Split-Belt Treadmill Adaptation Improves Spatial and Temporal Gait Symmetry in People with Multiple Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
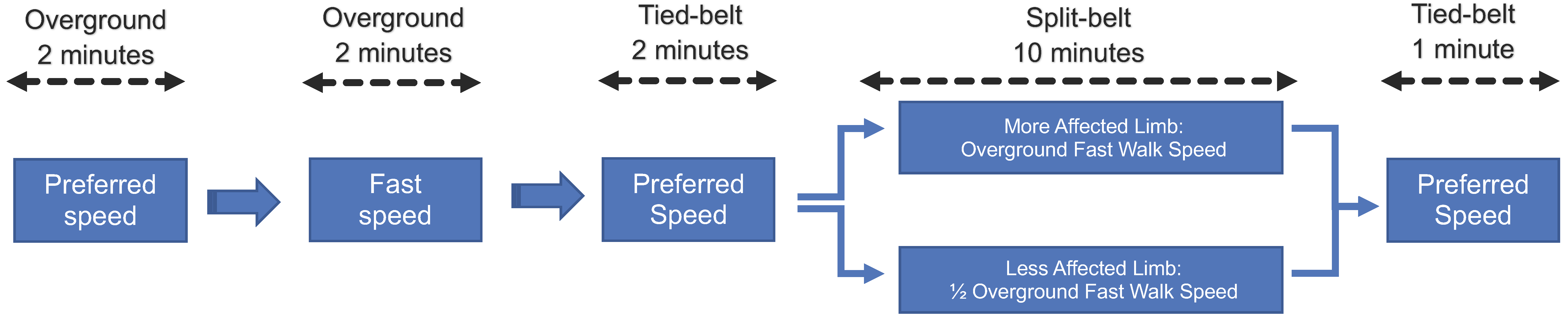
2.2. Split-Belt Treadmill Adaptation Paradigm
2.3. Gait Analysis
2.4. Data Processing
2.5. Statistical Methods
3. Results
3.1. Participants
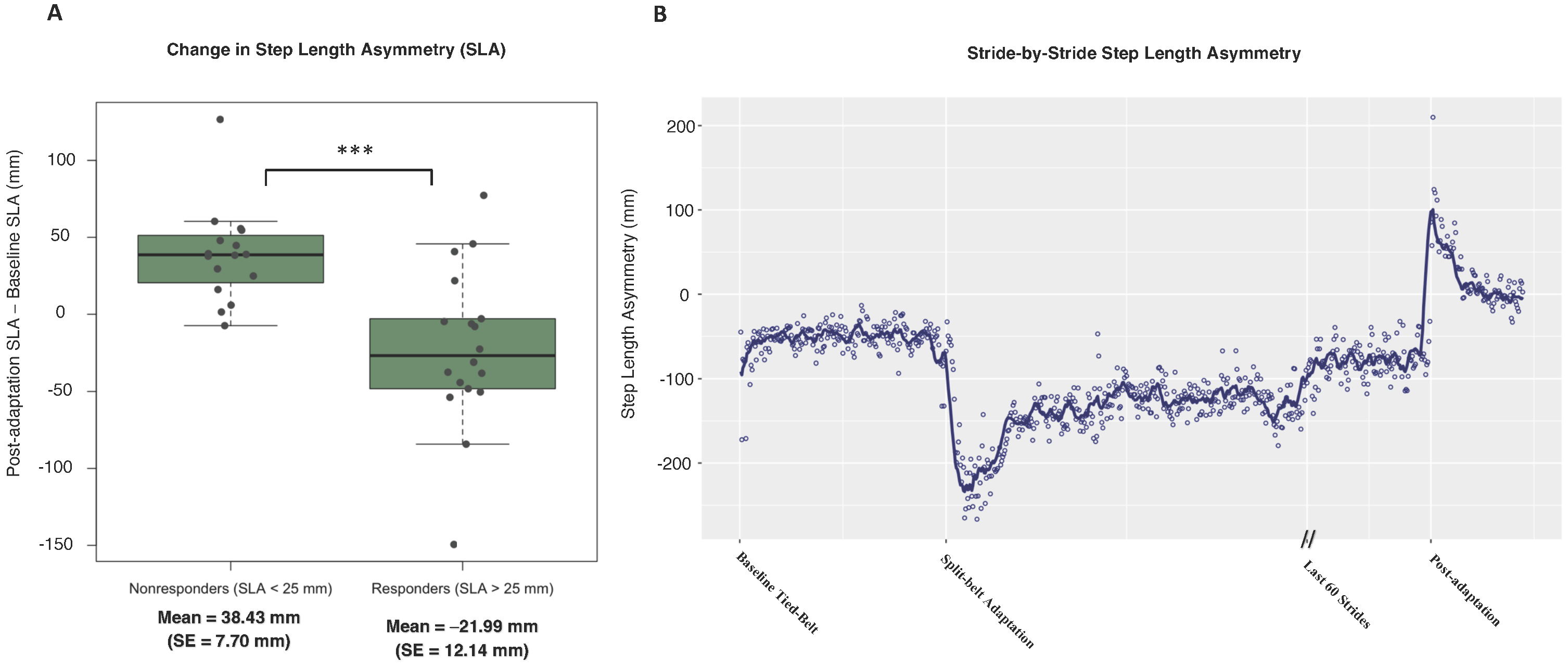
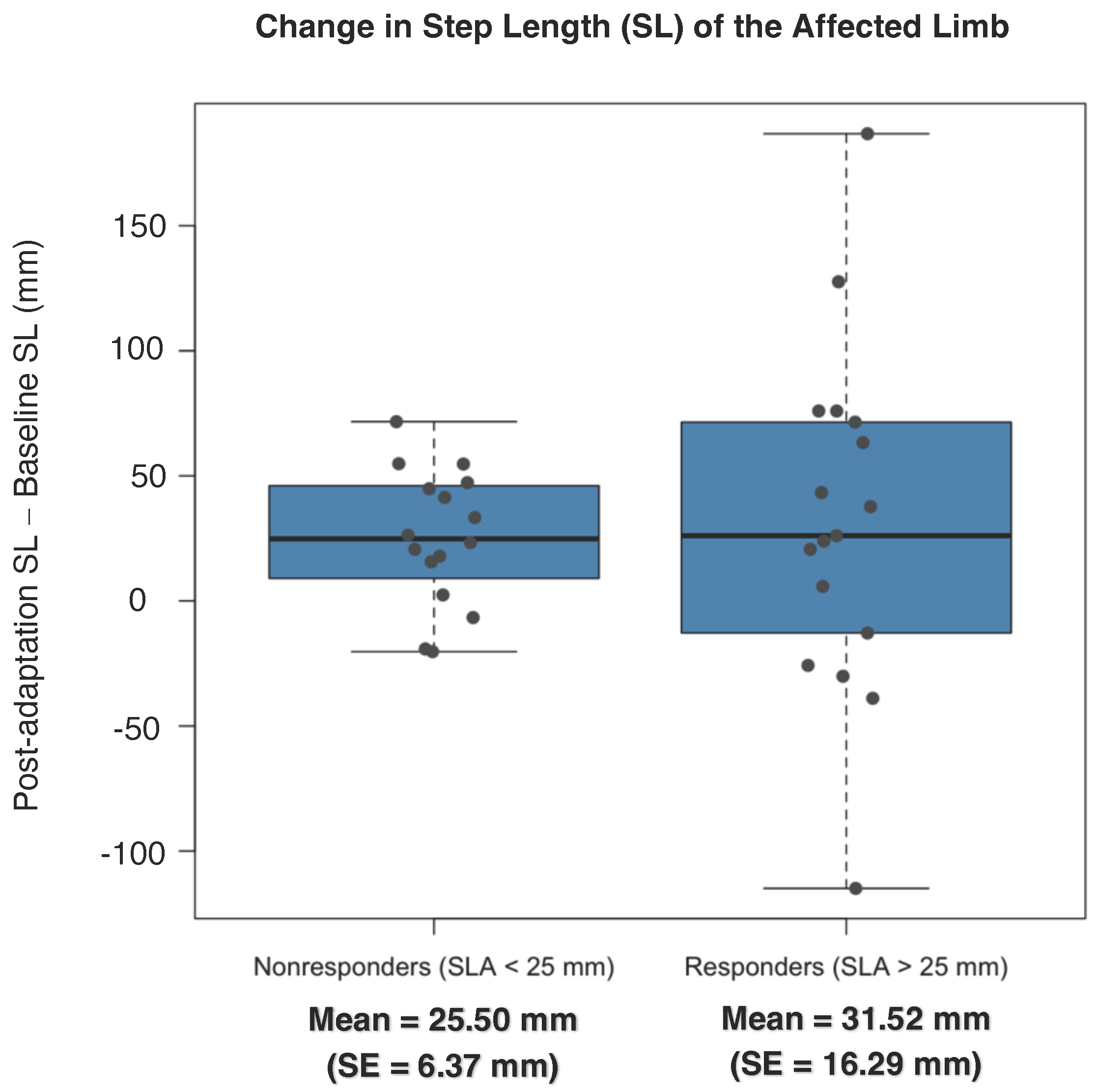
3.2. Spatial Symmetry
3.3. Temporal Symmetry
3.4. Correlation of Spatial Change and Temporal Change
4. Discussion
4.1. Baseline Asymmetry as a Predictor for Symmetry Improvements
4.2. Spatial and Temporal Independence
4.3. Neural Mechanisms of Adaptation in PwMS
4.4. Future Directions
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallin, M.T.; Culpepper, W.J.; Campbell, J.D.; Nelson, L.M.; Langer-Gould, A.; Marrie, R.A.; Cutter, G.R.; Kaye, W.E.; Wagner, L.; Tremlett, H.; et al. The Prevalence of MS in the United States: A Population-Based Estimate Using Health Claims Data. Neurology 2019, 92, E1029–E1040. [Google Scholar] [CrossRef] [PubMed]
- Bebo, B.; Cintina, I.; Larocca, N.; Ritter, L.; Talente, B.; Hartung, D.; Ngorsuraches, S.; Wallin, M.; Yang, G. The Economic Burden of Multiple Sclerosis in the United States. Neurology 2022, 98, e1810–e1817. [Google Scholar] [CrossRef] [PubMed]
- Confavreux, C.; Vukusic, S.; Moreau, T.; Adeleine, P. Relapses and Progression of Disability in Multiple Sclerosis. N. Engl. J. Med. 2000, 343, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.W.; Cho, C.C.; von Koch, L.; Finlayson, M.L. Injurious Falls among Middle Aged and Older Adults with Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2008, 89, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Finley, J.M.; Bastian, A.J.; Gottschall, J.S. Learning to Be Economical: The Energy Cost of Walking Tracks Motor Adaptation. J. Physiol. 2013, 591, 1081. [Google Scholar] [CrossRef]
- Buoite Stella, A.; Morelli, M.E.; Giudici, F.; Sartori, A.; Manganotti, P.; di Prampero, P.E. Comfortable Walking Speed and Energy Cost of Locomotion in Patients with Multiple Sclerosis. Eur. J. Appl. Physiol. 2020, 120, 551–566. [Google Scholar] [CrossRef]
- Oh, K.; Park, J.; Jo, S.H.; Hong, S.J.; Kim, W.S.; Paik, N.J.; Park, H.S. Improved Cortical Activity and Reduced Gait Asymmetry during Poststroke Self-Paced Walking Rehabilitation. J. Neuroeng. Rehabil. 2021, 18, 1–12. [Google Scholar] [CrossRef]
- Peterson, D.S.; Fling, B.W. How Changes in Brain Activity and Connectivity Are Associated with Motor Performance in People with MS. Neuroimage Clin. 2017, 17, 153–162. [Google Scholar] [CrossRef]
- Lewek, M.D.; Bradley, C.E.; Wutzke, C.J.; Zinder, S.M. The Relationship Between Spatiotemporal Gait Asymmetry and Balance in Individuals With Chronic Stroke. J. Appl. Biomech. 2014, 30, 31–36. [Google Scholar] [CrossRef]
- Comber, L.; Galvin, R.; Coote, S. Gait Deficits in People with Multiple Sclerosis: A Systematic Review and Meta-Analysis. Gait Posture 2017, 51, 25–35. [Google Scholar] [CrossRef]
- Bastian, A.J. Understanding Sensorimotor Adaptation and Learning for Rehabilitation. Curr. Opin. Neurol. 2008, 21, 628. [Google Scholar] [CrossRef]
- Izawa, J.; Rane, T.; Donchin, O.; Shadmehr, R. Motor Adaptation as a Process of Reoptimization. J. Neurosci. 2008, 28, 2883. [Google Scholar] [CrossRef]
- Prokop, T.; Berger, W.; Zijlstra, W.; Dietz, V. Adaptational and Learning Processes during Human Split-Belt Locomotion: Interaction between Central Mechanisms and Afferent Input. Exp. Brain Res. 1995, 106, 449–456. [Google Scholar] [CrossRef]
- Cui, C.K.; Lewis, S.J.G. Future Therapeutic Strategies for Freezing of Gait in Parkinson’s Disease. Front. Hum. Neurosci. 2021, 15, 741918. [Google Scholar] [CrossRef]
- Reisman, D.S.; Wityk, R.; Silver, K.; Bastian, A.J. Split-Belt Treadmill Adaptation Transfers to Overground Walking in Persons Poststroke. Neurorehabilit. Neural Repair 2009, 23, 735–744. [Google Scholar] [CrossRef]
- Dzewaltowski, A.C.; Hedrick, E.A.; Leutzinger, T.J.; Remski, L.E.; Rosen, A.B. The Effect of Split-Belt Treadmill Interventions on Step Length Asymmetry in Individuals Poststroke: A Systematic Review with Meta-Analysis. Neurorehabilit. Neural Repair 2021, 35, 563–575. [Google Scholar] [CrossRef]
- Seuthe, J.; D’Cruz, N.; Ginis, P.; Becktepe, J.S.; Weisser, B.; Nieuwboer, A.; Schlenstedt, C. The Effect of One Session Split-Belt Treadmill Training on Gait Adaptation in People With Parkinson’s Disease and Freezing of Gait. Neurorehabilit. Neural Repair 2020, 34, 954–963. [Google Scholar] [CrossRef]
- Seuthe, J.; D’Cruz, N.; Ginis, P.; Weisser, B.; Berg, D.; Deuschl, G.; Nieuwboer, A.; Schlenstedt, C. Split-Belt Treadmill Walking in Patients with Parkinson’s Disease: A Systematic Review. Gait Posture 2019, 69, 187–194. [Google Scholar] [CrossRef]
- Reisman, D.S.; McLean, H.; Keller, J.; Danks, K.A.; Bastian, A.J. Repeated Split-Belt Treadmill Training Improves Poststroke Step Length Asymmetry. Neurorehabilit. Neural Repair 2013, 27, 460–468. [Google Scholar] [CrossRef]
- Hulzinga, F.; Seuthe, J.; D’Cruz, N.; Ginis, P.; Nieuwboer, A.; Schlenstedt, C. Split-Belt Treadmill Training to Improve Gait Adaptation in Parkinson’s Disease. Mov. Disord. 2023, 38, 92–103. [Google Scholar] [CrossRef]
- Tomassini, V.; Johansen-Berg, H.; Leonardi, L.; Paixão, L.; Jbabdi, S.; Palace, J.; Pozzilli, C.; Matthews, P.M. Preservation of Motor Skill Learning in Patients with Multiple Sclerosis. Mult. Scler. J. 2010, 17, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Richmond, S.B.; Peterson, D.S.; Fling, B.W. Bridging the Callosal Gap in Gait: Corpus Callosum White Matter Integrity’s Role in Lower Limb Coordination. Brain Imaging Behav. 2022, 16, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Reisman, D.S.; Wityk, R.; Silver, K.; Bastian, A.J. Locomotor Adaptation on a Split-Belt Treadmill Can Improve Walking Symmetry Post-Stroke. Brain 2007, 130, 1861–1872. [Google Scholar] [CrossRef] [PubMed]
- Finley, J.M.; Long, A.; Bastian, A.J.; Torres-Oviedo, G. Spatial and Temporal Control Contribute to Step Length Asymmetry During Split-Belt Adaptation and Hemiparetic Gait. Neurorehabilit. Neural Repair 2015, 29, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Hoogkamer, W.; Bruijn, S.M.; Duysens, J. Stride Length Asymmetry in Split-Belt Locomotion. Gait Posture 2014, 39, 652–654. [Google Scholar] [CrossRef]
- Plotnik, M.; Giladi, N.; Hausdorff, J.M. A New Measure for Quantifying the Bilateral Coordination of Human Gait: Effects of Aging and Parkinson’s Disease. Exp. Brain Res. 2007, 181, 561–570. [Google Scholar] [CrossRef]
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R Package Version 0.7.2. 2023. Available online: https://CRAN.R-project.org/package=rstatix (accessed on 15 April 2023).
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988; ISBN 9780203771587. [Google Scholar]
- Klaren, R.E.; Motl, R.W.; Dlugonski, D.; Sandroff, B.M.; Pilutti, L.A. Objectively Quantified Physical Activity in Persons with Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2013, 94, 2342–2348. [Google Scholar] [CrossRef]
- Goldman, M.D.; Ward, M.D.; Motl, R.W.; Jones, D.E.; Pula, J.H.; Cadavid, D. Identification and Validation of Clinically Meaningful Benchmarks in the 12-Item Multiple Sclerosis Walking Scale. Mult. Scler. 2017, 23, 1405. [Google Scholar] [CrossRef]
- Gera, G.; Fling, B.W.; Van Ooteghem, K.; Cameron, M.; Frank, J.S.; Horak, F.B. Postural Motor Learning Deficits in People With MS in Spatial but Not Temporal Control of Center of Mass. Neurorehabilit. Neural Repair 2016, 30, 722–730. [Google Scholar] [CrossRef]
- Malone, L.A.; Bastian, A.J. Spatial and Temporal Asymmetries in Gait Predict Split-Belt Adaptation Behavior in Stroke. Neurorehabilit. Neural Repair 2014, 28, 230. [Google Scholar] [CrossRef]
- Christogianni, A.; Bibb, R.; Davis, S.L.; Jay, O.; Barnett, M.; Evangelou, N.; Filingeri, D. Temperature Sensitivity in Multiple Sclerosis: An Overview of Its Impact on Sensory and Cognitive Symptoms. Temperature 2018, 5, 208–223. [Google Scholar] [CrossRef]
- Odom, A.D.; Richmond, S.B.; Fling, B.W. White Matter Microstructure of the Cerebellar Peduncles Is Associated with Balance Performance during Sensory Re-Weighting in People with Multiple Sclerosis. Cerebellum 2021, 20, 92–100. [Google Scholar] [CrossRef]
- Pearson, K.G. Proprioceptive Regulation of Locomotion. Curr. Opin. Neurobiol. 1995, 5, 786–791. [Google Scholar] [CrossRef]
- Fling, B.W.; Dutta, G.G.; Schlueter, H.; Cameron, M.H.; Horak, F.B. Associations between Proprioceptive Neural Pathway Structural Connectivity and Balance in People with Multiple Sclerosis. Front. Hum. Neurosci. 2014, 8, 814. [Google Scholar] [CrossRef]
- Kuhman, D.; Moll, A.; Reed, W.; Rosenblatt, N.; Visscher, K.; Walker, H.; Hurt, C.P. Effects of Sensory Manipulations on Locomotor Adaptation to Split-Belt Treadmill Walking in Healthy Younger and Older Adults. IBRO Neurosci. Rep. 2022, 12, 149–156. [Google Scholar] [CrossRef]
- Forssberg, H.; Grillner, S.; Halbertsma, J.; Rossignol, S. The Locomotion of the Low Spinal Cat. II. Interlimb Coordination. Acta Physiol. Scand. 1980, 108, 283–295. [Google Scholar] [CrossRef]
- Morton, S.M.; Bastian, A.J. Cerebellar Contributions to Locomotor Adaptations during Splitbelt Treadmill Walking. J. Neurosci. 2006, 26, 9107–9116. [Google Scholar] [CrossRef]
- Chambers, W.W.; Sprague, J.M. Functional Localization in the Cerebellum: Somatotopic Organization in Cortex and Nuclei. AMA Arch. Neurol. Psychiatry 1955, 74, 653–680. [Google Scholar] [CrossRef]
- Yanagihara, D.; Udo, M. Climbing Fiber Responses in Cerebellar Vermal Purkinje Cells during Perturbed Locomotion in Decerebrate Cats. Neurosci. Res. 1994, 19, 245–248. [Google Scholar] [CrossRef]
- Jayaram, G.; Galea, J.M.; Bastian, A.J.; Celnik, P. Human Locomotor Adaptive Learning Is Proportional to Depression of Cerebellar Excitability. Cereb. Cortex 2011, 21, 1901–1909. [Google Scholar] [CrossRef]
- Choi, J.T.; Vining, E.P.G.; Reisman, D.S.; Bastian, A.J. Walking Flexibility after Hemispherectomy: Split-Belt Treadmill Adaptation and Feedback Control. Brain 2009, 132, 722. [Google Scholar] [CrossRef] [PubMed]
- Bastian, A.J. Learning to Predict the Future: The Cerebellum Adapts Feedforward Movement Control. Curr. Opin. Neurobiol. 2006, 16, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Hinton, D.C.; Thiel, A.; Soucy, J.P.; Bouyer, L.; Paquette, C. Adjusting Gait Step-by-Step: Brain Activation during Split-Belt Treadmill Walking. Neuroimage 2019, 202, 116095. [Google Scholar] [CrossRef] [PubMed]
- Celnik, P. Understanding and Modulating Motor Learning with Cerebellar Stimulation. Cerebellum 2015, 14, 171–174. [Google Scholar] [CrossRef]
- Nguemeni, C.; Hiew, S.; Kögler, S.; Homola, G.A.; Volkmann, J.; Zeller, D. Split-Belt Training but Not Cerebellar Anodal TDCS Improves Stability Control and Reduces Risk of Fall in Patients with Multiple Sclerosis. Brain Sci. 2022, 12, 63. [Google Scholar] [CrossRef]
- Alenazy, M.; Daneshgar Asl, S.; Petrigna, L.; Feka, K.; Alvarez, E.; Almuklass, A.M.; Enoka, R.M. Treatment with Electrical Stimulation of Sensory Nerves Improves Motor Function and Disability Status in Persons with Multiple Sclerosis: A Pilot Study. J. Electromyogr. Kinesiol. 2021, 61, 102607. [Google Scholar] [CrossRef]
- Malone, L.A.; Bastian, A.J. Thinking About Walking: Effects of Conscious Correction Versus Distraction on Locomotor Adaptation. J. Neurophysiol. 2010, 103, 1954. [Google Scholar] [CrossRef]
- Sasikumar, S.; Sorrento, G.; Lang, A.E.; Strafella, A.P.; Fasano, A. Cognition Affects Gait Adaptation after Split-Belt Treadmill Training in Parkinson’s Disease. Neurobiol. Dis. 2023, 181, 106109. [Google Scholar] [CrossRef]


| Participant Characteristic | Mean | SD |
|---|---|---|
| N | 35 | |
| Age | 51.66 | 12.02 |
| Sex | 61% Female | |
| BMI | 25.37 | 4.19 |
| Activity (min per week) | 289.4 | 266.8 |
| Years since diagnosis | 13.85 | 10.73 |
| Falls in last 6 months | 0.65 | 1.02 |
| Reported neuropathy | 85% | |
| EDSS | 3.57 | 1.03 |
| MFIS | 31.39 | 14.98 |
| MSWS-12 | 21.89 | 12.08 |
| BDI | 7.63 | 7.06 |
| MOCA | 27.25 | 2.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagen, A.C.; Acosta, J.S.; Geltser, C.S.; Fling, B.W. Split-Belt Treadmill Adaptation Improves Spatial and Temporal Gait Symmetry in People with Multiple Sclerosis. Sensors 2023, 23, 5456. https://doi.org/10.3390/s23125456
Hagen AC, Acosta JS, Geltser CS, Fling BW. Split-Belt Treadmill Adaptation Improves Spatial and Temporal Gait Symmetry in People with Multiple Sclerosis. Sensors. 2023; 23(12):5456. https://doi.org/10.3390/s23125456
Chicago/Turabian StyleHagen, Andrew C., Jordan S. Acosta, Chaia S. Geltser, and Brett W. Fling. 2023. "Split-Belt Treadmill Adaptation Improves Spatial and Temporal Gait Symmetry in People with Multiple Sclerosis" Sensors 23, no. 12: 5456. https://doi.org/10.3390/s23125456
APA StyleHagen, A. C., Acosta, J. S., Geltser, C. S., & Fling, B. W. (2023). Split-Belt Treadmill Adaptation Improves Spatial and Temporal Gait Symmetry in People with Multiple Sclerosis. Sensors, 23(12), 5456. https://doi.org/10.3390/s23125456








