A Dynamical Systems Approach to Characterizing Brain–Body Interactions during Movement: Challenges, Interpretations, and Recommendations
Abstract
1. Introduction
2. EEG Signal Acquisition and Preprocessing
2.1. Re-Referencing
2.2. Artifact Removal
3. Interpretation of EEG-Measured Oscillatory Activity
3.1. Brain Dynamics: From Spiking to Scalp-Measured Oscillations
3.2. Delta/Theta (1–7 Hz)
3.3. Alpha (8–14 Hz)
3.4. Beta (15–30 Hz)
3.5. Low Gamma (30–50 Hz)
4. A Dynamical Systems Approach to Brain–Body Interactions
4.1. Modeling Brain–Body Interactions
4.2. Dynamics of a Single System
4.3. Coupling between Two Systems
5. Worked Examples
5.1. Known Systems: Van Der Pol Oscillators
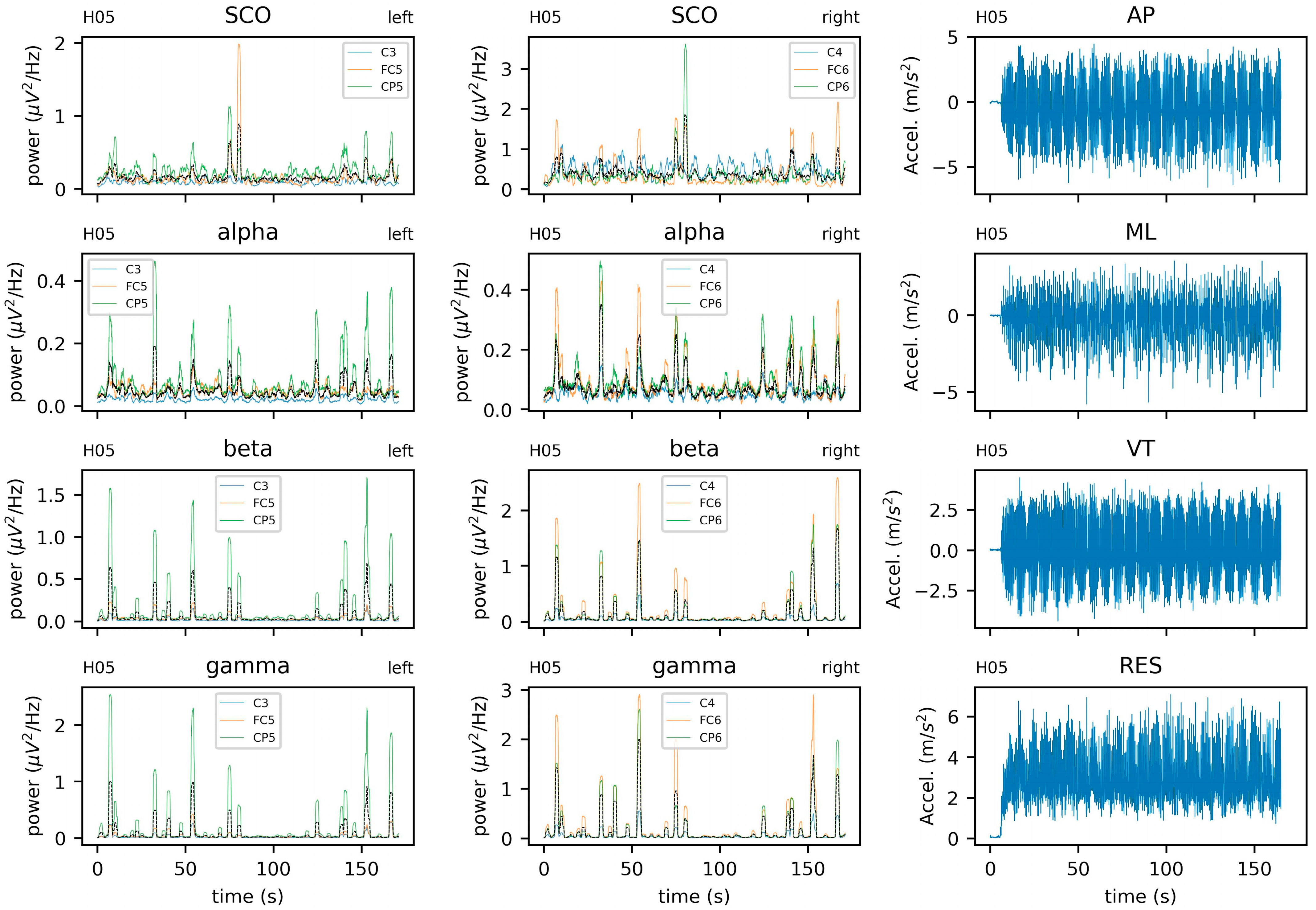
5.2. Empirical Data: EEG and Center of Mass Acceleration during Walking
5.3. Data Acquisition
5.3.1. EEG Data Processing
5.3.2. COM Data Processing
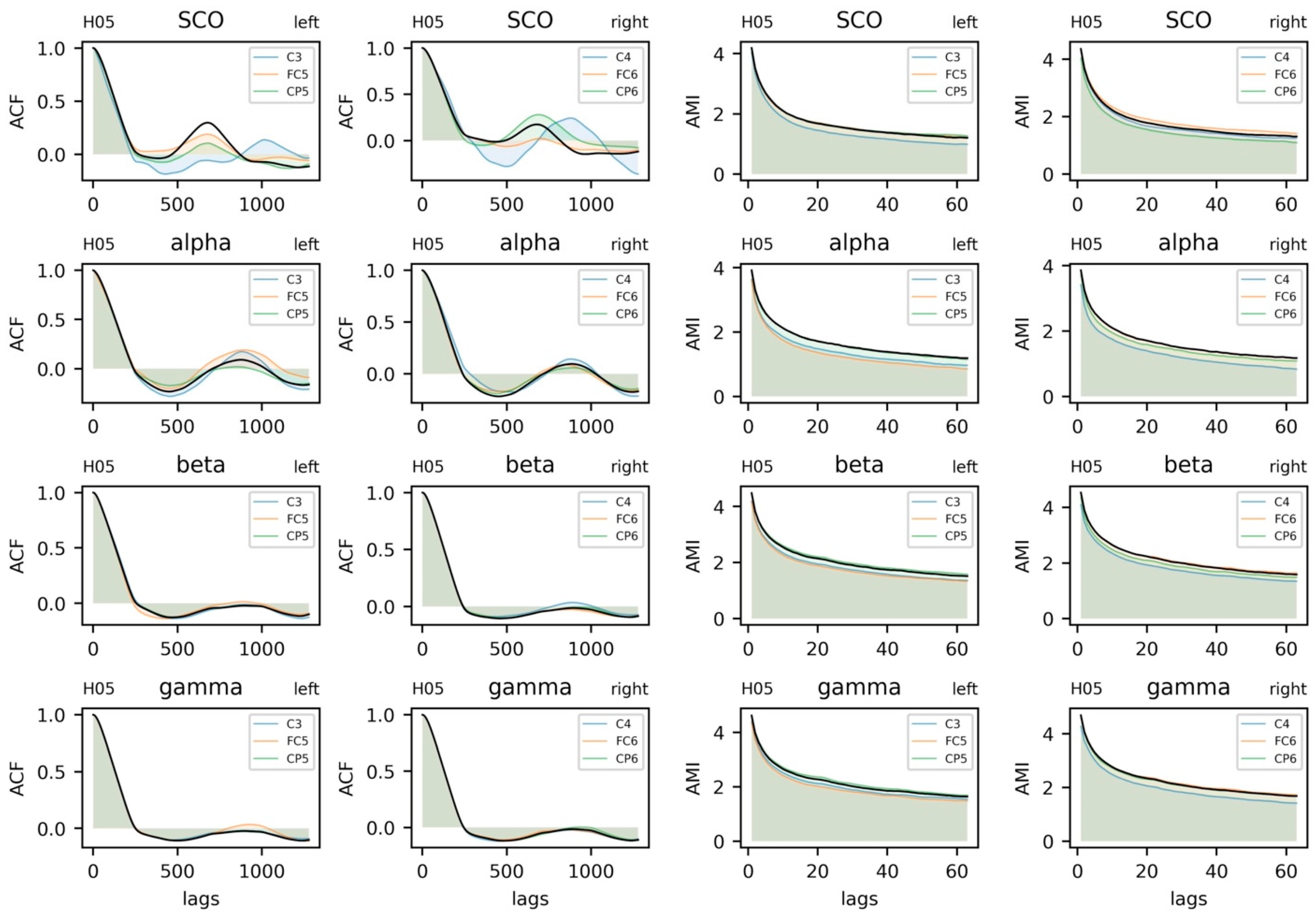
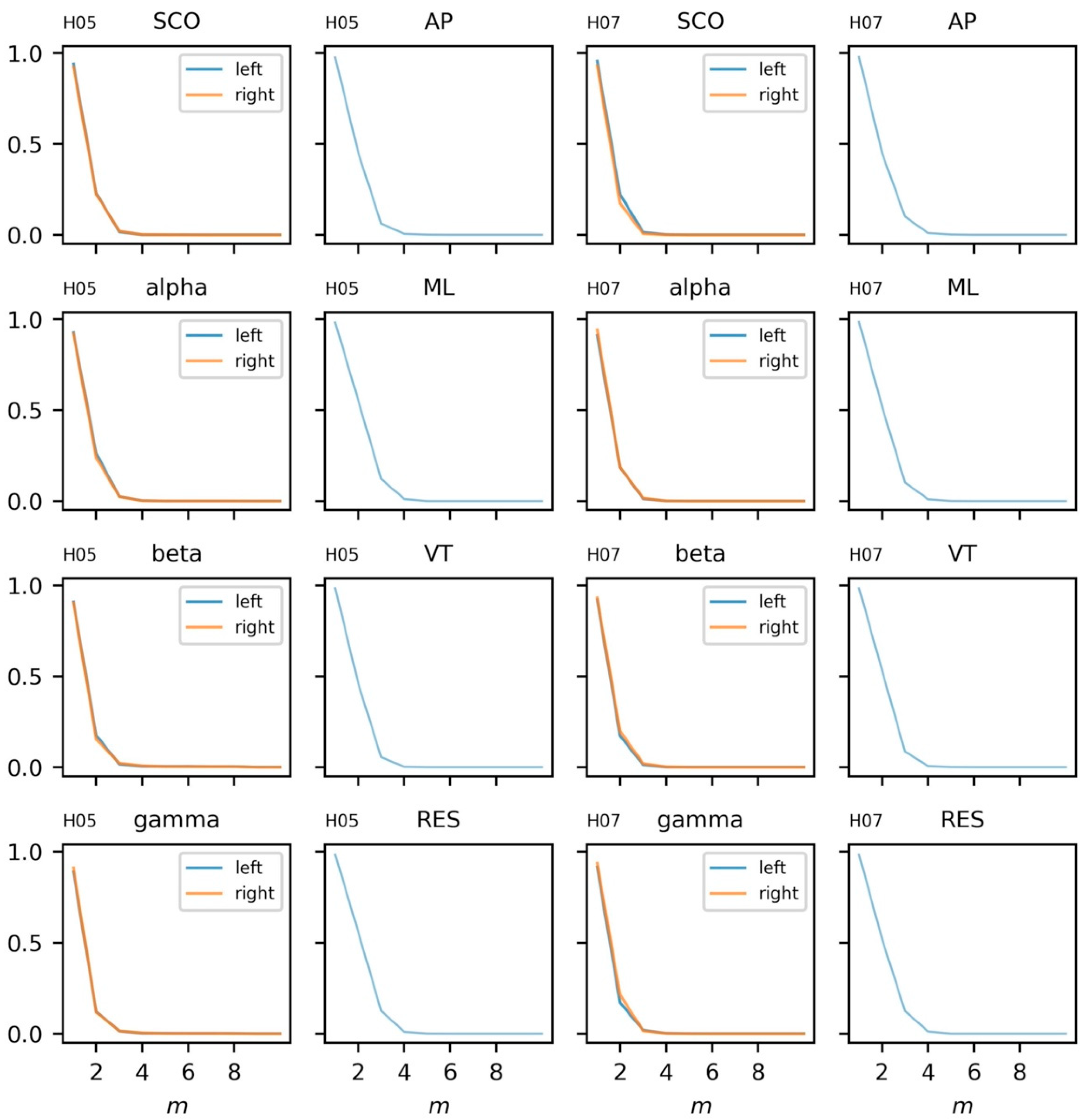
5.3.3. Parameter Selection
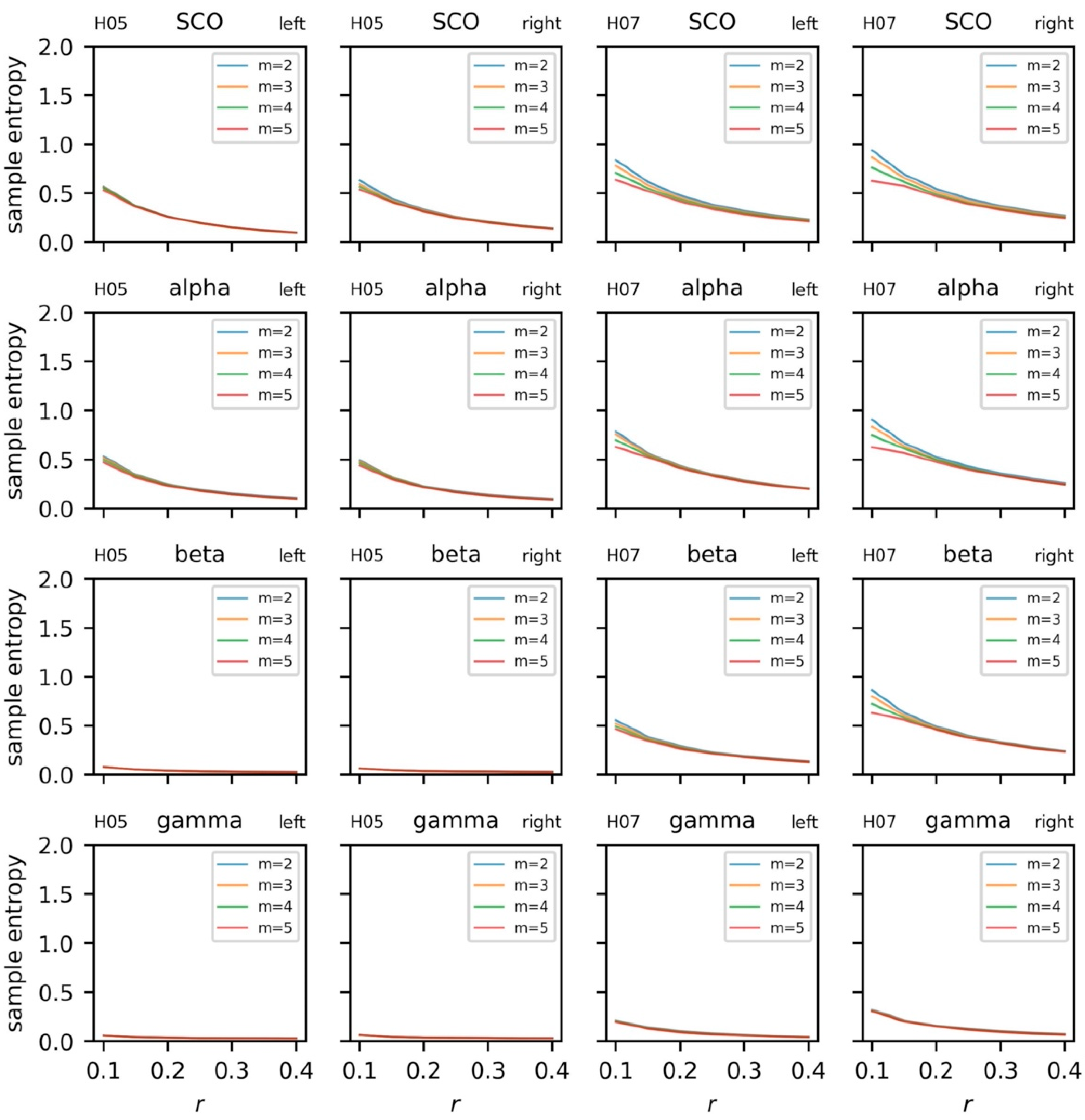
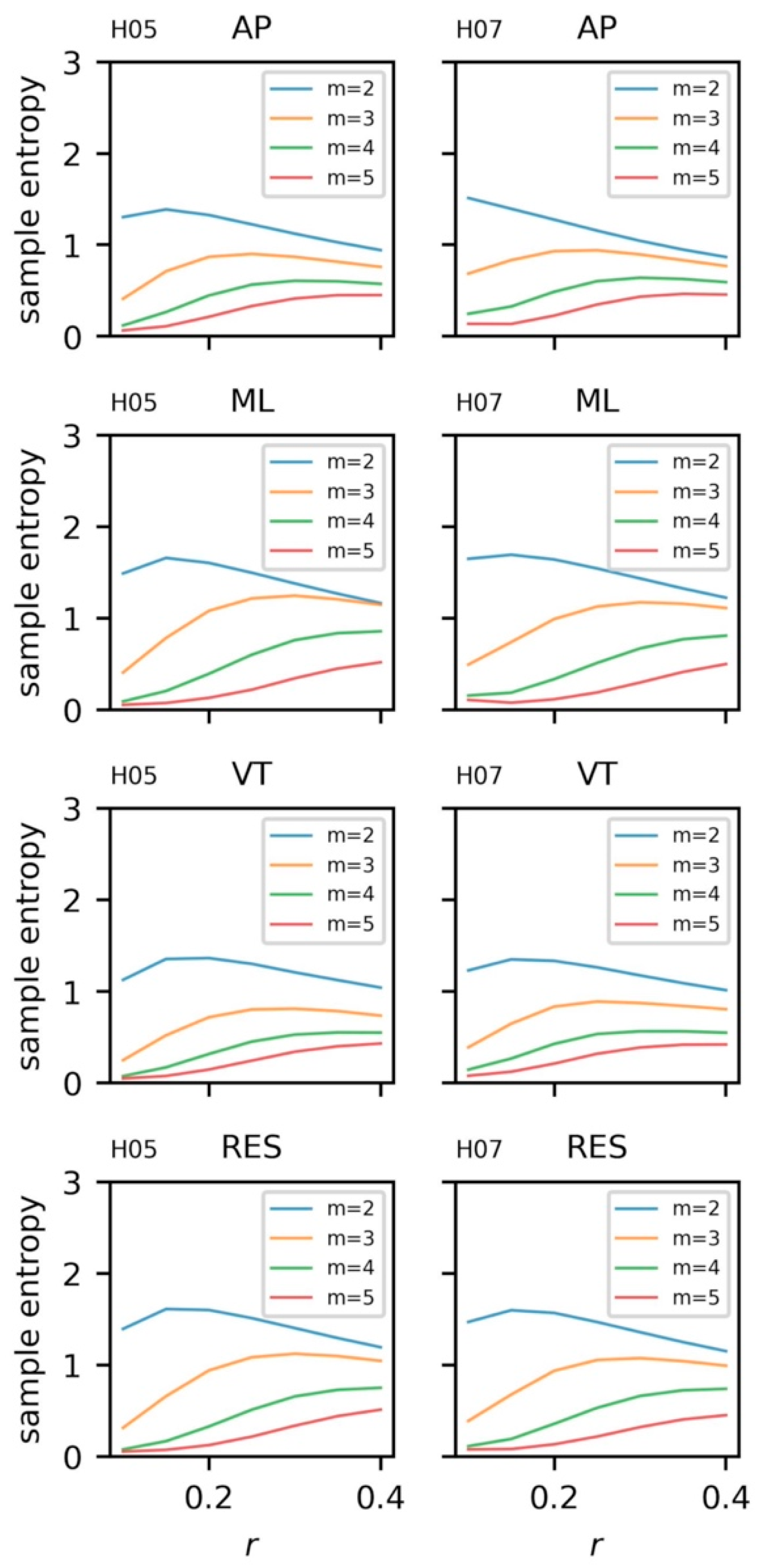
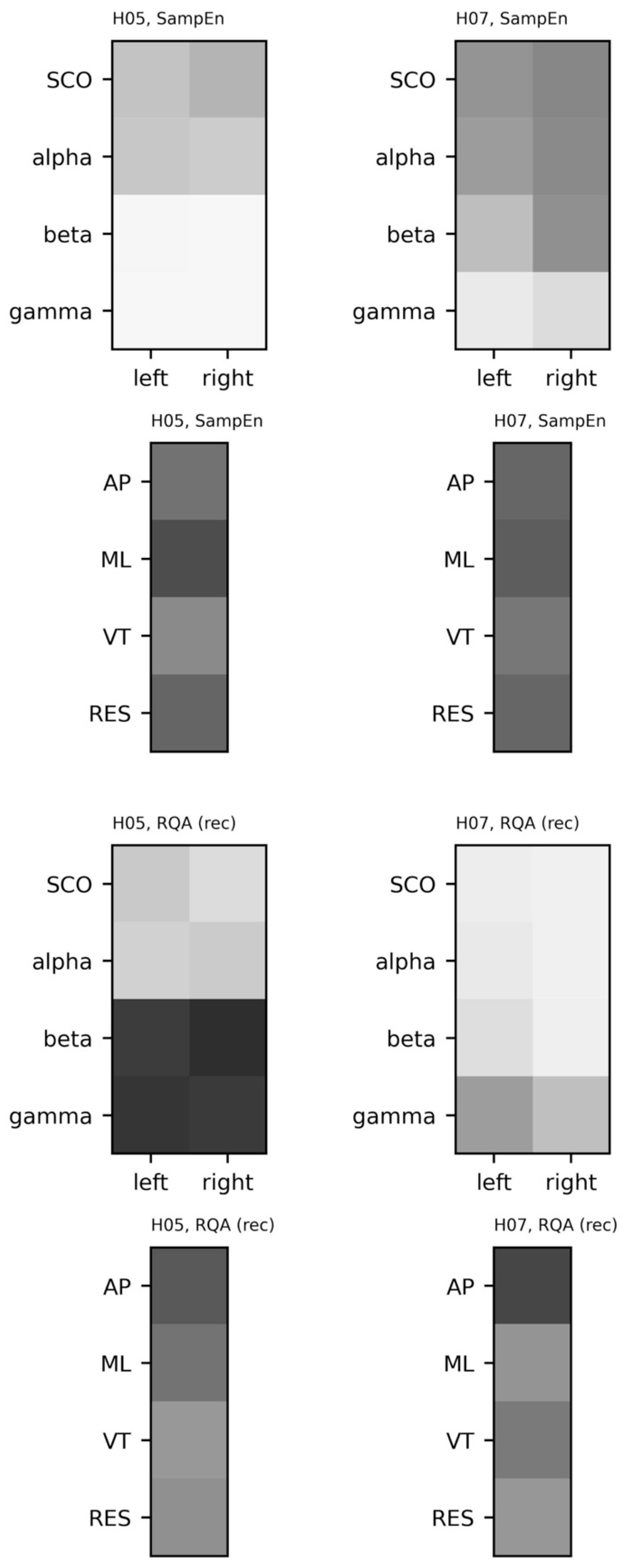
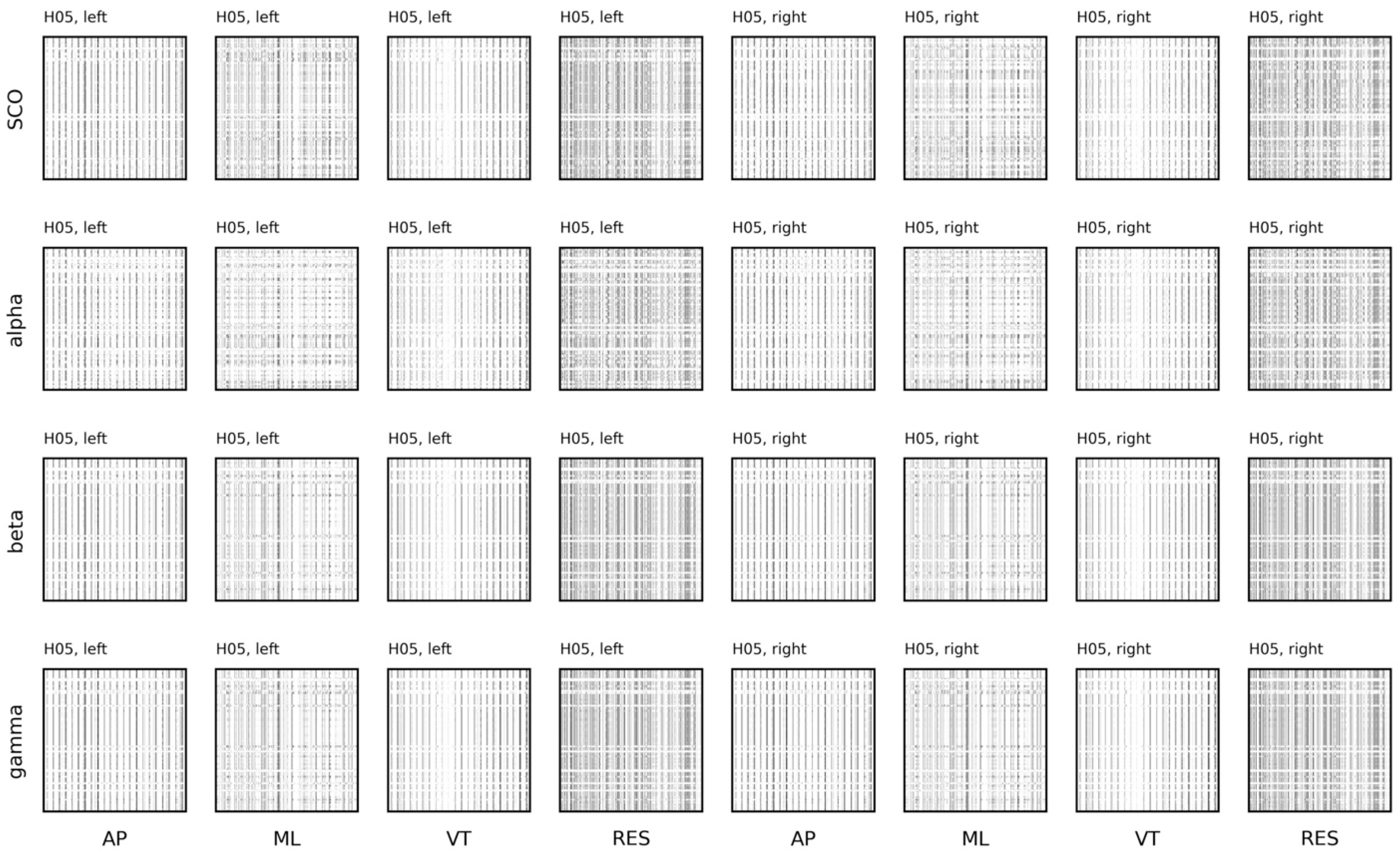
5.3.4. Sample Entropy and Recurrence Quantification Analysis
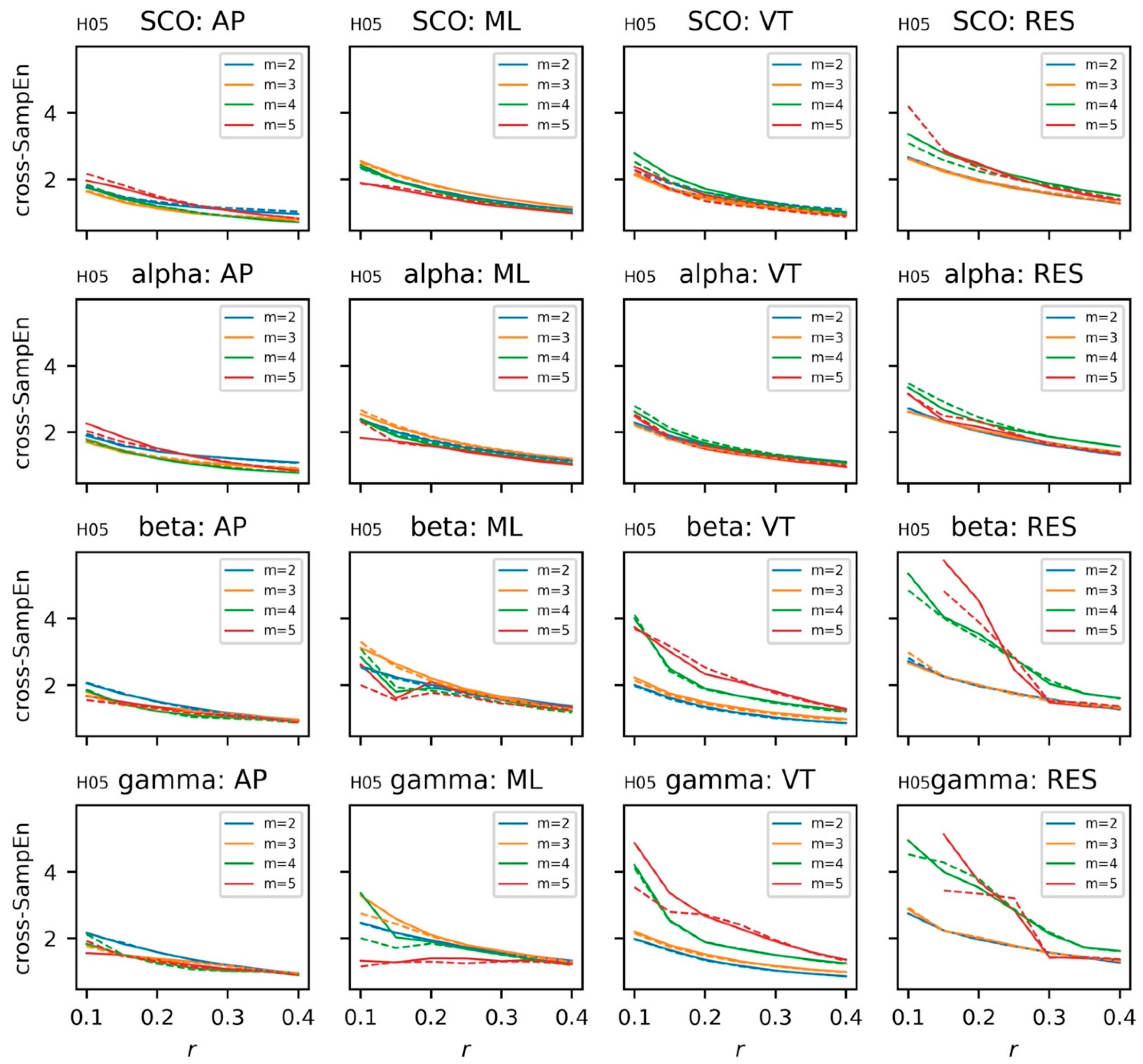
5.3.5. Coupling between Brain and Body Systems
6. Future Directions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gwin, J.T.; Gramann, K.; Makeig, S.; Ferris, D.P. Electrocortical activity is coupled to gait cycle phase during treadmill walking. NeuroImage 2011, 54, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Carvalhaes, C.; de Barros, J.A. The surface Laplacian technique in EEG: Theory and methods. Int. J. Psychophysiol. 2015, 97, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Theiler, J.; Eubank, S.; Longtin, A.; Galdrikian, B.; Doyne Farmer, J. Testing for nonlinearity in time series: The method of surrogate data. Phys. D: Nonlinear Phenom. 1981, 58, 77–94. [Google Scholar] [CrossRef]
- van Rossum, G.; Drake, F.L. Python Reference Manual; Centrum voor Wiskunde en Informatica: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Steriade, M.; Nunez, A.; Amzica, F. Intracellular analysis of relations between the slow (<1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J. Neurosci. 1993, 13, 3266–3283. [Google Scholar] [CrossRef] [PubMed]
- Klug, M.; Jeung, S.; Wunderlich, A.; Gehrke, L.; Protzak, J.; Djebbara, Z.; Argubi-Wollesen, A.; Wollesen, B.; Gramann, K. The BeMoBIL Pipeline for automated analyses of multimodal mobile brain and body imaging data. bioRxiv 2022, 2022.09.29.510051. [Google Scholar] [CrossRef]
- Delorme, A. EEG is better left alone. Sci. Rep. 2023, 13, 2372. [Google Scholar] [CrossRef]
- Castermans, T.; Duvinage, M.; Cheron, G.; Dutoit, T. About the cortical origin of the low-delta and high-gamma rhythms observed in EEG signals during treadmill walking. Neurosci. Lett. 2014, 561, 166–170. [Google Scholar] [CrossRef]
- Nordin, A.D.; Hairston, W.D.; Ferris, D.P. Faster Gait Speeds Reduce Alpha and Beta EEG Spectral Power From Human Sensorimotor Cortex. IEEE Trans. Biomed. Eng. 2020, 67, 842–853. [Google Scholar] [CrossRef]
- Lopes da Silva, F. Neural mechanisms underlying brain waves: From neural membranes to networks. Electroencephalogr. Clin. Neurophysiol. 1991, 79, 81–93. [Google Scholar] [CrossRef]
- van Emmerik, R.E.A.; Ducharme, S.W.; Amado, A.C.; Hamill, J. Comparing dynamical systems concepts and techniques for biomechanical analysis. J. Sport Health Sci. 2016, 5, 3–13. [Google Scholar] [CrossRef]
- Poulet, J.F.A.; Petersen, C.C.H. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature 2008, 454, 7206. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-C.; Luu, P.; Morgan, K.K.; Dow, M.; Davey, C.; Song, J.; Malony, A.D.; Tucker, D.M. Localizing Movement-Related Primary Sensorimotor Cortices with Multi-Band EEG Frequency Changes and Functional MRI. PLoS ONE 2014, 9, e112103. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.N.; Fetz, E.E. Synchronization of neurons during local field potential oscillations in sensorimotor cortex of awake monkeys. J. Neurophysiol. 1996, 76, 3968–3982. [Google Scholar] [CrossRef]
- Anders, P.; Müller, H.; Skjæret-Maroni, N.; Vereijken, B.; Baumeister, J. The influence of motor tasks and cut-off parameter selection on artifact subspace reconstruction in EEG recordings. Med. Biol. Eng. Comput. 2020, 58, 2673–2683. [Google Scholar] [CrossRef] [PubMed]
- Gwin, J.T.; Ferris, D. Beta- and gamma-range human lower limb corticomuscular coherence. Front. Hum. Neurosci. 2012, 6, 258. [Google Scholar] [CrossRef]
- Nordin, A.D.; Hairston, W.D.; Ferris, D.P. Dual-electrode motion artifact cancellation for mobile electroencephalography. J. Neural Eng. 2018, 15, 056024. [Google Scholar] [CrossRef]
- Jensen, O.; Goel, P.; Kopell, N.; Pohja, M.; Hari, R.; Ermentrout, B. On the human sensorimotor-cortex beta rhythm: Sources and modeling. NeuroImage 2005, 26, 347–355. [Google Scholar] [CrossRef]
- Lopes da Silva, F.H.; Pijn, J.P.; Velis, D.; Nijssen, P.C.G. Alpha rhythms: Noise, dynamics and models. Int. J. Psychophysiol. 1997, 26, 237–249. [Google Scholar] [CrossRef]
- Pikovsky, A.; Rosenblum, M.; Kurths, J. Synchronization: A Universal Concept in Nonlinear Science. Am. J. Phys. 2002, 70, 655. [Google Scholar] [CrossRef]
- Donoghue, T.; Haller, M.; Peterson, E.J.; Varma, P.; Sebastian, P.; Gao, R.; Noto, T.; Lara, A.H.; Wallis, J.D.; Knight, R.T.; et al. Parameterizing neural power spectra into periodic and aperiodic components. Nat. Neurosci. 2020, 23, 1655–1665. [Google Scholar] [CrossRef]
- Wainio-Theberge, S.; Wolff, A.; Northoff, G. Dynamic relationships between spontaneous and evoked electrophysiological activity. Commun. Biol. 2021, 4, 1. [Google Scholar] [CrossRef]
- Stergiou, N.; Decker, L.M. Huma? movement variability, nonlinear dynamics, and pathology: Is there a connection? Hum. Mov. Sci. 2011, 30, 869–888. [Google Scholar] [CrossRef]
- Bressler, S.L.; Kelso, J.A.S. Cortical coordination dynamics and cognition. Trends Cogn. Sci. 2001, 5, 26–36. [Google Scholar] [CrossRef]
- Niedermeyer, E.; da Silva, F.H.L. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Softky, W.R.; Koch, C. The highly irregular firing of cortical cells is inconsistent with temporal integration of random EPSPs. J. Neurosci. 1993, 13, 334–350. [Google Scholar] [CrossRef]
- Goldberger, A.L.; West, B.J. Fractals in physiology and medicine. Yale J. Biol. Med. 1987, 60, 421–435. [Google Scholar]
- Rawald, T.; Sips, M.; Marwan, N. PyRQA—Conducting recurrence quantification analysis on very long time series efficiently. Comput. Geosci. 2017, 104, 101–108. [Google Scholar] [CrossRef]
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.-M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011, 2011, 156869. [Google Scholar] [CrossRef]
- Cohen, M.X. Where Does EEG Come From and What Does It Mean? Trends Neurosci. 2017, 40, 208–218. [Google Scholar] [CrossRef]
- Schoffelen, J.-M.; Poort, J.; Oostenveld, R.; Fries, P. Selective Movement Preparation Is Subserved by Selective Increases in Corticomuscular Gamma-Band Coherence. J. Neurosci. 2011, 31, 6750–6758. [Google Scholar] [CrossRef]
- Takens, F. Detecting strange attractors in turbulence. In Dynamical Systems and Turbulence, Warwick 1980; Rand, D., Young, L.-S., Eds.; Springer: Berlin/Heidelberg, Germany, 1981; Volume 898, pp. 366–381. [Google Scholar] [CrossRef]
- Leutheuser, H.; Gabsteiger, F.; Hebenstreit, F.; Reis, P.; Lochmann, M.; Eskofier, B. Comparison of the AMICA and the InfoMax algorithm for the reduction of electromyogenic artifacts in EEG data. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 6804–6807. [Google Scholar] [CrossRef]
- Strogatz, S.H. Nonlinear Dynamics and Chaos: With Applications to Physics, Biology, Chemistry, and Engineering, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar] [CrossRef]
- Gorjan, D.; Gramann, K.; Pauw, K.D.; Marusic, U. Removal of movement-induced EEG artifacts: Current state of the art and guidelines. J. Neural Eng. 2022, 19, 011004. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Edelberg, H.K.; Mitchell, S.L.; Goldberger, A.L.; Wei, J.Y. Increased gait unsteadiness in community-dwelling elderly fallers. Arch. Phys. Med. Rehabil. 1997, 78, 278–283. [Google Scholar] [CrossRef]
- Klug, M.; Gramann, K. Identifying key factors for improving ICA-based decomposition of EEG data in mobile and stationary experiments. Eur. J. Neurosci. 2021, 54, 8406–8420. [Google Scholar] [CrossRef]
- Van Orden, G.C.; Jansen Op Haar, M.A.; De Bosman, A.M.T. Complex Dynamic Systems also Predict Dissociations, but They Do Not Reduce to Autonomous Components. Cogn. Neuropsychol. 1997, 14, 131–165. [Google Scholar] [CrossRef]
- Srinivasan, R.; Winter, W.R.; Nunez, P.L. Source analysis of EEG oscillations using high-resolution EEG and MEG. In Progress in Brain Research; Neuper, C., Klimesch, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 159, pp. 29–42. [Google Scholar] [CrossRef]
- Combrisson, E.; Perrone-Bertolotti, M.; Soto, J.L.; Alamian, G.; Kahane, P.; Lachaux, J.-P.; Guillot, A.; Jerbi, K. From intentions to actions: Neural oscillations encode motor processes through phase, amplitude and phase-amplitude coupling. NeuroImage 2017, 147, 473–487. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Stancák, A.; Neuper, C. Event-related synchronization (ERS) in the alpha band—An electrophysiological correlate of cortical idling: A review. Int. J. Psychophysiol. 1996, 24, 39–46. [Google Scholar] [CrossRef]
- Ombao, H.; Lindquist, M.; Thompson, W.; Aston, J. (Eds.) Electroencephalography (EEG): Neurophysics, Experimental Methods, and Signal Processing. In Handbook of Neuroimaging Data Analysis; Chapman and Hall/CRC: London, UK, 2016; pp. 215–242. [Google Scholar] [CrossRef]
- Gollo, L.L.; Zalesky, A.; Hutchison, R.M.; van den Heuvel, M.; Breakspear, M. Dwelling quietly in the rich club: Brain network determinants of slow cortical fluctuations. Philos. Trans. R. Soc. B: Biol. Sci. 2015, 370, 20140165. [Google Scholar] [CrossRef]
- Başar, E.; Schürmann, M.; Başar-Eroglu, C.; Karakaş, S. Alpha oscillations in brain functioning: An integrative theory. Int. J. Psychophysiol. 1997, 26, 5–29. [Google Scholar] [CrossRef]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 7825. [Google Scholar] [CrossRef]
- Alamia, A.; VanRullen, R. Alpha oscillations and traveling waves: Signatures of predictive coding? PLoS Biol. 2019, 17, e3000487. [Google Scholar] [CrossRef]
- Fu, C.; Suzuki, Y.; Kiyono, K.; Morasso, P.; Nomura, T. An intermittent control model of flexible human gait using a stable manifold of saddle-type unstable limit cycle dynamics. J. R. Soc. Interface 2014, 11, 20140958. [Google Scholar] [CrossRef]
- Nunez, P. Neocortical Dynamics and Human EEG Rhythms; Oxford University Press: New York, NY, USA, 1995. [Google Scholar]
- Gwin, J.T.; Gramann, K.; Makeig, S.; Ferris, D.P. Removal of Movement Artifact From High-Density EEG Recorded During Walking and Running. J. Neurophysiol. 2010, 103, 3526–3534. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T. Coupled Van der Pol oscillators? A model of excitatory and inhibitory neural interactions. Biol. Cybern. 1980, 39, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, P.; Karmos, G.; Mehta, A.D.; Ulbert, I.; Schroeder, C.E. Entrainment of Neuronal Oscillations as a Mechanism of Attentional Selection. Science 2008, 320, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Flood, M.W.; Grimm, B. EntropyHub: An open-source toolkit for entropic time series analysis. PLoS ONE 2021, 16, e0259448. [Google Scholar] [CrossRef]
- Sherman, M.A.; Lee, S.; Law, R.; Haegens, S.; Thorn, C.A.; Hämäläinen, M.S.; Moore, C.I.; Jones, S.R. Neural mechanisms of transient neocortical beta rhythms: Converging evidence from humans, computational modeling, monkeys, and mice. Proc. Natl. Acad. Sci. USA 2016, 113, E4885–E4894. [Google Scholar] [CrossRef]
- Cassim, F.; Monaca, C.; Szurhaj, W.; Bourriez, J.-L.; Defebvre, L.; Derambure, P.; Guieu, J.-D. Does post-movement beta synchronization reflect an idling motor cortex? NeuroReport 2001, 12, 3859. [Google Scholar] [CrossRef]
- Delval, A.; Bayot, M.; Defebvre, L.; Dujardin, K. Cortical Oscillations during Gait: Wouldn’t Walking Be So Automatic? Brain Sci. 2020, 10, 90. [Google Scholar] [CrossRef]
- Sinha, M.; Narayanan, R. Active Dendrites and Local Field Potentials: Biophysical Mechanisms and Computational Explorations. Neuroscience 2022, 489, 111–142. [Google Scholar] [CrossRef]
- Bressler, S.L.; Freeman, W.J. Frequency analysis of olfactory system EEG in cat, rabbit, and rat. Electroencephalogr. Clin. Neurophysiol. 1980, 50, 19–24. [Google Scholar] [CrossRef]
- Buzsáki, G.; Wang, X.-J. Mechanisms of Gamma Oscillations. Annu. Rev. Neurosci. 2012, 35, 203–225. [Google Scholar] [CrossRef]
- von Stein, A.; Sarnthein, J. Different frequencies for different scales of cortical integration: From local gamma to long range alpha/theta synchronization. Int. J. Psychophysiol. 2000, 38, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Halgren, M.; Ulbert, I.; Bastuji, H.; Fabó, D.; Erőss, L.; Rey, M.; Devinsky, O.; Doyle, W.K.; Mak-McCully, R.; Halgren, E.; et al. The generation and propagation of the human alpha rhythm. Proc. Natl. Acad. Sci. USA 2019, 116, 23772–23782. [Google Scholar] [CrossRef] [PubMed]
- Seeber, M.; Scherer, R.; Wagner, J.; Solis-Escalante, T.; Müller-Putz, G.R. EEG beta suppression and low gamma modulation are different elements of human upright walking. Front. Hum. Neurosci. 2014, 8, 485. [Google Scholar] [CrossRef] [PubMed]
- Shelhamer, M. Nonlinear Dynamics in Physiology: A State-Space Approach; World Scientific: Singapore, 2007. [Google Scholar]
- Wagner, J.; Solis-Escalante, T.; Grieshofer, P.; Neuper, C.; Müller-Putz, G.; Scherer, R. Level of participation in robotic-assisted treadmill walking modulates midline sensorimotor EEG rhythms in able-bodied subjects. NeuroImage 2012, 63, 1203–1211. [Google Scholar] [CrossRef]
- Bourguignon, M.; Jousmäki, V.; Dalal, S.S.; Jerbi, K.; De Tiège, X. Coupling between human brain activity and body movements: Insights from non-invasive electromagnetic recordings. NeuroImage 2019, 203, 116177. [Google Scholar] [CrossRef]
- Scholz, J.P.; Kelso, J.A.S.; Schöner, G. Nonequilibrium phase transitions in coordinated biological motion: Critical slowing down and switching time. Phys. Lett. A 1987, 123, 390–394. [Google Scholar] [CrossRef]
- Warren, W.H. The dynamics of perception and action. Psychol. Rev. 2006, 113, 358–389. [Google Scholar] [CrossRef]
- Roenneberg, T.; Dragovic, Z.; Merrow, M. Demasking biological oscillators: Properties and principles of entrainment exemplified by the Neurospora circadian clock. Proc. Natl. Acad. Sci. USA 2005, 102, 7742–7747. [Google Scholar] [CrossRef]
- Suzuki, M.; Wasaka, T.; Inui, K.; Kakigi, R. Reappraisal of field dynamics of motor cortex during self-paced finger movements. Brain Behav. 2013, 3, 747–762. [Google Scholar] [CrossRef]
- van Vreeswijk, C.; Sompolinsky, H. Chaos in Neuronal Networks with Balanced Excitatory and Inhibitory Activity. Science 1996, 274, 1724–1726. [Google Scholar] [CrossRef]
- Goldman-Rakic, P.S. Regional and cellular fractionation of working memory. Proc. Natl. Acad. Sci. USA 1996, 93, 13473–13480. [Google Scholar] [CrossRef] [PubMed]
- West, B.J. The Fractal Tapestry of Life: A Review of Fractal Physiology. Nonlinear Dyn. Psychol. Life Sci. 2021, 25, 261–296. [Google Scholar]
- Devaney, R.L. A First Course in Chaotic Dynamical Systems: Theory and Experiment; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Manning, J.R.; Jacobs, J.; Fried, I.; Kahana, M.J. Broadband Shifts in Local Field Potential Power Spectra Are Correlated with Single-Neuron Spiking in Humans. J. Neurosci. 2009, 29, 13613–13620. [Google Scholar] [CrossRef] [PubMed]
- The Pandas Development Team. Pandas-Dev/Pandas: Pandas; Zenodo: Genève, Switzerland, 2023. [Google Scholar] [CrossRef]
- Jackson, A.F.; Bolger, D.J. The neurophysiological bases of EEG and EEG measurement: A review for the rest of us. Psychophysiology 2014, 51, 1061–1071. [Google Scholar] [CrossRef]
- Kennel, M.B.; Brown, R.; Abarbanel, H.D.I. Determining embedding dimension for phase-space reconstruction using a geometrical construction. Phys. Rev. A 1992, 45, 3403–3411. [Google Scholar] [CrossRef]
- Engel, A.K.; Fries, P. Beta-band oscillations—Signalling the status quo? Curr. Opin. Neurobiol. 2010, 20, 156–165. [Google Scholar] [CrossRef]
- Severens, M.; Nienhuis, B.; Desain, P.; Duysens, J. Feasibility of measuring event Related Desynchronization with electroencephalography during walking. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 2764–2767. [Google Scholar] [CrossRef]
- Pincus, S.M.; Gladstone, I.M.; Ehrenkranz, R.A. A regularity statistic for medical data analysis. J. Clin. Monit. 1991, 7, 335–345. [Google Scholar] [CrossRef]
- Sandhaeger, F.; von Nicolai, C.; Miller, E.K.; Siegel, M. Monkey EEG links neuronal color and motion information across species and scales. eLife 2019, 8, e45645. [Google Scholar] [CrossRef]
- Anishchenko, V.S.; Vadivasova, T.E.; Postnov, D.E.; Safonova, M.A. Synchronization of chaos. Int. J. Bifurc. Chaos 1992, 2, 633–644. [Google Scholar] [CrossRef]
- Cheron, G.; Duvinage, M.; De Saedeleer, C.; Castermans, T.; Bengoetxea, A.; Petieau, M.; Seetharaman, K.; Hoellinger, T.; Dan, B.; Dutoit, T.; et al. From Spinal Central Pattern Generators to Cortical Network: Integrated BCI for Walking Rehabilitation. Neural Plast. 2012, 2012, e375148. [Google Scholar] [CrossRef]
- Courtiol, J.; Perdikis, D.; Petkoski, S.; Müller, V.; Huys, R.; Sleimen-Malkoun, R.; Jirsa, V.K. The multiscale entropy: Guidelines for use and interpretation in brain signal analysis. J. Neurosci. Methods 2016, 273, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Mitra, P.P.; Pesaran, B. Analysis of Dynamic Brain Imaging Data. Biophys. J. 1999, 76, 691–708. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Lopes da Silva, F.H. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef] [PubMed]
- Dingwell, J.B.; Kang, H.G. Differences Between Local and Orbital Dynamic Stability During Human Walking. J. Biomech. Eng. 2006, 129, 586–593. [Google Scholar] [CrossRef]
- Garcia, J.O.; Ashourvan, A.; Thurman, S.M.; Srinivasan, R.; Bassett, D.S.; Vettel, J.M. Reconfigurations within resonating communities of brain regions following TMS reveal different scales of processing. Netw. Neurosci. 2020, 4, 611–636. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, J.M.; Peng, C.K.; Ladin, Z.; Wei, J.Y.; Goldberger, A.L. Is walking a random walk? Evidence for long-range correlations in stride interval of human gait. J. Appl. Physiol. 1995, 78, 349–358. [Google Scholar] [CrossRef]
- Hussain, L.; Aziz, W.; Alshdadi, A.A.; Abbasi, A.A.; Majid, A.; Marchal, A.R. Multiscale entropy analysis to quantify the dynamics of motor movement signals with fist or feet movement using topographic maps. Technol. Health Care 2020, 28, 259–273. [Google Scholar] [CrossRef]
- van den Broek, S.P.; Reinders, F.; Donderwinkel, M.; Peters, M.J. Volume conduction effects in EEG and MEG. Electroencephalogr. Clin. Neurophysiol. 1998, 106, 522–534. [Google Scholar] [CrossRef]
- Thomas, K.S.; Russell, D.M.; Van Lunen, B.L.; Colberg, S.R.; Morrison, S. The impact of speed and time on gait dynamics. Hum. Mov. Sci. 2017, 54, 320–330. [Google Scholar] [CrossRef]
- Freyer, F.; Roberts, J.A.; Ritter, P.; Breakspear, M. A Canonical Model of Multistability and Scale-Invariance in Biological Systems. PLoS Comput. Biol. 2012, 8, e1002634. [Google Scholar] [CrossRef]
- Zduniak, A.; Łusakowski, J. Autocorrelation Function and Mutual Information from Short Experimental Time Series. Acta Phys. Pol. A 1995, 1, 257–260. [Google Scholar] [CrossRef]
- Shockley, K.; Butwill, M.; Zbilut, J.P.; Webber, C.L. Cross recurrence quantification of coupled oscillators. Phys. Lett. A 2002, 305, 59–69. [Google Scholar] [CrossRef]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol.-Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef] [PubMed]
- Cao, L. Practical method for determining the minimum embedding dimension of a scalar time series. Phys. D Nonlinear Phenom. 1997, 110, 43–50. [Google Scholar] [CrossRef]
- Kiebel, S.J.; Daunizeau, J.; Friston, K.J. A Hierarchy of Time-Scales and the Brain. PLoS Comput. Biol. 2008, 4, e1000209. [Google Scholar] [CrossRef]
- Izhikevich, E.M. Dynamical Systems in Neuroscience: The Geometry of Excitability and Bursting; MIT Press: Cambridge, MA, USA, 2006. [Google Scholar] [CrossRef]
- Uchida, N.; Kepecs, A.; Mainen, Z.F. Seeing at a glance, smelling in a whiff: Rapid forms of perceptual decision making. Nat. Rev. Neurosci. 2006, 7, 6. [Google Scholar] [CrossRef]
- Pion-Tonachini, L.; Kreutz-Delgado, K.; Makeig, S. ICLabel: An automated electroencephalographic independent component classifier, dataset, and website. NeuroImage 2019, 198, 181–197. [Google Scholar] [CrossRef]
- Riley, M.A.; Turvey, M.T. Variability and Determinism in Motor Behavior. J. Mot. Behav. 2002, 34, 99–125. [Google Scholar] [CrossRef]
- Yentes, J.M.; Hunt, N.; Schmid, K.K.; Kaipust, J.P.; McGrath, D.; Stergiou, N. The Appropriate Use of Approximate Entropy and Sample Entropy with Short Data Sets. Ann. Biomed. Eng. 2013, 41, 349–365. [Google Scholar] [CrossRef]
- Eckmann, J.-P.; Kamphorst, S.O.; Ruelle, D. Recurrence Plots of Dynamical Systems. Europhys. Lett. 1987, 4, 973. [Google Scholar] [CrossRef]
- Zbilut, J.; Webber, C. Embeddings and delays as derived from quantification of recurrence plots. Phys. Lett. A 1992, 171, 199–203. [Google Scholar] [CrossRef]
- Miskovic, V.; MacDonald, K.J.; Rhodes, L.J.; Cote, K.A. Changes in EEG multiscale entropy and power-law frequency scaling during the human sleep cycle. Hum. Brain Mapp. 2019, 40, 538–551. [Google Scholar] [CrossRef] [PubMed]
- Sipp, A.R.; Gwin, J.T.; Makeig, S.; Ferris, D.P. Loss of balance during balance beam walking elicits a multifocal theta band electrocortical response. J. Neurophysiol. 2013, 110, 2050–2060. [Google Scholar] [CrossRef]
- West, B.J.; Scafetta, N. Nonlinear dynamical model of human gait. Phys. Rev. E 2003, 67, 051917. [Google Scholar] [CrossRef] [PubMed]
- West, B.J.; Scafetta, N. A Multifractal Dynamical Model of Human Gait. In Fractals in Biology and Medicine; Losa, G.A., Merlini, D., Nonnenmacher, T.F., Weibel, E.R., Eds.; Birkhäuser: Basel, Switzerland, 2005; pp. 131–140. [Google Scholar] [CrossRef]
- Kelso, J.A.S. Dynamic Patterns: The Self-Organization of Brain and Behavior; MIT Press: Cambridge, MA, USA, 1995. [Google Scholar]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Peng, C.-K.; Costa, M.; Goldberger, A.L. Adaptive data analysis of complex fluctuations in physiologic time series. Adv. Adapt. Data Anal. 2009, 01, 61–70. [Google Scholar] [CrossRef]
- Mullen, T.R.; Kothe, C.A.E.; Chi, Y.M.; Ojeda, A.; Kerth, T.; Makeig, S.; Jung, T.-P.; Cauwenberghs, G. Real-time neuroimaging and cognitive monitoring using wearable dry EEG. IEEE Trans. Biomed. Eng. 2015, 62, 2553–2567. [Google Scholar] [CrossRef]
- Schmidt, R.A. Motor Schema Theory after 27 Years: Reflections and Implications for a New Theory. Res. Q. Exerc. Sport 2003, 74, 366–375. [Google Scholar] [CrossRef]
- Hasson, U.; Yang, E.; Vallines, I.; Heeger, D.J.; Rubin, N. A Hierarchy of Temporal Receptive Windows in Human Cortex. J. Neurosci. 2008, 28, 2539–2550. [Google Scholar] [CrossRef]
- Troy, M.L.; Joseph, T.G.; Daniel, P.F. How Many Electrodes Are Really Needed for EEG-Based Mobile Brain Imaging? J. Behav. Brain Sci. 2012, 2, 387–393. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, C.; Morari, M. The false nearest neighbors algorithm: An overview. Comput. Chem. Eng. 1997, 21, S1149–S1154. [Google Scholar] [CrossRef]
- Fraser, A.M.; Swinney, H.L. Independent coordinates for strange attractors from mutual information. Phys. Rev. A 1986, 33, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S. Dynamical Systems with Applications Using Python; Springer International Publishing: Berlin, Germany, 2018. [Google Scholar] [CrossRef]
- Friston, K. Prediction, perception and agency. Int. J. Psychophysiol. 2012, 83, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Breakspear, M. Dynamic models of large-scale brain activity. Nat. Neurosci. 2017, 20, 3. [Google Scholar] [CrossRef]
- Abdullah, N.F.; Tang, T.B.; Ho, E.T.W. Discrete Attractor Pattern Recognition During Resting State in EEG Signal. In Proceedings of the 2021 International Conference on Intelligent Cybernetics Technology & Applications (ICICyTA), Virtual, 1–2 December 2021; pp. 117–121. [Google Scholar] [CrossRef]
- McCamley, J.; Denton, W.; Lyden, E.; Yentes, J.M. Measuring Coupling of Rhythmical Time Series Using Cross Sample Entropy and Cross Recurrence Quantification Analysis. Comput. Math. Methods Med. 2017, 2017, e7960467. [Google Scholar] [CrossRef]
- Winawer, J.; Kay, K.N.; Foster, B.L.; Rauschecker, A.M.; Parvizi, J.; Wandell, B.A. Asynchronous Broadband Signals Are the Principal Source of the BOLD Response in Human Visual Cortex. Curr. Biol. 2013, 23, 1145–1153. [Google Scholar] [CrossRef]
- Chong, R.K.Y.; Chastan, N.; Welter, M.-L.; Do, M.-C. Age-related changes in the center of mass velocity control during walking. Neurosci. Lett. 2009, 458, 23–27. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monroe, D.C.; Berry, N.T.; Fino, P.C.; Rhea, C.K. A Dynamical Systems Approach to Characterizing Brain–Body Interactions during Movement: Challenges, Interpretations, and Recommendations. Sensors 2023, 23, 6296. https://doi.org/10.3390/s23146296
Monroe DC, Berry NT, Fino PC, Rhea CK. A Dynamical Systems Approach to Characterizing Brain–Body Interactions during Movement: Challenges, Interpretations, and Recommendations. Sensors. 2023; 23(14):6296. https://doi.org/10.3390/s23146296
Chicago/Turabian StyleMonroe, Derek C., Nathaniel T. Berry, Peter C. Fino, and Christopher K. Rhea. 2023. "A Dynamical Systems Approach to Characterizing Brain–Body Interactions during Movement: Challenges, Interpretations, and Recommendations" Sensors 23, no. 14: 6296. https://doi.org/10.3390/s23146296
APA StyleMonroe, D. C., Berry, N. T., Fino, P. C., & Rhea, C. K. (2023). A Dynamical Systems Approach to Characterizing Brain–Body Interactions during Movement: Challenges, Interpretations, and Recommendations. Sensors, 23(14), 6296. https://doi.org/10.3390/s23146296





