Objective Methods of Monitoring Usage of Orthotic Devices for the Extremities: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
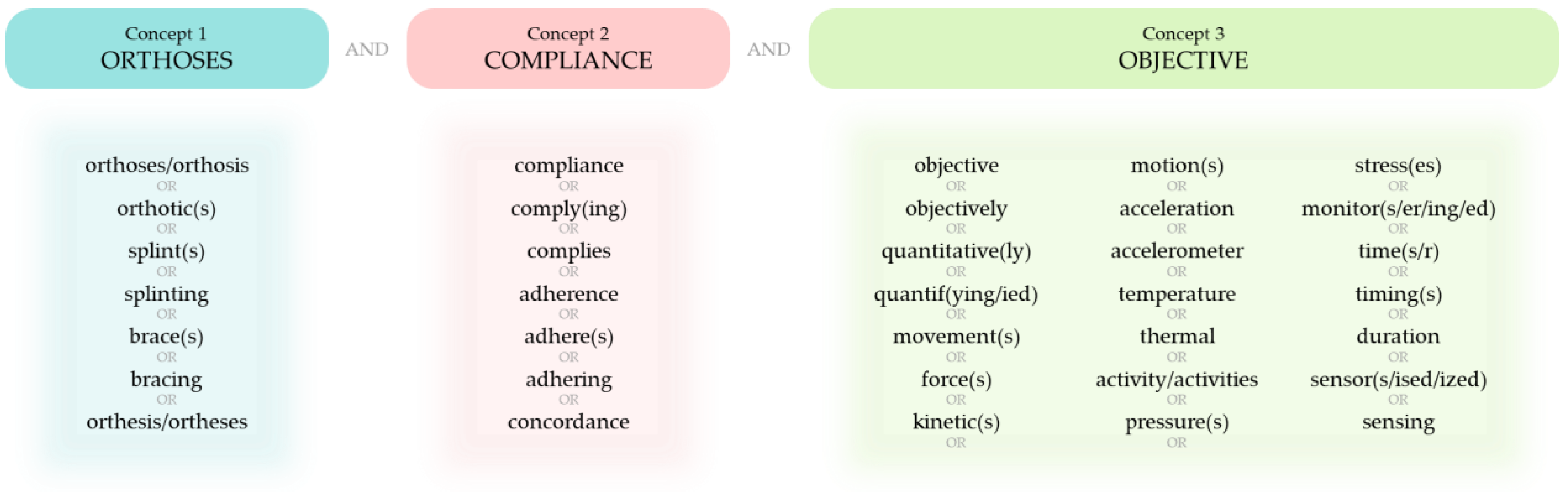
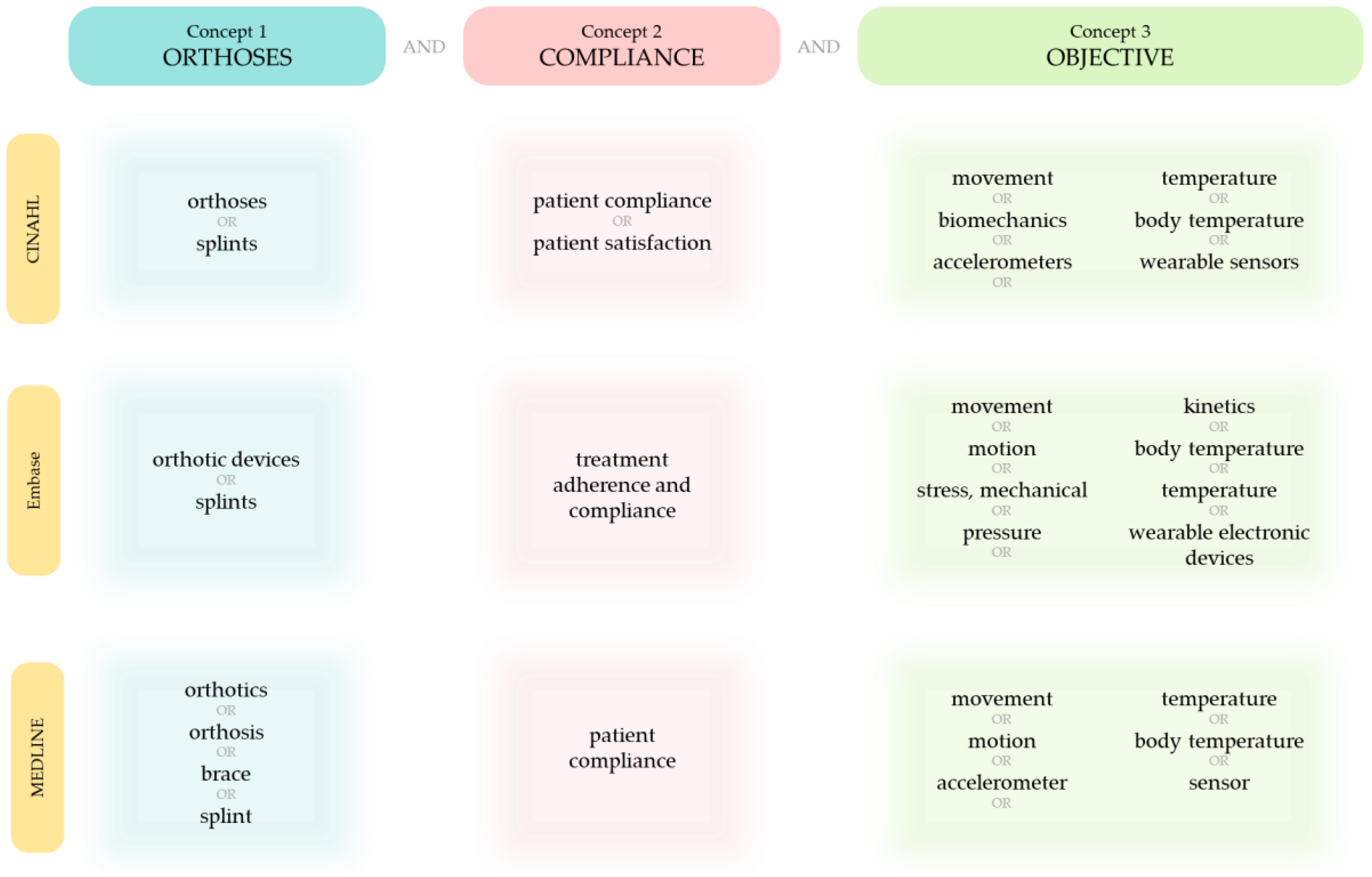
2.1. Literature Search
2.2. Eligibility Criteria
2.3. Screening and Selection
2.4. Data Extraction
2.5. Data Analysis
3. Results
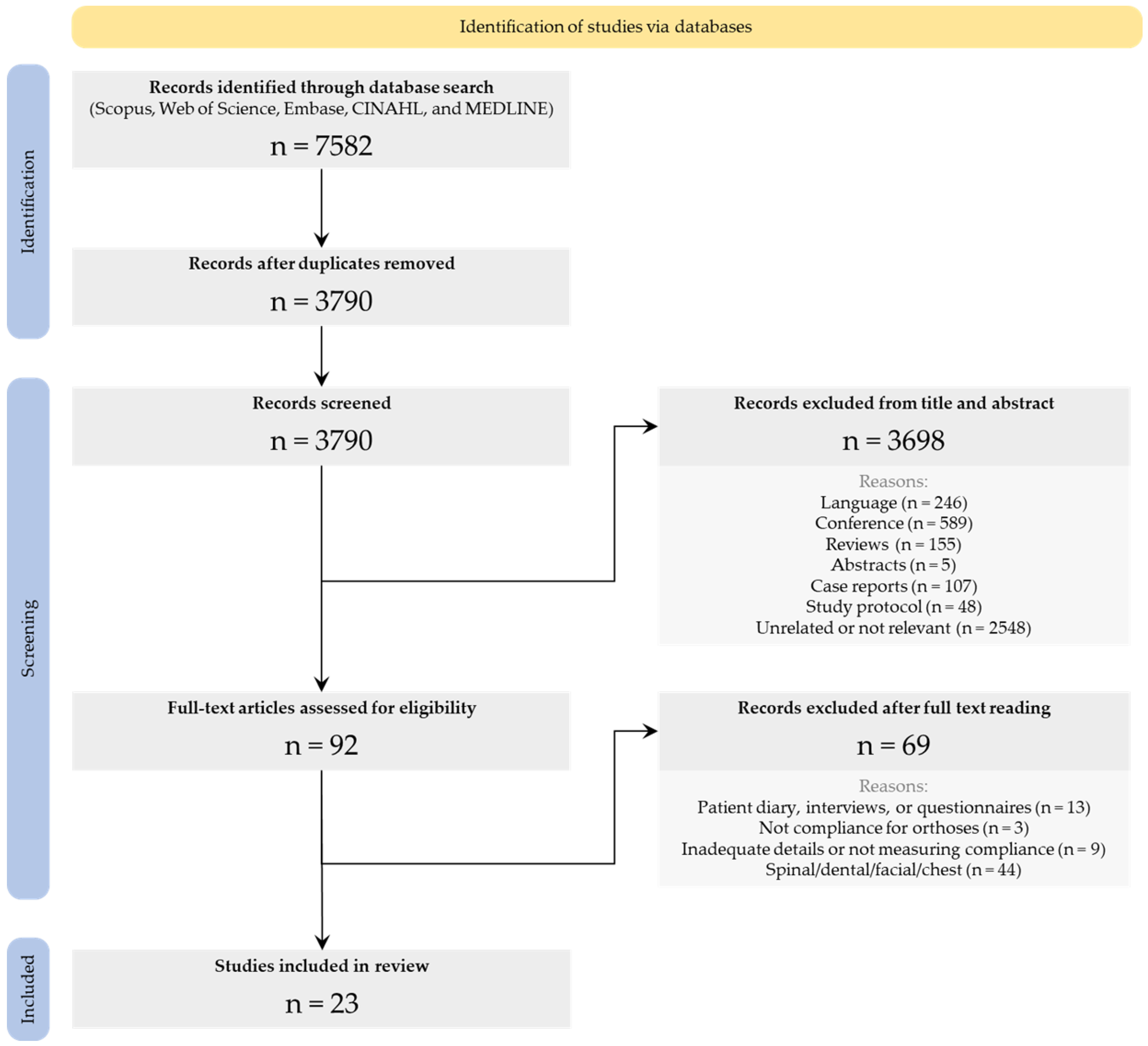
3.1. Literature Search
3.2. Study Demographics
3.3. Compliance Monitoring
3.4. Temperature Sensors
| Author and Year | Orthosis Prescribed | Study Aim(s) | Population Demographics | Description of Technology | Instructions for Use | Wear Time Estimation | Pros of Method | Cons of Method |
|---|---|---|---|---|---|---|---|---|
| Sangiorgio et al., 2016 [50] | Mitchell Ponseti brace sandals for clubfoot | Assess difference between prescribed and measured brace use, difference between parent-reported use and measured brace use, extent to which brace compliance affects risk of relapse. | 48 patients (37 male and 11 female) aged 6 months to 4 years | Two wireless temperature loggers (SmartButton, ACR Systems, Surrey, BC, Canada) consisting of programmable data acquisition, a 3 V battery, and data storage capacity of 2048 readings for data collection every 90 min by each sensor, offset by 45 min, for 4 months. Sensors attached to outside of braces and above the heel. | Wear brace during night and naps. | Data imported to MATLAB and data from both sensors were synchronised to make a single dataset for each patient. A baseline temperature was established using the mean temperature of the dataset, and wear time was determined by finding data points above the baseline. | Objectively measured compliance. Beneficial to clinicians when interpreting parental reports of brace use. | Sampling rate was limited. |
| Ehrmann et al., 2018 [48] | Insoles for diabetes | Determine when patients become nonadherent to diabetic footwear. Observe possible effects of gender on adherence. | 26 patients (18 male and 8 female) aged 59–76 years | Temperature sensor (Orthotimer, Rollerwerk Medical Engineering & Consulting, Balingen, Germany) was embedded into the longitudinal arch of one insole. Temperature was measured within footwear every 15 min. Sensors stored data for 100 days before overwriting the oldest data. | - | An optimal cutoff temperature of 25 °C was found by testing the sensor in healthy participants. Temperatures higher than cutoff temperature were classified as worn and lower temperatures as not worn. | Monitored compliance objectively. Improved long-term adherence and early detection of non-compliance. Patients could not feel the sensor. | No information regarding patient activity and mobility. Temperatures in footwear could have exceeded 25 °C due to environmental factors and not just wear. |
| Grubhofer et al., 2019 [21] | Abduction shoulder brace for post-arthroscopic rotator cuff repair | Analyse abduction brace wearing behaviour in patients who underwent arthroscopic rotator cuff surgery. | 50 patients (23 male and 27 female) aged 28–79 years | Temperature sensor (Orthotimer, Rollerwerk Medical Engineering, Balingen, Germany) was invisibly placed in abdominal belt of brace. Sensor recorded surrounding temperature every 15 min. | 23 h/day wear time for 6 weeks postoperatively. | If the measured temperature was above 35 °C, it was recorded as wear time. Data from the sensors were read out using computer software and displayed wear time for each day since sensor activation. | Clear to see overestimation in self-reported wearing time, so useful to have invisible sensor. | Self-reported wear time was done by patient estimation in outpatient visit; questionnaires would have been better to compare with temperature sensor. Software only shows hours worn per day and not when during the day. |
| Richards et al., 2020 [49] | Abduction brace for clubfoot | Observe daily orthosis wear time in patients successfully treated with the Ponseti method. Determine compliance of patient caretaker with prescribed brace treatment. | 124 patients (83 male and 41 female) aged less than 3 months | Temperature sensor (iButton, Maxim Integrated Products, San Jose, CA, USA) embedded in shoe recorded temperature every 15 min. Held up to 3 months of data. | Prescribed 22 h/day for the first 90 days, then 12 h/night until 2 years old. | - | Highlighted true usage of braces. | Awareness of foot temperature being measured could have influenced brace wear. Few sensors failed to record data during a time interval. |
| Sood et al., 2021 [45] | Shoulder sling for postoperative use | Investigate accuracy of temperature sensors positioned in shoulder slings. Assess whether sensor could discern difference between body temperature and hot environment. | 4 healthy participants (3 male and 1 female) aged 25–32 years | Compact (3.35 × 5.64 × 1.8 cm; 12.75 g) data loggers (Onset HOBO MX2201, Onset Computer Corporation, Bourne, MA, USA) with internal microprocessor, data storage, and sensors to measure contact temperature. Data was sampled every 15 min and transferred via Bluetooth. Sensors were in three locations within the sling: inner region of the bolster touching the abdomen, medial elbow region, and palmar surface of the carpometacarpal joint. | Wear the sling as much as possible but free to remove the sling to perform daily activities. | An algorithm was used to estimate wear time. Start of a wear period was determined by a temperature increase. Temperature had to remain above a threshold value for at least 30 min and not exceed a maximum threshold value. End of a wear period was categorised by a fall in temperature and the temperature being lower than a threshold value for 30 min. | Accurately measured compliance (>99% accuracy). Algorithm could discern temperature difference when donned/doffed. | Data recorded at 15 min intervals, so could underestimate wear time. Sensors with less body contact overestimated compliance. User could “cheat” by leaving sling in a warm environment. Hawthorne effect could alter patient behaviour if they are aware they are being monitored. |
| Swarup et al., 2021 [47] | Abduction brace for residual acetabular dysplasia | Validate efficacy of part-time bracing. Determine relationship between brace wear time and correction of pathology. | 26 patients around 6 months of age | Temperature sensor (iButton, Maxim Integrated Products, San Jose, USA), costing USD 75, placed in the posterior thigh region of the abduction brace. | Wear brace for nights/naps and return in 6 months for follow-up. | Temperature higher that 75 °F was defined as the orthosis being worn and less than or equal to 75 °F was defined was not worn. | Used differences in body and ambient temperatures, and wear patterns to determine temperature thresholds. | Inaccuracies could arise from temperature sensor depending on whether the brace was worn over or under clothing. |
| Grubhofer et al., 2022 [46] | Abduction shoulder brace for post-arthroscopic rotator cuff repair | Investigate whether compliance with immobilisation influences healing. Define compliance rate associated with tendon-repair post rotator cuff repair. | 50 patients (23 male and 27 female) aged 28–79 years | Temperature sensor (Orthotimer, Rollerwerk Medical Engineering, Balingen, Germany) was invisibly placed in abdominal belt of brace. Sensor recorded surrounding temperature every 15 min. | 23 h/day wear time for 6 weeks postoperatively. | If the measured temperature was above 35 °C, it was recorded as wear time. Data from the sensors was read out using computer software and displayed wear time for each day since sensor activation. | Monitored compliance objectively. | Patient not informed about sensor until after 6 weeks—could affect behaviour. |
| Haarman et al., 2022 [34] | Upper limb orthoses for impairment of the shoulder, arm, or hands | Validate method to estimate orthosis wear time using temperature sensors attached to the upper body. Assess if two temperature sensors are better than one to estimate wear time. Investigate the effect of sampling time on wear time estimation. | 15 healthy participants (7 male and 8 female) aged 24–67 years | Miniature (diameter: 17 mm, height: 6 mm) data loggers (DS1922L Thermochron iButtons, Maxim Integrated Products, San Jose, USA) that measure and store temperature. Attached to the body using elastic straps positioned around the chest and forearm. Data were sampled at 1 min. Android smartphone application cued user to don or doff. | Remove and re-attach straps at specified time-points. Sixteen hours of non-use and eight hours of donning and doffing as instructed by the smartphone app (intervals between cues ranged from 15–60 min). | Data obtained from temperature sensors were used to train decision tree classification algorithm to estimate wear time. | Accurate wear time estimation without direct sensor–skin contact. Accurate estimation during donning and doffing. Algorithm was evaluated with unseen data to minimise bias. | As sampling time increased, the data stored increased, and wear time error and estimation error range increased. Potential discrepancy between actual and reported timestamp due to reaction time of user (on smartphone app). Data not collected on warm days, so not trained for high temperatures. |
3.5. Pressure Sensors
| Author and Year | Orthosis Prescribed | Study Aim (s) | Population Demographics | Description of Technology | Instructions for Use | Wear Time Estimation | Pros of Method | Cons of Method |
|---|---|---|---|---|---|---|---|---|
| Morgenstein et al., 2015 [31] | Denis-Brown bar and shoes for clubfoot | Investigate brace wear rates. Determine if a sensor influenced wear rates. | 67 patients (47 male and 20 female) aged 0–3 years | 1.5 inch-square pressure sensor (Interlink Electronics, Camarillo, CA, USA) attached to sole of shoe and connected to data logger box containing circuit board by wires. Data logger collected voltage readings from the pressure sensor that were then exported to Microsoft Excel for processing. | Wear the device for 24 h per day for 3 months (except when bathing). | Baseline voltage readings collected for 10 min while participant wore the orthosis. Baseline data were used to set a threshold value using an algorithm. At all data points above the threshold, brace was considered to be worn. | Clear to see overestimation in self-reported compliance. Presence of sensor did not influence the reported rates of wear. | Does not address different types of braces used for clubfoot. |
| Döbele et al., 2016 [35] | Walking boot for lower extremity fracture recovery | Assess and evaluate validity and reliability of activity-monitoring device for real-time feedback and long-term measurement of partial weight bearing. | 20 healthy participants under the age of 50 years | Insole sensor system consisting of 15 sensors (13 pressure sensors, a temperature sensor, and a measuring acceleration. Only pressure sensors were used to obtain data for this study. Included embedded battery power supply. Data collected recorded on data chip and downloaded wirelessly (ANT Technology). Sample rate of 50 Hz. | Wear walking boot instrumented with insole and complete course of 500 m containing stairs with partial weight bearing (15 kg). | MATLAB script used to identify steps taken and analysed maximum force of every step. | User did not have to operate insole themselves. Data recording was automatically activated as soon as sole was in motion and entered standby mode when not in motion. No problems encountered when fitting into orthotic boot. Wireless data read out was reliable. Maintained accuracy regardless of load applied. | Data were not recorded for extended periods of time (a week and more), so full potential of device was not explored. |
| Najafi et al., 2017 [52] | Footwear for diabetes | Observe adherence to alert-based offloading with a pressure-sensitive insole system. | 12 patients aged 52–71 years | Smart insole system (SurroSense Rx system, Orpyx Medical Technologies Inc., Calgary, AB, Canada) consisting of two pressure-sensing insoles and a smartwatch. Pressure data collected from the plantar surface of foot through insoles transmitted to smartwatch. Each insole contained eight pressure sensors. Provided alerts when safe thresholds for pressure were exceeded. | Wear device for 3 months. | Adherence was defined as the time when footwear with the insole and smartwatch were worn together. Pressure data were obtained from the smartwatch. | Objectively monitored daily adherence during a long period of time (3 months). Self-reported adherence was higher than recorded by sensors. Technology was accepted by patients. | System can be removed by patient. Cannot tell if patient was not wearing prescribed footwear, wearing inserts with no watch, or if they removed insert from footwear. No data collected when device was not worn. |
| Abbott et al., 2019 [32] | Insoles for diabetes | Investigate effectiveness of active insole system in preventing diabetic foot ulcer recurrence. | 58 patients (51 male and 7 female) aged 50–76 years | Plantar pressure measuring insole system (SurroSense Rx, Orpyx Medical Technologies, Calgary, Canada) weighing 45 g, consisting of 0.6 mm flexible, pressure-sensing inserts connected to a smartwatch using ANT+ wireless communication protocol. Each insert contained eight pressure sensors located at plantar surface of foot. Sampling rate of 8 Hz. Smartwatch provided alerts encouraging patients to offload and had a battery life of 2 days (350 mAh rechargeable). Battery life of sensing inserts was 1 week (80 mAh rechargeable). | Wear footwear throughout day-to-day life. | Compliance data obtained when insole was worn inside shoes were connected to smartwatch. For every minute of wear, readings from the previous 15 min were categorised as high, medium, and low pressure. | Measured cumulative pressure applied over time. Intervention was self-directed. Provided high-pressure alerts to allow patients to take action and offload. | Calculation of wear time did not include times when patient wore shoes with insoles without being connected to the smartwatch or when shoes were worn without insoles. Low perceived aesthetic value and problems engaging with smartwatch technology reported as reasons for non-adherence. Device required charging every other day and needed to be connected to smartwatch every time shoes were worn. Did not fit optimally in custom-made shoes. Sampling rate not adequate to measure peak pressures. |
| Lajevardi-Khosh et al., 2019 [51] | Walking boot for lower extremity fracture recovery | Observe patient compliance towards weight-bearing protocols when recovering from lower extremity fractures. | 11 patients (5 male and 6 female) aged 19–64 years | Load-monitoring insole (Ambulatory Tibial Load Analysis System) using piezoelectric pressure sensors in silicone gel within silicone elastomer case inserted into base of walking boot. Insole contained three individually cased load sensors—two under the medial and lateral metatarsal heads and one under the heel. | Wear device at all times except sleeping and during ROM exercises. | Average daily weight bearing (from pressure sensors) compared to expected amount of weight bearing to determine compliance. To determine overall compliance, the total number of compliant days was divided by total number of days that the protocol was prescribed for. | Continuous, objective out-of-clinic monitoring for up to 6 weeks on a single set of batteries. Data collected over long periods, so more representative of patient behaviour (patient’s own environment and outside the lab). | Small number of patients and lacking in patient diversification as more weight-bearing protocols exist. |
3.6. Step Counters
3.7. Accelerometers
| Author and Year | Orthosis Prescribed | Study Aim(s) | Population Demographics | Description of Technology | Instructions for Use | Wear Time Estimation | Pros of Method | Cons of Method |
|---|---|---|---|---|---|---|---|---|
| Sykes et al., 1996 [36] | Reciprocating gait orthoses (RGO) for spinal cord lesions | Objectively measure the number of steps taken while wearing the orthosis. Objectively measure home use of the orthosis, with and without electrical stimulation. | 5 patients aged 24–37 years | Contact switch combined with electronic counter (Syrelec, Farnell, Leeds, UK). Switch consisted of two pieces of aluminium foil (30 mm × 30 mm) separated by plastic sponge foam (4 mm thick). Switch was enclosed in vinyl pocket and attached to heel area of the base of an ankle foot orthosis on the RGO. Switch was connected to a counter attached upright to the thigh area of the RGO. Counter, powered by lithium battery, could display up to 999,999 steps. | Monitoring of RGO use at home for 18 months. | When the contact switch is compressed, one step is registered using the counter. Readings were taken at intervals and the previous recordings were subtracted from the latest recording. | Reliability of step counter was good in laboratory setting. Low use of orthosis was seen in number of steps taken and in patient diaries. | Accuracy decreased when subject started using RGO at home (possibly due to tightness of shoelaces or deterioration of sponge in switch mechanism). Participants were reluctant to provide information. |
| Author and Year | Orthosis Prescribed | Study Aim(s) | Population Demographics | Description of Technology | Instructions for Use | Wear Time Estimation | Pros of Method | Cons of Method |
|---|---|---|---|---|---|---|---|---|
| Weber et al., 2013 [38] | HEPHAISTOS unloading orthoses for calf muscle unloading | Observe compliance and adherence to the orthoses. | 11 healthy male participants aged 20–45 years | Portable three-axis digital accelerometers with replaceable battery (X1-6A, Gulf Coast Data Concepts, Waveland, MS, USA) fixed to shaft of orthosis using Velcro® strips. Set up to automatically start and stop recording data every day (5 a.m.–12 p.m.) on a built-in SD card. New file created every two hours and data retrieved during weekly visit using USB connector. | Wear orthosis during all daily activities for 56 days. | Acceleration analysis using R program. Moving window over 40 samples with no overlap was applied to longitudinal axis each day of each week. For each window, standard deviation (SD) of accelerometer values was calculated. Task-related previously identified SD values (sitting ≈ 0.03 G, standing ≈ 0.01 G, walking ≈ 0.3 G, stair ascending ≈ 0.4 G, and descending ≈ 0.55 G). Activities classified by number of samples ≥ 0.1 G in daytime SD samples compared to total number of SD samples. | Provided control tool. Showed participants wore orthosis during light activities (less locomotive). | No direct proof activity monitor data showed good compliance with protocol. |
| Griffiths et al., 2022 [37] | Abduction brace for clubfoot | Validate brace wear monitoring method in an infant population. | 11 patients aged less than 1 year old | Three-axis accelerometer (activPAL, PAL Technologies, Glasgow, UK) with sampling rate of 20 Hz attached to centre of foot abduction orthoses. Algorithm used summation of accelerometer readings from three axes. | Wear for up to 7 days. | Algorithm used to calculate activity count from raw acceleration to quantify amount of movement. Each time period assigned to wear, and non-wear based on activity count threshold obtained from initial analysis of data from three participants. Algorithm provided wear status every second. | High agreement between results and diary. Showed movement missed by diary. Low cost and easy to attach accelerometers, so no need to create or buy new devices. Method easily reproducible. | Non-wear related movements (e.g., travelling with device that is not worn) could cause errors in wear time estimation. Algorithm needed to be validated against more robust measures. More participants should be recruited. |
3.8. Combination of Sensors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kloppenburg, M.; Kroon, F.P.; Blanco, F.J.; Doherty, M.; Dziedzic, K.S.; Greibrokk, E.; Haugen, I.K.; Herrero-Beaumont, G.; Jonsson, H.; Kjeken, I.; et al. 2018 Update of the EULAR Recommendations for the Management of Hand Osteoarthritis. Ann. Rheum. Dis. 2019, 78, 16–24. [Google Scholar] [CrossRef]
- Nordenskiöld, U. Elastic Wrist Orthoses. Reduction of Pain and Increase in Grip Force for Women with Rheumatoid Arthritis. Arthritis Care Res. 1990, 3, 158–162. [Google Scholar] [PubMed]
- Herrnstadt, G.; Alavi, N.; Randhawa, B.K.; Boyd, L.A.; Menon, C. Bimanual Elbow Robotic Orthoses: Preliminary Investigations on an Impairment Force-Feedback Rehabilitation Method. Front. Hum. Neurosci. 2015, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- Barroso, P.N.; Vecchio, S.D.; Xavier, Y.R.; Sesselmann, M.; Araújo, P.A.; Pinotti, M. Improvement of Hand Function in Children with Cerebral Palsy via an Orthosis That Provides Wrist Extension and Thumb Abduction. Clin. Biomech. 2011, 26, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Burtner, P.A.; Poole, J.L.; Torres, T.; Medora, A.M.; Abeyta, R.; Keene, J.; Qualls, C. Effect of Wrist Hand Splints on Grip, Pinch, Manual Dexterity, and Muscle Activation in Children with Spastic Hemiplegia: A Preliminary Study. J. Hand Ther. 2008, 21, 36–42. [Google Scholar] [CrossRef]
- Nadler, M.; Pauls, M. Shoulder Orthoses for the Prevention and Reduction of Hemiplegic Shoulder Pain and Subluxation: Systematic Review. Clin. Rehabil. 2017, 31, 444–453. [Google Scholar] [CrossRef]
- Horne, R.; Weinman, J.; Barber, N.; Elliott, R.; Morgan, M.; Cribb, A.; Kellar, I. Concordance, Adherence and Compliance in Medicine Taking. London NCCSDO 2005, 2005, 6. [Google Scholar]
- Egan, M.Y.; Brousseau, L. Splinting for Osteoarthritis of the Carpometacarpal Joint: A Review of the Evidence. Am. J. Occup. Ther. 2007, 61, 70–78. [Google Scholar] [CrossRef]
- Adams, J.; Barratt, P.; Arden, N.K.; Barbosa Bouças, S.; Bradley, S.; Doherty, M.; Dutton, S.; Dziedzic, K.; Gooberman-Hill, R.; Hislop Lennie, K.; et al. The Osteoarthritis Thumb Therapy (OTTER) II Trial: A Study Protocol for a Three-Arm Multi-Centre Randomised Placebo Controlled Trial of the Clinical Effectiveness and Efficacy and Cost-Effectiveness of Splints for Symptomatic Thumb Base Osteoarthritis. BMJ Open 2019, 9, e028342. [Google Scholar] [CrossRef]
- Rivett, L.; Rothberg, A.; Stewart, A.; Berkowitz, R. The Relationship between Quality of Life and Compliance to a Brace Protocol in Adolescents with Idiopathic Scoliosis: A Comparative Study. BMC Musculoskelet. Disord. 2009, 10, 5. [Google Scholar] [CrossRef]
- Down, K.; Stead, E. Assistive Technology Workforce Development; Foundation for Assistive Technology: London, UK, 2007. [Google Scholar]
- Chockalingam, N.; Eddison, N.; Healy, A. Cross-Sectional Survey of Orthotic Service Provision in the UK: Does Where You Live Affect the Service You Receive? BMJ Open 2019, 9, e028186. [Google Scholar] [CrossRef]
- Felton, K. The Use of Adherence Monitors with Orthoses. J. Prosthet. Orthot. 1999, 11, 98–100. [Google Scholar] [CrossRef]
- Houwen-van Opstal, S.L.S.; van den Elzen, Y.M.E.M.; Jansen, M.; Willemsen, M.A.A.P.; Cup, E.H.C.; De Groot, I.J.M. Facilitators and Barriers to Wearing Hand Orthoses by Adults with Duchenne Muscular Dystrophy: A Mixed Methods Study Design. J. Neuromuscul. Dis. 2020, 7, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, E.; Kerckhofs, E. Compliance of Patients Wearing an Orthotic Device or Orthopedic Shoes: A Systematic Review. J. Bodyw. Mov. Ther. 2015, 19, 759–770. [Google Scholar] [CrossRef]
- Davies, G.; Yeomans, D.; Tolkien, Z.; Kreis, I.A.; Potter, S.; Gardiner, M.D.; Jain, A.; Henderson, J.; Blazeby, J.M. Methods for Assessment of Patient Adherence to Removable Orthoses Used after Surgery or Trauma to the Appendicular Skeleton: A Systematic Review. Trials 2020, 21, 507. [Google Scholar] [CrossRef]
- Thatipelli, S.; Arun, A.; Chung, P.; Etemadi, M.; Heller, J.A.; Kwiat, D.; Imamura-Ching, J.; Harrison, M.R.; Roy, S. Review of Existing Brace Adherence Monitoring Methods to Assess Adherence. J. Prosthet. Orthot. 2016, 28, 126–135. [Google Scholar] [CrossRef]
- Liavaag, S.; Brox, J.I.; Pripp, A.H.; Enger, M.; Soldal, L.A.; Svenningsen, S. Immobilization in External Rotation after Primary Shoulder Dislocation Did Not Reduce the Risk of Recurrence: A Randomized Controlled Trial. J. Bone Jt. Surg. Am. Vol. 2011, 93, 897–904. [Google Scholar] [CrossRef]
- Silverio, L.M.; Cheung, E.V. Patient Adherence with Postoperative Restrictions after Rotator Cuff Repair. J. Shoulder Elb. Surg. 2014, 23, 508–513. [Google Scholar] [CrossRef]
- McGrath, M.S.; Ulrich, S.D.; Bonutti, P.M.; Smith, J.M.; Seyler, T.M.; Mont, M.A. Evaluation of Static Progressive Stretch for the Treatment of Wrist Stiffness. J. Hand Surg. Am. 2008, 33, 1498–1504. [Google Scholar] [CrossRef]
- Grubhofer, F.; Gerber, C.; Meyer, D.C.; Wieser, K.; Ernstbrunner, L.; Catanzaro, S.; Bouaicha, S. Compliance with Wearing an Abduction Brace after Arthroscopic Rotator Cuff Repair: A Prospective, Sensor-Controlled Study. Prosthet. Orthot. Int. 2019, 43, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Horne, R.; Weinman, J. Patients’ Beliefs about Prescribed Medicines and Their Role in Adherence to Treatment in Chronic Physical Illness. J. Psychosom. Res. 1999, 47, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Haynes, R.B.; Ackloo, E.; Sahota, N.; McDonald, H.P.; Yao, X. Interventions for Enhancing Medication Adherence. Cochrane Database Syst. Rev. 2008, CD000011. [Google Scholar] [CrossRef]
- Vandal, S.; Rivard, C.; Bradet, R. Measuring the Compliance Behavior of Adolescents Wearing Orthopedic Braces. Issues Compr. Pediatr. Nurs. 1999, 22, 59–73. [Google Scholar] [CrossRef]
- Mak, I.; Lou, E.; Raso, J.V.; Hill, D.; Parent, E.; Mahood, J.K.; Moreau, M.J.; Hedden, D. The Effect of Time on Qualitative Compliance in Brace Treatment for AIS. Prosthet. Orthot. Int. 2008, 32, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Benish, B.M.; Smith, K.J.; Schwartz, M.H. Validation of a Miniature Thermochron for Monitoring Thoracolumbosacral Orthosis Wear Time. Spine 2012, 37, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Hunter, L.N.; Sison-Williamson, M.; Mendoza, M.M.; McDonald, C.M.; Molitor, F.; Mulcahey, M.J.; Betz, R.R.; Vogel, L.C.; Bagley, A. The Validity of Compliance Monitors to Assess Wearing Time of Thoracic-Lumbar-Sacral Orthoses in Children with Spinal Cord Injury. Spine 2008, 33, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Takemitsu, M.; Bowen, J.R.; Rahman, T.; Glutting, J.J.; Scott, C.B. Compliance Monitoring of Brace Treatment for Patients with Idiopathic Scoliosis. Spine 2004, 29, 2070–2074. [Google Scholar] [CrossRef]
- Lou, E.; Hill, D.; Hedden, D.; Mahood, J.; Moreau, M.; Raso, J. An Objective Measurement of Brace Usage for the Treatment of Adolescent Idiopathic Scoliosis. Med. Eng. Phys. 2011, 33, 290–294. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Morgenstein, A.; Davis, R.; Talwalkar, V.; Iwinski, H., Jr.; Walker, J.; Milbrandt, T.A. A Randomized Clinical Trial Comparing Reported and Measured Wear Rates in Clubfoot Bracing Using a Novel Pressure Sensor. J. Pediatr. Orthop. 2015, 35, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Abbott, C.A.; Chatwin, K.E.; Foden, P.; Hasan, A.N.; Sange, C.; Rajbhandari, S.M.; Reddy, P.N.; Vileikyte, L.; Bowling, F.L.; Boulton, A.J.M. Innovative Intelligent Insole System Reduces Diabetic Foot Ulcer Recurrence at Plantar Sites: A Prospective, Randomised, Proof-of-Concept Study. Lancet Digit. Health 2019, 1, e308–e318. [Google Scholar] [CrossRef]
- Wong, M.S.; Wu, H.D.; Beygi, B.H.; Zhang, Q.; Lin, Y.; Chan, W.S.; Lou, E. Compliance Study of Hip Protector Users for Prevention of Fragility Fracture: A Pilot Randomized Trial. Prosthet. Orthot. Int. 2022, 46, E392–E397. [Google Scholar] [CrossRef] [PubMed]
- Haarman, C.J.W.; Hekman, E.E.G.; Rietman, J.S.; van der Kooij, H. Accurate Estimation of Upper Limb Orthosis Wear Time Using Miniature Temperature Loggers. J. Rehabil. Med. 2022, 54, 43. [Google Scholar] [CrossRef] [PubMed]
- Dobele, S.; Deininger, C.; Sandmann, G.H.; Schmitt, A.; Freude, T.; Stockle, U.; Lucke, M. New Method for Monitoring Partial Weight Bearing (PWB) of Outpatients with an Independent Insole Sensor System. Nov. Metod. Pro Monit. Castecneho Zatezovani U Ambul. Pacientu S Nezavis. Vlozkovym Senzorickym Syst. 2016, 83, 88–93. [Google Scholar]
- Sykes, L.; Ross, E.R.; Powell, E.S.; Edwards, J. Objective Measurement of Use of the Reciprocating Gait Orthosis (RGO) and the Electrically Augmented RGO in Adult Patients with Spinal Cord Lesions. Prosthet. Orthot. Int. 1996, 20, 182–190. [Google Scholar] [CrossRef][Green Version]
- Griffiths, B.; Silver, N.; Granat, M.H.; Lebel, E. Measuring Foot Abduction Brace Wear Time Using a Single 3-Axis Accelerometer. Sensors 2022, 22, 2433. [Google Scholar] [CrossRef]
- Weber, T.; Ducos, M.; Yang, P.; Jos, D.; Frings-Meuthen, P.; Bruggemann, G.-P.; Bloch, W.; Rittweger, J. The HEPHAISTOS Study: Compliance and Adherence with a Novel Orthotic Device for Calf Muscle Unloading. J. Musculoskelet. Neuronal Interact. 2013, 13, 487–495. [Google Scholar] [PubMed]
- Dinis, E.; Gonçalves, R.; Rodrigues, I.; Mendes, B.; Quintão, C.; Vigário, R.; Quaresma, C. Ortho-Monitorizer: A Portable Device to Monitor Pressure and Temperature During the Use of Upper Limb Orthoses. SN Comput. Sci. 2023, 4, 34. [Google Scholar] [CrossRef]
- Bus, S.A.; Waaijman, R.; Nollet, F. New Monitoring Technology to Objectively Assess Adherence to Prescribed Footwear and Assistive Devices during Ambulatory Activity. Arch. Phys. Med. Rehabil. 2012, 93, 2075–2079. [Google Scholar] [CrossRef]
- Waaijman, R.; Keukenkamp, R.; de Haart, M.; Polomski, W.P.; Nollet, F.; Bus, S.A. Adherence to Wearing Prescription Custom-Made Footwear in Patients with Diabetes at High Risk for Plantar Foot Ulceration. Diabetes Care 2013, 36, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
- Telfer, S.; Munguia, J.; Pallari, J.; Dalgarno, K.; Steultjens, M.; Woodburn, J. Personalized Foot Orthoses with Embedded Temperature Sensing: Proof of Concept and Relationship with Activity. Med. Eng. Phys. 2014, 36, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Menz, H.B.; Bonanno, D.R. Objective Measurement of Adherence to Wearing Foot Orthoses Using an Embedded Temperature Sensor. Med. Eng. Phys. 2021, 88, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Miller Renfrew, L.; Rafferty, D.; Lord, A.; Hunter, R.; Paul, L. Determining Validity of the PALite and ODFS PACE Activity Logger for Measuring Step Count in Healthy Adults. Gait Posture 2020, 80, 315–317. [Google Scholar] [CrossRef]
- Sood, A.; Klein, A.; Kaveeshwar, S.; Jones, D.L.; Duvall, G.; Hovis, J.P.; Weir, T.B.; Enobun, B.; Hasan, S.A.; Henn, R.F.; et al. An Accurate Method of Measuring Shoulder Sling Compliance: A Validation Study. BMC Musculoskelet. Disord. 2021, 22, 524. [Google Scholar] [CrossRef]
- Grubhofer, F.; Ernstbrunner, L.; Gerber, C.; Hochreiter, B.; Schwihla, I.; Wieser, K.; Bouaicha, S. Effect of Abduction Brace Wearing Compliance on the Results of Arthroscopic Rotator Cuff Repair. JBJS Open Access 2022, 7, e21.00148. [Google Scholar] [CrossRef]
- Swarup, I.; Talwar, D.; Sankar, W.N. Part-Time Abduction Bracing in Infants With Residual Acetabular Dysplasia: Does Compliance Monitoring Support a Dose-Dependent Relationship? J. Pediatr. Orthop. 2021, 41, e125–e129. [Google Scholar] [CrossRef]
- Ehrmann, D.; Spengler, M.; Jahn, M.; Niebuhr, D.; Haak, T.; Kulzer, B.; Hermanns, N. Adherence Over Time: The Course of Adherence to Customized Diabetic Insoles as Objectively Assessed by a Temperature Sensor. J. Diabetes Sci. Technol. 2018, 12, 695–700. [Google Scholar] [CrossRef]
- Richards, B.S.; Faulks, S.; Felton, K.; Karacz, C.M. Objective Measurement of Brace Wear in Successfully Ponseti-Treated Clubfeet: Pattern of Decreasing Use in the First 2 Years. J. Am. Acad. Orthop. Surg. 2020, 28, 383–387. [Google Scholar] [CrossRef]
- Sangiorgio, S.N.; Ho, N.C.; Morgan, R.D.; Ebramzadeh, E.; Zionts, L.E. The Objective Measurement of Brace-Use Adherence in the Treatment of Idiopathic Clubfoot. J. Bone Jt. Surg. Am. Vol. 2016, 98, 1598–1605. [Google Scholar] [CrossRef]
- Lajevardi-Khosh, A.; Stuart, A.; Ackerman, M.; Rothberg, D.; Kubiak, E.; Petelenz, T. Characterization of Compliance to Weight-Bearing Protocols and Patient Weight-Bearing Behavior during the Recovery Period in Lower Extremity Fractures: A Pilot Study. Curr. Orthop. Pract. 2019, 30, 395–402. [Google Scholar] [CrossRef]
- Najafi, B.; Ron, E.; Enriquez, A.; Marin, I.; Razjouyan, J. Smarter Sole Survival: Will Neuropathic Patients at High Risk for Ulceration Use a Smart Insole-Based Foot Protection System? J. Diabetes Sci. Technol. 2017, 11, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Guilak, F. Biomechanical Factors in Osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2011, 25, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Chaudri, N.A. Adherence to Long-Term Therapies Evidence for Action. Ann. Saudi Med. 2004, 24, 221–222. [Google Scholar] [CrossRef]
- McGillicuddy, J.W.; Weiland, A.K.; Frenzel, R.M.; Mueller, M.; Brunner-Jackson, B.M.; Taber, D.J.; Baliga, P.K.; Treiber, F.A. Patient Attitudes toward Mobile Phone-Based Health Monitoring: Questionnaire Study among Kidney Transplant Recipients. J. Med. Internet Res. 2013, 15, e6. [Google Scholar] [CrossRef] [PubMed]
- Bros, J.S.; Poulet, C.; Arnol, N.; Deschaux, C.; Gandit, M.; Charavel, M. Acceptance of Telemonitoring Among Patients with Obstructive Sleep Apnea Syndrome: How Is the Perceived Interest by and for Patients? Telemed. e-Health 2018, 24, 351–359. [Google Scholar] [CrossRef]
- van Netten, J.J.; Dijkstra, P.U.; Geertzen, J.H.B.; Postema, K. What Influences a Patient’s Decision to Use Custom-Made Orthopaedic Shoes? BMC Musculoskelet. Disord. 2012, 13, 92. [Google Scholar] [CrossRef]
- Rudd, P. In Search of the Gold Standard for Compliance Measurement. Arch. Intern. Med. 1979, 139, 627–628. [Google Scholar] [CrossRef]
- Vermeire, E.; Hearnshaw, H.; Van Royen, P.; Denekens, J. Patient Adherence to Treatment: Three Decades of Research. A Comprehensive Review. J. Clin. Pharm. Ther. 2001, 26, 331–342. [Google Scholar] [CrossRef]
- Farmer, K.C. Methods for Measuring and Monitoring Medication Regimen Adherence in Clinical Trials and Clinical Practice. Clin. Ther. 1999, 21, 1074–1090; discussion 1073. [Google Scholar] [CrossRef]
| Study Characteristic | Number of Studies | |
|---|---|---|
| Design | Randomised controlled trial | 3 |
| Observational | 20 | |
| Country | United Kingdom | 5 |
| Netherlands | 3 | |
| Switzerland | 2 | |
| Germany | 3 | |
| Portugal | 1 | |
| United States of America | 7 | |
| China | 1 | |
| Australia | 1 | |
| Sample | Patient | 15 |
| Healthy | 7 | |
| Patient and healthy | 1 | |
| Sample size | Range | 2–124 |
| Age (years) | Range | 0.25–86 |
| Orthosis application region | Upper limb | 5 |
| Hip | 2 | |
| Lower limb | 16 | |
| Compliance-monitoring method | Temperature sensors | 8 |
| Pressure sensors | 5 | |
| Step counters | 1 | |
| Accelerometers | 2 | |
| Temperature sensors and accelerometers | 3 | |
| Temperature and pressure sensors | 1 | |
| Temperature sensors and step counters | 2 | |
| Accelerometers and step counters | 1 | |
| Medical condition orthosisis used for | Diabetes | 6 |
| Clubfoot | 4 | |
| Postoperative (shoulder surgery) | 3 | |
| Carpel tunnel syndrome | 1 | |
| Hip fracture prevention | 1 | |
| Hip dysplasia | 1 | |
| Lower limb fracture recovery | 2 | |
| Foot drop | 1 | |
| Spinal cord injury | 1 | |
| No specific condition | 3 | |
| Author and Year | Orthosis Prescribed | Study Aim(s) | Population Demographics | Description of Technology | Instructions for Use | Wear Time Estimation | Pros of Method | Cons of Method |
|---|---|---|---|---|---|---|---|---|
| Bus et al., 2012 [40] | Footwear for diabetes | Assess validity and feasibility of temperature-based adherence monitor to monitor footwear use. | 11 healthy participants (8 male, 3 female, mean age 42.0 ± 9.4 years) and 14 patients (11 male, 3 female, mean age 56.2 ± 12.9 years) | Temperature-based adherence monitor (@monitor) measuring 35 × 15 × 5 mm consisting of two temperature sensors, battery, and data logger. Temperature collected every minute (maximum sampling rate) at the inner lateral shoe border. Sensors placed in a foam pad and affixed using tape. Number of steps taken recorded using step activity monitor (StepWatch, Orthocare Innovations, Edmonds, WA, USA) attached to the ankle. | Healthy: Don/doff several times while wearing monitor for one day in climate-controlled hospital setting. Subset of healthy participants instructed to wear device between 4 and 7 days while recording donning/doffing times. Patients: tested in own prescription footwear for 7-day period with step activity monitor. Instructed to wear at all times apart from when taking a shower/bath. | Average temperature difference between both temperature sensors was calculated in all samples that showed a difference greater than ≥0.3 °C. When the difference between the two sensors in sample was >25% of average, the shoes were classified as being worn and not worn when <25%. Number of steps taken and the time shoes were worn was calculated for each day. Adherence calculated as percentage of daily steps taken whilst wearing footwear and averaged over number of days of data collection. | Valid and feasible adherence data obtained from temperature sensors. Two sensors reading temperature could contribute to higher accuracy and improve sensitivity to temperature change. Small and light device. Performance of temperature sensors checked during the summer and autumn and no difference was found. | Some overestimations of donning and doffing times when data obtained over multiple days. Few issues with usability. Experienced loss of data due to an error on temperature-monitoring device. Removal or incorrect placement of step activity monitor by patient could cause incomplete data. Cycling activity could not be distinguished from ambulatory activity. Discomfort experienced when wearing step activity monitor. System performance in extreme temperatures unknown. Only sampled once every minute so donning or doffing within 1 min period may not be registered. Not tested in orthoses. May not be enough space to fit monitor in a total contact device. |
| Waaijman et al., 2013 [41] | Custom-made footwear for diabetes | Objectively assess adherence to prescribed footwear during ambulation in diabetic patients at high risk for ulceration. Evaluate determinants of adherence in patients. | 107 patients (93 male and 14 female) aged 54–73 years | Temperature-based adherence monitor (@monitor) measures 35 × 15 × 5 mm and consists of two temperature sensors, battery, and data logger. Temperature collected every minute (maximum sampling rate) at the inner lateral shoe border below the ankle. Number of steps taken recorded every minute using step activity monitor (StepWatch, Orthocare Innovations, Edmonds, USA) strapped to the ankle. | Footwear use for 7 consecutive days. Patients asked to step activity monitor at all times apart from when taking a shower or bath or when facing discomfort. | Adherence calculated as total number of steps whilst wearing the prescribed footwear divided by total number of steps. When step activity was recorded but @monitor did not record use, it was assumed patient was walking barefoot or in non-prescribed footwear. Time away from home was reported in daily log and used to classify wear time data for periods at home and away from home. | Objective data collected on adherence. Helped to understand predictive factors of adherence and improve treatment. | Adherence was not measured when standing. Still dependent on daily logs to determine wear time (subjective). Methods needed to ensure patients do not take off step activity monitor. |
| Telfer et al., 2014 [42] | Footwear for diabetes | Assess feasibility of foot orthoses with embedded temperature sensors for monitoring foot temperatures. Determine if temperature measurements could be used to detect periods of activity. | 10 healthy participants aged 22–46 years | Temperature sensor (iButton DS1920Z, Maxim Integrated Products, San Jose, USA) with ability to store 2048 individual measurements and resolution of ±0.125 °C enclosed in stainless steel shell (16.25 mm diameter, 5.95 mm thick) positioned distal to medial arch in contact with foot under first and second metatarsal heads. Data collected at 3 min intervals to allow for 4 days of temperature measurements. Activity monitor (ActivPAL, PAL Technologies, Glasgow, UK) worn on thigh to give estimate on energy expenditure based on steps taken and posture. | Wear for 4 days. | Algorithm developed to predict high activity from temperature data using the rate of change of the difference between plantar surface and ambient temperatures. Optimum threshold rate of change, averaging of temperature data, and offset of temperature data in relation to activity data (to account for delays in response of foot temperatures to activity) used to optimise performance of algorithm. | Demonstrated feasibility. System could be used to measure compliance with foot orthosis interventions. Potential to increase understanding of how activity affects plantar and in-shoe temperatures. | Limitations with respects to data storage and resolution. Low sampling rate to measure temperatures for several days. Orthosis needed to be removed to download data from sensors. |
| Miller Renfrew et al., 2020 [44] | Ankle foot orthoses for foot drop | Assess validity and level of agreement between PALite and Odstock Dropped Foot Stimulator (ODFS) Pace activity in measuring step count. | 16 healthy participants (9 male and 7 female) aged 18–65 years | Accelerometer (PALite, PAL Technologies, Ltd., Glasgow, UK) placed on lateral aspect of lower leg with waterproof dressing. Step counter (ODFS Pace activity logger, OML) was worn on waistband (no stimulation delivered), and steps were counted using a pressure sensitive switch taped to heel of insole inserted into participant’s shoe. | Walk for 5 min at 3 speeds: normal (1.3 ms−1), slow (0.4 ms−1), and fast (1.7–2.0 ms−1). | PALite uses accelerometer values to detect movement of the leg shank during the step cycle. ODFS Pace activity logger records heel contacts on the pressure switch during heel strike. | PALite measured step count more accurately than ODFS. Validity for both in measuring step count. PALite could be used to monitor adherence with other orthotic devices. | Devices agreed less at normal and slow walking speeds than at fast walking speed. |
| Menz & Bonanno 2021 [43] | Foot orthoses for lower limb disorders | Validate a temperature sensor for monitoring adherence to foot orthoses | 10 healthy participants (7 male and 3 female) aged 38–50 years | Miniature (9 × 13 × 4.5 mm) temperature sensor (Orthotimer, Rollerwerk Medical Engineering, Balingen, Germany) embedded into proximal region of medial longitudinal arch. Wearable accelerometer (Fitbit Zip, Fitbit Inc., San Francisco, CA, USA) attached to the exterior of right shoe. USB temperature data logger (Instrument Choice, Adelaide, Australia) recorded ambient temperature. Smartphone application (HoursTracker, Cribasoft, Round Rock, TX, USA) used to record the time the orthoses were placed in the shoes and when they were removed. Temperature recorded at one-minute intervals and physical activity every 15 min. | Wear orthosis for between one and seven hours per day over a five-day period. Then remove orthoses while keeping shoes on. Wear time for each day was randomised for each participant. Instructed to always keep USB ambient temperature data logger near them. | Nine algorithms were evaluated. Four used absolute cut-off temperature values from the sensor, four used temperature values from the sensor relative to ambient temperature. The ninth algorithm identified the largest one-minute increases and decreases in temperature from the sensor corresponding to donning and doffing. | Provided valid orthosis wear time data. Ambient temperature found not to affect temperature sensor readings. | Sensor accuracy tested in only one location. Testing conducted in largely sedentary conditions rather than physically active populations. Sensor embedded in orthosis and not in shoe, so identification of shoe removal vs. orthosis removal may differ. Further evaluation needed to determine accuracy at lower sampling rates. |
| Wong et al., 2022 [33] | Hip protector for hip fracture prevention | Quantify compliance with hip protector use in an elderly population. Investigate effects of different hip protector holders on compliance. | 13 patients aged 76–86 years | Temperature sensor (to differentiate between the ambient and body temperatures), three-axis accelerometer (to detect falls), flash memory (256,000-sample storage capacity), battery, and backup battery. For each data point, the date, time, temperature, and acceleration in each axis were recorded. Data were sampled every 30 s and were transferred wirelessly to a computer. | 24 h for 4 weeks (except when bathing). | Wear time was assessed using both temperature sensor and accelerometer. If the temperature was higher than the personalised threshold (determined as 1 °C less than temperature recorded after an individual wore the hip protector for 15 min), and if changes in the acceleration between the previous two points were higher than the threshold value of 0.023 g, the hip protector was considered to be worn. | Demonstrated feasibility of compliance quantification. Clear to see overestimation in self-reported compliance. Provided understanding of actual usage and efficacy. | Some devices failed to extract data, meaning a potential risk in damage to the monitor. |
| Dinis et al., 2023 [39] | Upper limb orthoses for carpal tunnel syndrome | Investigate device that monitors orthosis use, tightness, and potential inflammation reaction. | 2 healthy participants | Arduino ® UNO R3 connected to three resistive force (pressure) sensors (0.3 mm thickness) and three temperature sensors. Data transfer performed through Bluetooth and data shown in Android application while saved in database in real-time. | Wear for 2 consecutive days. | Temperature and pressure increase when orthosis worn and decrease when unused. | Objectively measured compliance. Pressure and temperature sensors were low cost. | Sensors needed to be kept still for optimal results. Internet connection problems when recording data. Compliance difficult to measure with temperature sensor alone, so pressure also needed to be measured. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devanand, D.B.; Kedgley, A.E. Objective Methods of Monitoring Usage of Orthotic Devices for the Extremities: A Systematic Review. Sensors 2023, 23, 7420. https://doi.org/10.3390/s23177420
Devanand DB, Kedgley AE. Objective Methods of Monitoring Usage of Orthotic Devices for the Extremities: A Systematic Review. Sensors. 2023; 23(17):7420. https://doi.org/10.3390/s23177420
Chicago/Turabian StyleDevanand, Devi Baruni, and Angela E. Kedgley. 2023. "Objective Methods of Monitoring Usage of Orthotic Devices for the Extremities: A Systematic Review" Sensors 23, no. 17: 7420. https://doi.org/10.3390/s23177420
APA StyleDevanand, D. B., & Kedgley, A. E. (2023). Objective Methods of Monitoring Usage of Orthotic Devices for the Extremities: A Systematic Review. Sensors, 23(17), 7420. https://doi.org/10.3390/s23177420






