ. Biotechnol. 2012, 53, 428–433. [Google Scholar] [CrossRef]
- Fan, C.; Yao, G. 3D imaging of tomato seeds using frequency domain optical coherence tomography. In Proc. SPIE 8369, Sensing for Agriculture and Food Quality and Safety IV, 83690F; SPIE: Bellingham, WA, USA, 2012. [Google Scholar] [CrossRef]
- Bharti; Taeil, Y.; Byeong, H.L. Identification of Fungus-infected Tomato Seeds Based on Full-Field Optical Coherence Tomography. Curr. Opt. Photonics 2019, 3, 571–576. [Google Scholar] [CrossRef]
- Joshi, D.; Butola, A.; Kanade, S.R.; Prasad, D.K.; Amitha Mithra, V.; Singh, N.K.; Bisht, D.S.; Mehta, D.S. Label-free non-invasive classification of rice seeds using optical coherence tomography assisted with deep neural network. Opt. Laser Technol. 2021, 137, 106861. [Google Scholar] [CrossRef]
- De Silva, Y.S.K.; Rajagopalan, M.; Kadono, H.; Li, D. Positive and negative phenotyping of increasing Zn concentrations by Biospeckle Optical Coherence Tomography in speedy monitoring on lentil (Lens culinaris) seed germination and seedling growth. Plant Stress 2021, 2, 100041. [Google Scholar] [CrossRef]
- Knüttel, A.R.; Boehlau-Godau, M. Spatially confined and temporally resolved refractive index and scattering evaluation in human skin performed with optical coherence tomography. J. Biomed. Opt. 2000, 5, 83–92. [Google Scholar] [CrossRef]
- Leitgeb, R.A.; Villiger, M.; Bachmann, A.H.; Steinmann, L.; Lasser, T. Extended focus depth for Fourier domain optical coherence microscopy. Opt. Lett. 2006, 31, 2450–2452. [Google Scholar] [CrossRef]
- De Boer, J.F.; Leitgeb, R.; Wojtowski, M. Twenty-five years of optical coherence tomography: The paradigm shift in sensitivity and speed provided by Fourier domain OCT. Biomed. Opt. Express 2017, 8, 3248–3280. [Google Scholar] [CrossRef]
- Steinvurzel, P.; Tantiwanichapan, K.; Goto, M.; Ramachandran, S. Fiber-based Bessel beams with controllable diffraction-resistant distance. Opt. Lett. 2011, 36, 4671–4673. [Google Scholar] [CrossRef] [PubMed]
- Blatter, C.; Grajciar, B.; Eigenwillig, C.M.; Wieser, W.; Biedermann, B.R.; Huber, R.; Leitgeb, R.A. Extended focus high-speed swept source OCT with self-reconstructive illumination. Opt. Express 2011, 19, 12141–12155. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Gambin, S.; Jiménez, N.; Benlloch, J.M.; Camarena, F. Generating Bessel beams with broad depth-of-field by using phase-only acoustic holograms. Sci. Rep. 2019, 9, 20104. [Google Scholar] [CrossRef] [PubMed]
- Sen, D.; Classen, A.; Fernandez, A.; Gruner-Nielsen, L.; Gibbs, H.C.; Esmaeili, S.; Hemmer, P.; Baltuska, A.; Sokolov, A.V.; Leitgeb, R.A.; et al. Extended focal depth Fourier domain optical coherence microscopy with a Bessel-beam—LP02 mode—from a higher order mode fiber. Biomed. Opt. Express 2021, 12, 7327–7337. [Google Scholar] [CrossRef] [PubMed]
- Rooney, W.L. Registration of Tx2921 through Tx2928 Sorghum Germplasm Lines. Crop Sci. 2003, 43, 443–444. [Google Scholar] [CrossRef]
- Miller, F.R. Registration of RTx430 Sorghum Parental Line. Crop Sci. 1984, 24, 1224. [Google Scholar] [CrossRef]
- Verhoef, A.J.; Zhu, L.; Israelsen, S.M.; Gruner-Nielsen, L.; Unterhuber, A.; Kautek, W.; Rottwitt, K.; Baltuska, A.; Fernandez, A. Sub-100 fs pulses from an all-polarization maintaining Yb-fiber oscillator with an anomalous dispersion higher-order-mode fiber. Opt. Express 2015, 23, 26139–26145. [Google Scholar] [CrossRef]
- Grüner-Nielsen, L.; Ramachandran, S.; Jespersen, K.G.; Ghalmi, S.; Garmund, M.; Pálsdóttir, B. Optimization of higher order mode fibers for dispersion management of femtosecond fiber lasers. In Proc. SPIE 6873, Fiber Lasers V: Technology, Systems, and Applications, 68730Q; SPIE: Bellingham, WA, USA, 2008. [Google Scholar] [CrossRef]
- Zhu, L.; Verhoef, A.J.; Jespersen, K.G.; Kalashnikov, V.L.; Grüner-Nielsen, L.; Lorenc, D.; Baltuška, A.; Fernández, A. Generation of high fidelity 62-fs, 7-nJ pulses at 1035 nm from a net normal-dispersion Yb-fiber laser with anomalous dispersion higher-order-mode fiber. Opt. Express 2013, 21, 16255–16262. [Google Scholar] [CrossRef]
- MIMMS 2.1 (2020). Available online: https://www.janelia.org/open-science/mimms-21-2020 (accessed on 22 December 2022).
- Tian, J.; Varga, B.; Somfai, G.M.; Lee, W.-H.; Smiddy, W.E.; Cabrera DeBuc, D. Real-Time Automatic Segmentation of Optical Coherence Tomography Volume Data of the Macular Region. PLoS ONE 2015, 10, e0133908. [Google Scholar] [CrossRef]
- Mahapatra, P.K.; Thareja, R.; Kaur, M.; Kumar, A.A. Machine Vision System for Tool Positioning and Its Verification. Meas. Control 2015, 48, 249–260. [Google Scholar] [CrossRef]
- Sadovsky, A.J.; Kruskal, P.B.; Kimmel, J.M.; Ostmeyer, J.; Neubauer, F.B.; MacLean, J.N. Heuristically optimal path scanning for high-speed multiphoton circuit imaging. J. Neurophysiol. 2011, 106, 1591–1598. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sen, D.; Fernández, A.; Crozier, D.; Henrich, B.; Sokolov, A.V.; Scully, M.O.; Rooney, W.L.; Verhoef, A.J. Non-Destructive Direct Pericarp Thickness Measurement of Sorghum Kernels Using Extended-Focus Optical Coherence Microscopy. Sensors 2023, 23, 707. https://doi.org/10.3390/s23020707
Sen D, Fernández A, Crozier D, Henrich B, Sokolov AV, Scully MO, Rooney WL, Verhoef AJ. Non-Destructive Direct Pericarp Thickness Measurement of Sorghum Kernels Using Extended-Focus Optical Coherence Microscopy. Sensors. 2023; 23(2):707. https://doi.org/10.3390/s23020707
Chicago/Turabian StyleSen, Dipankar, Alma Fernández, Daniel Crozier, Brian Henrich, Alexei V. Sokolov, Marlan O. Scully, William L. Rooney, and Aart J. Verhoef. 2023. "Non-Destructive Direct Pericarp Thickness Measurement of Sorghum Kernels Using Extended-Focus Optical Coherence Microscopy" Sensors 23, no. 2: 707. https://doi.org/10.3390/s23020707
APA StyleSen, D., Fernández, A., Crozier, D., Henrich, B., Sokolov, A. V., Scully, M. O., Rooney, W. L., & Verhoef, A. J. (2023). Non-Destructive Direct Pericarp Thickness Measurement of Sorghum Kernels Using Extended-Focus Optical Coherence Microscopy. Sensors, 23(2), 707. https://doi.org/10.3390/s23020707