2-Mercaptobenzimidazole Functionalized Copper Nanoparticles Fluorescence Probe for Sensitivity and Selectivity Detection of Cys in Serum
Abstract
1. Introduction
2. Experiment
2.1. Chemical Reagents
2.2. Instrument
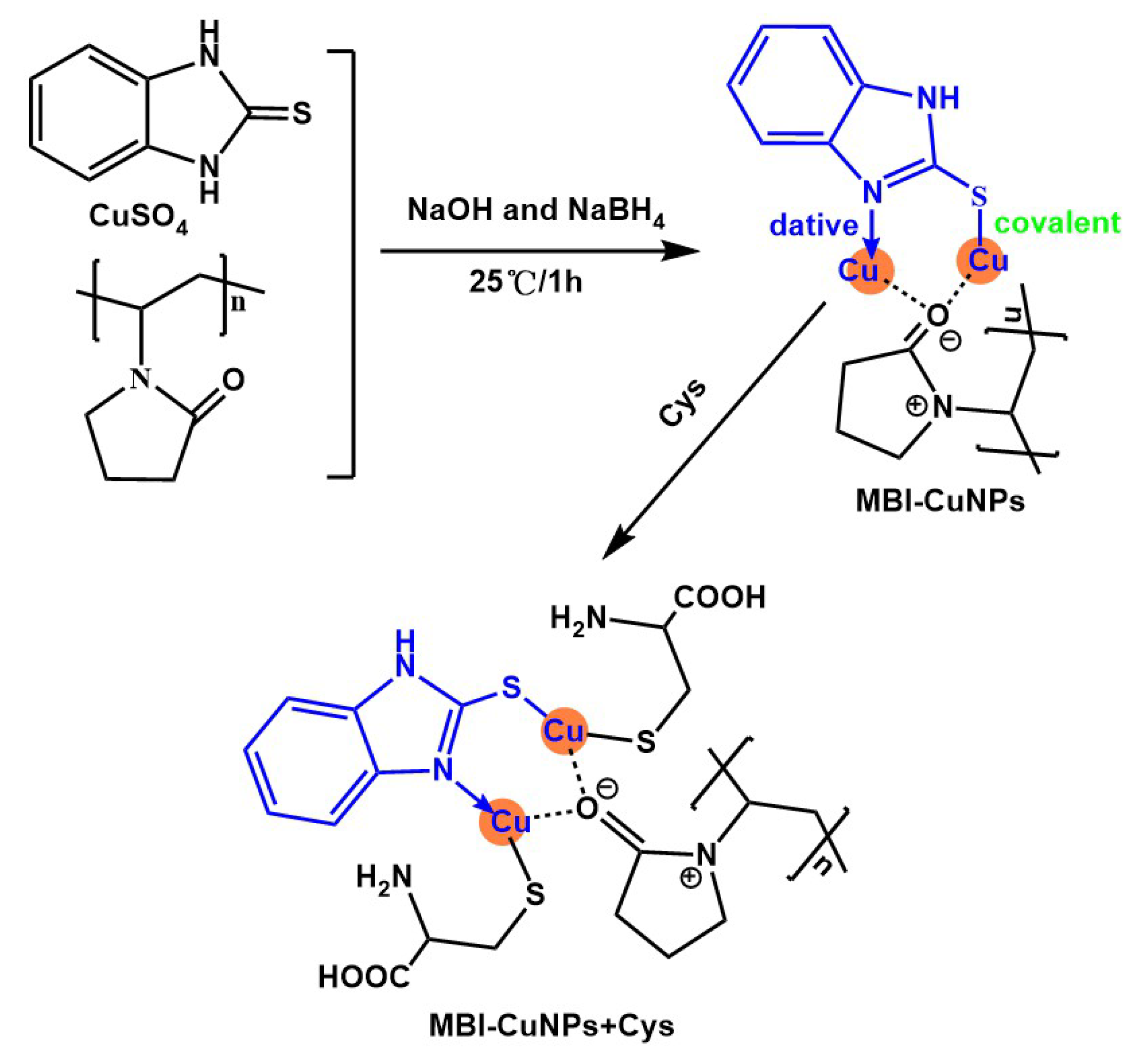
2.3. Preparation of MBI-CuNPs
2.4. Detection of Cys
2.5. Detection of Cys in Serum
3. Results and Discussion
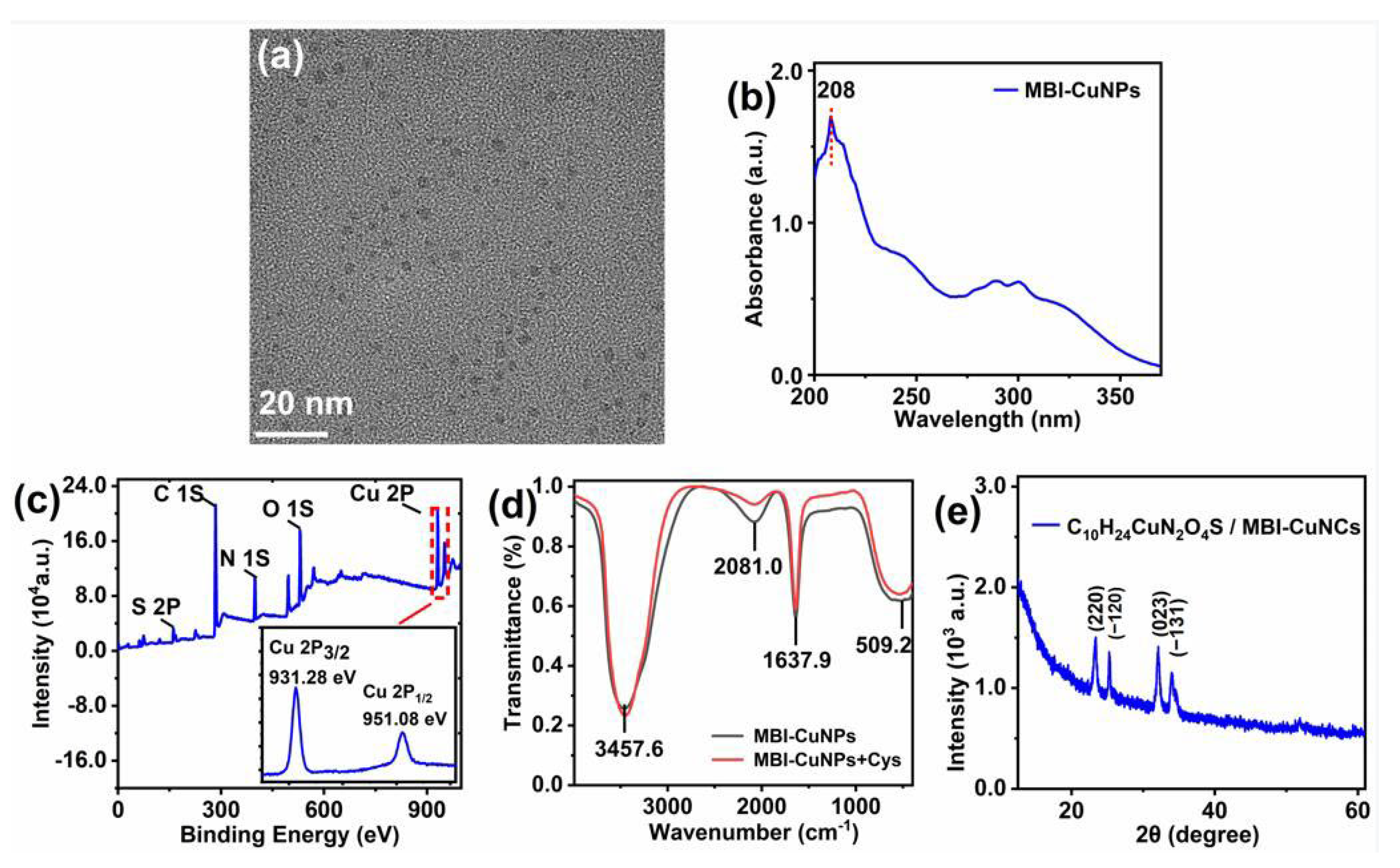
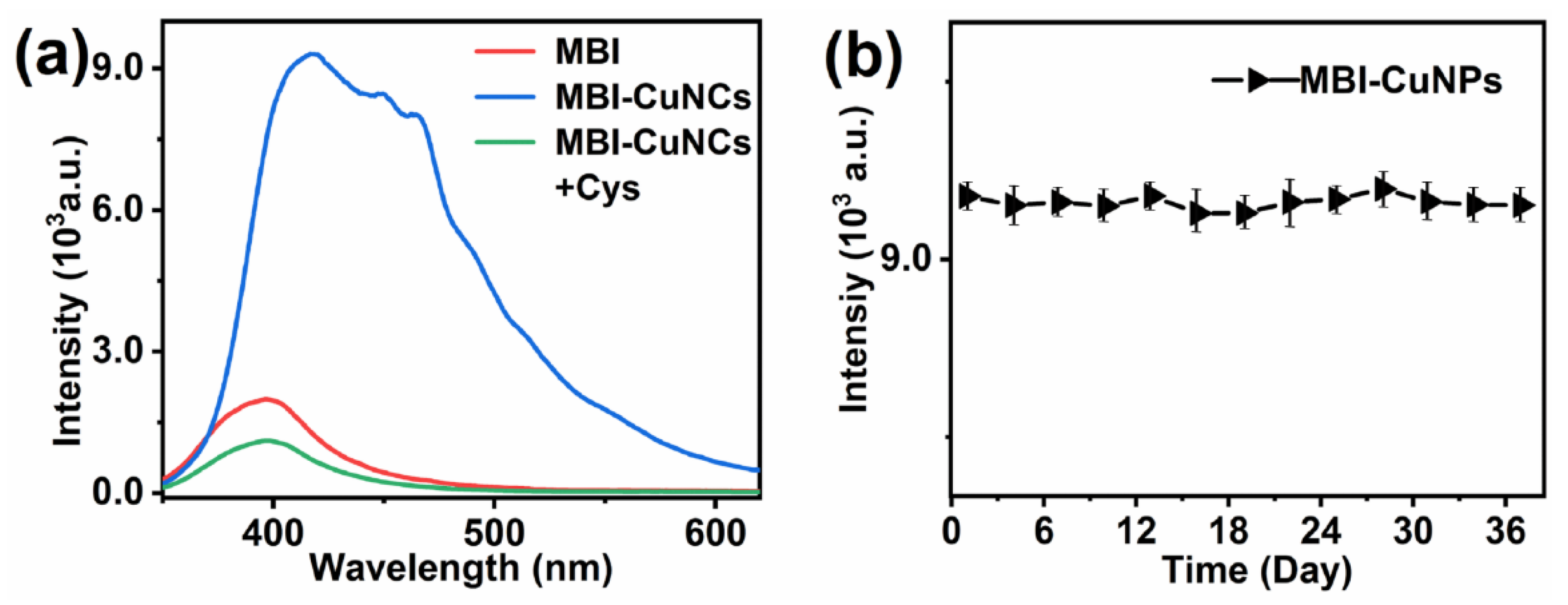
3.1. Characterization of MBI-CuNPs
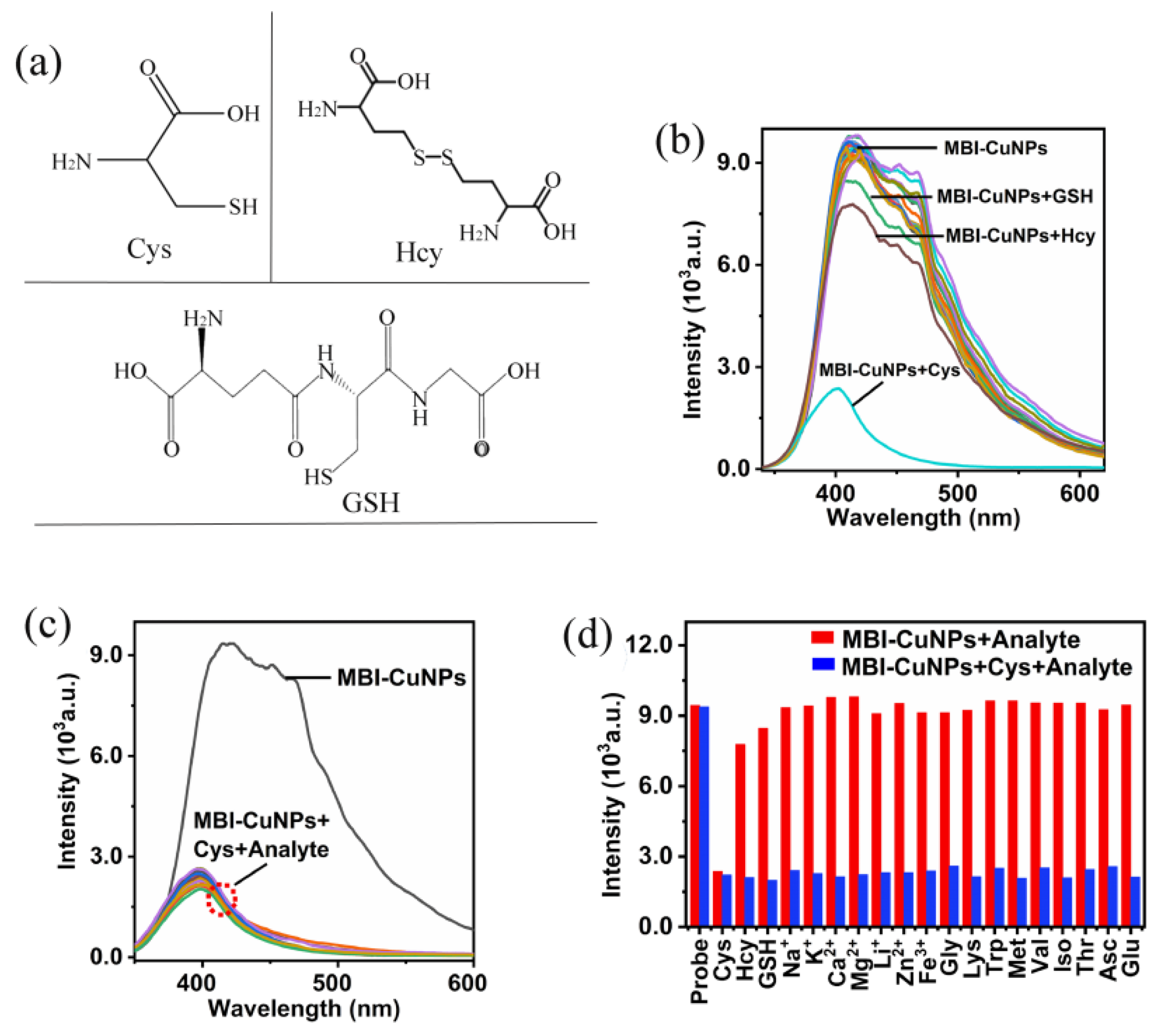
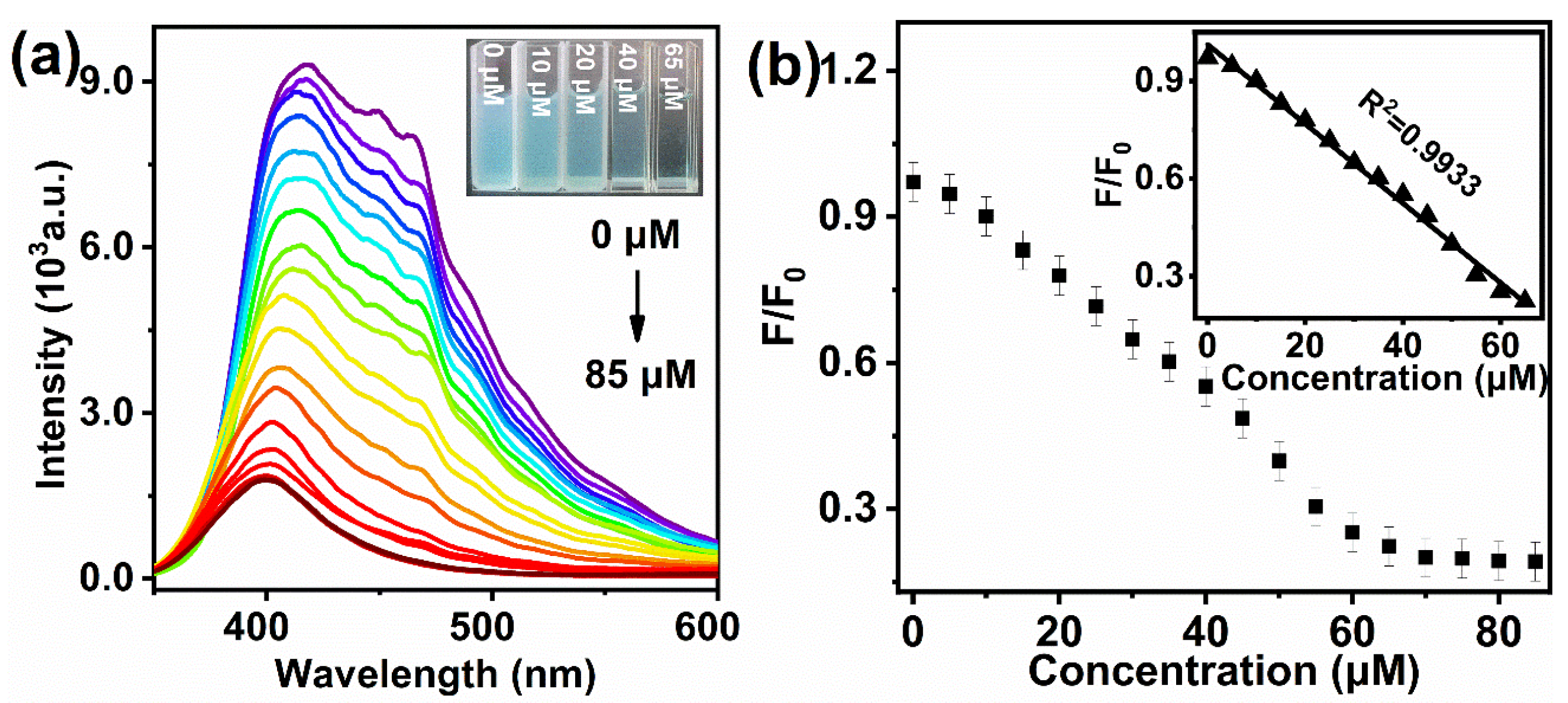
3.2. Response of MBI-CuNPs Probe to Cys
3.3. Analysis of Cys in Serum Samples
3.4. Research on Response Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Liu, W.; Zhang, P.; Zhang, H.; Wu, J.; Ge, J.; Wang, P. A fluorescent probe for the efficient discrimination of Cys, Hcy and GSH based on different cascade reactions. Biosens. Bioelectron. 2016, 11, 11021. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, T.; Ma, Y.; Lin, W. Discriminating Cys from GSH/H2S in vitro and in vivo with a NIR fluorescent probe. Sens. Actuators B Chem. 2019, 127, 127202. [Google Scholar] [CrossRef]
- Liu, X.-L.; Niu, L.-Y.; Chen, Y.-Z.; Yang, Y.; Yang, Q.-Z. A Multi-emissive Fluorescent Probe for the Discrimination of Glutathione and Cysteine. Biosens. Bioelectron. 2016, 6, 06076. [Google Scholar] [CrossRef] [PubMed]
- Rani, B.K.; John, S.A. A novel pyrene based fluorescent probe for selective detection of cysteine in presence of other bio-thiols in living cells. Biosens. Bioelectron. 2016, 4, 04013. [Google Scholar] [CrossRef]
- Xue, S.; Ding, S.; Zhai, Q. A readily available colorimetric and near-infrared fluorescent turn-on probe for rapid and selective detection of cysteine in living cells. Biosens. Bioelectron. 2015, 68, 316–321. [Google Scholar] [CrossRef]
- Nehra, N.; Ghule, V.D.; Tittal, R.K. Simpler fluorescent probe for homocysteine selective detection. J. Mol. Struct. 2022, 1250, 131755. [Google Scholar] [CrossRef]
- Yin, K.; Yu, F.; Zhang, W.; Chen, L. A near-infrared ratiometric fluorescent probe for cysteine detection over glutathione indicating mitochondrial oxidative stress in vivo. Biosens. Bioelectron. 2015, 6, 06039. [Google Scholar] [CrossRef]
- Xie, J.-H.; Mu, R.; Fang, M.-X.; Cheng, Y.-F.; Senchyna, F.; Moreno, A.; Banaeibcd, N.; Rao, J.-H. A dual-caged resorufin probe for rapid screening of infections resistant to lactam antibiotics. Chem. Sci. 2021, 12, 9153–9161. [Google Scholar] [CrossRef]
- Nrhra, N.; Kaushik, R.; Vikas, G.; Tittal, R.K. Simpler Molecular Structure as Selective & Sensitive ESIPT-based Fluorescent Probe for Cysteine and Homocysteine Detection with DFT Studies. Mol. Struct. 2020, 1207, 127839. [Google Scholar]
- Zhang, X.; He, N.; Huang, Y.; Yu, F.; Li, B.; Lv, L.; Chen, L. Mitochondria-targeting near-infrared ratiometric fluorescent probe for selective imaging of cysteine in orthotopic lung cancer mice. Sens. Actuators B 2018, 56, 320100. [Google Scholar] [CrossRef]
- Lu, X.; Wu, M.; Wang, S.; Qin, J.; Li, P. Development of a NIR fluorescent probe for the detection of intracellular cysteine and glutathione and the monitoring of the drug resistance. Talanta 2021, 235, 122771. [Google Scholar] [CrossRef]
- Yang, X.-Z.; Wei, X.-R.; Sun, R.; Wu, Y.-J.; Ge, J.-F. Benzoxazine-based fluorescent probes with different auxochrome groups for cysteine detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 226, 117582. [Google Scholar] [CrossRef]
- Zhao, G.; Yang, W.; Li, F.; Deng, Z.; Hu, Y. A turn-on fluorescent probe for real-time detection of endogenous cysteine in living cells. J. Lumin. 2020, 226, 117506. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, X.; Gu, W.; Cheng, T.; Wang, B.; Jiang, Y.; Shen, J. A novel naphthalene-based fluorescent probe for highly selective detection of cysteine with a large Stokes shift and its application in bioimaging. R. Soc. Chemstry 2018, 42, 18109–18116. [Google Scholar] [CrossRef]
- Cao, C.; Feng, Y.; Li, H.; Yang, Y.; Song, X.; Wang, Y.; Zhang, G.; Dou, W.; Liu, W. A simple highly selective probe for discriminative visualization of endogenous cysteine, homocysteine and glutathione in living cells via three separated fluorescence channels. Talanta 2020, 219, 121353. [Google Scholar] [CrossRef]
- Qi, S.; Zhang, H.; Wang, X.; Lv, J.; Liu, D.; Shen, W.; Li, Y.; Du, J.; Yang, Q. Development of a NIR fluorescent probe for highly selective and sensitive detection of cysteine in living cells and in vivo. Talanta 2021, 234, 122685. [Google Scholar] [CrossRef]
- Qiao, L.; Yang, Y.; Cai, J.; Lv, X.; Hao, J.; Li, Y. Long wavelength emission fluorescent probe for highly selective detection of cysteine in living cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 264, 120247. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, C.; Jiao, X.; Cai, S.; He, S.; Zhao, L.; Zeng, X.; Wang, T. Lysosome-targeted near-infrared fluorescent dye and its application in designing of probe for sensitive detection of cysteine in living cells. Dye. Pigment. 2021, 190, 109203. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, Y.; Li, H.; Shen, R.; Wang, Y.; Song, X.; Cao, C.; Zhang, G.; Liu, W. Hydro-soluble NIR fluorescent probe with multiple sites and multiple excitations for distinguishing visualization of endogenous Cys/Hcy, and GSH. Sens. Actuators B Chem. 2020, 129, 129189. [Google Scholar] [CrossRef]
- Hu, Q.; Yu, C.; Xia, X.; Zeng, F.; Wu, S. A fluorescent probe for simultaneous discrimination of GSH and Cys/Hcy in human serum samples via distinctly-separated emissions with independent excitations. Biosens. Bioelectron. 2016, 81, 341–348. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Y.; Huo, F.G.; Liu, Y.; Yin, C. Dual-channel red fluorescent probe for detection of Cys/Hcy and GSH in plants. Sens. Actuators B Chem. 2019, 127, 127123. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, B.; Yang, W.; Jing, J.; Zhang, X. A fluorescent probe for differentiating Cys, Hcy and GSH via a stepwise interaction. Sens. Actuators B Chem. 2018, 1, 01181. [Google Scholar] [CrossRef]
- Angarano, V.; Akkermans, S.; Smet, C.; Chieffi, A.; Van Impe, J.F.M. The potential of violet, blue, green and red light for the inactivation of P. fluorescens as planktonic cells, individual cells on a surface and biofilms. Food Bioprod. Process. 2020, 19, 304818. [Google Scholar] [CrossRef]
- Becerra-Ruiz, M.; Vargas, V.; Jara, P.; Tirapegui, C.; Carrasco, C.; Nuñez, M.; Lezana, N.; Galdámez, A.; Vilches-Herrera, M. Blue-fluorescent probes for lipid droplets based on pyrazolepyridine or pyrrolopyridine fused dihydrochromeno. Eur. J. Org. Chem. 2017, 10, 701633. [Google Scholar]
- Ford, P.C.; Cariati, E.; Bourassa, J. Photoluminescence Properties of Multinuclear Copper(I) Compounds. Chem. Rev. 1999, 99, 3625–3647. [Google Scholar] [CrossRef] [PubMed]
- Kozlica, D.K.; Kokalj, A.; Milošev, I. Synergistic effect of 2-mercaptobenzimidazole and octylphosphonic acid as corrosion inhibitors for copper and aluminium–An electrochemical, XPS, FTIR and DFT study. Corros. Sci. 2020, 5, 109082. [Google Scholar] [CrossRef]
- Granata, G.; Onoguchi, A.; Tokoro, C. Preparation of copper nanoparticles for metal-metal bonding by aqueous reduction with D-glucose and PVP. Chem. Eng. Sci. 2019, 209, 115210. [Google Scholar] [CrossRef]
- Cheng, Y.; Deng, S.; Sun, F.; Zhou, Y.-H. Synthesis of luminescent Cu9S5 nanoclusters from copper-2,5-dimercapto-1, 3,4-thiadiazole coordination polymer as pH sensor. J. Lumin. 2019, 210, 38–46. [Google Scholar] [CrossRef]
- Momeni, S.; Ahmadi, R.; Safavi, A.; Nabipour, I. Blue-emitting copper nanoparticles as a fluorescent probe for detection of cyanide ions. Talanta 2017, 175, 514–521. [Google Scholar] [CrossRef]
- Aparna, R.S.; Devi, J.S.A.; Anjana, R.R.; Nebu, J.; George, S. Zn(II) ion modulated red emitting copper nanocluster probe for the fluorescence turn on sensing of RDX. Sens. Actuators B Chem. 2019, 291, 298–305. [Google Scholar] [CrossRef]
- Manimozhi, T.; Archana, J.; Navaneethan, M.; Ramamurthi, K. Morphology and phase controlled synthesis of PVP-assisted copper antimony sulfide microstructures using solvothermal method and their properties. Mater. Sci. Semicond. Process. 2019, 103, 104606. [Google Scholar] [CrossRef]
- Lin, L.; Hu, Y.; Zhang, L.; Huang, Y.; Zhao, S. Photoluminescence Light-up Detection of Zinc Ion and Imaging in Living Cells Based on the Aggregation Induced Emission Enhancement of Glutathione-capped Copper Nanoclusters. Biosens. Bioelectron. 2017, 17, 703038. [Google Scholar] [CrossRef]
- Chen, Q.; Zhan, Y.; Zhen, Y.; Lin, X.; Zheng, Q. Poly(N-vinyl-2-pyrrolidone)-capped CdS Nanocrystals: Polyol Synthesis, Characterization and Visible-Hght Photocatalysis. J. Inorg. Chem. 2008, 24, 605–609. [Google Scholar]
- Gayathri, R.; Rajalakshmi, P.S.; Thomas, A.; Rengan, A.K. Doxorubicin loaded polyvinylpyrrolidone-copper sulfide nanoparticles enabling mucoadhesiveness and chemo-photothermal synergism for effective killing of breast cancer cells. Materialia 2021, 19, 101195. [Google Scholar]
- Li, L.; Chen, J.; Li, Y.; Song, N.; Zhu, L.; Li, Z. Synthesis of fluorescent pink emitting copper nanoparticles and sensitive detection of a-naphthaleneacetic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 224, 117433. [Google Scholar] [CrossRef]
- Rahimpour, E.; Hosseini, M.B.; Jouyban, A. Copper nanocluster-based sensor for determination of vancomycin in exhaled breath condensate: A synchronous fluorescence spectroscopy. J. Pharm. Biomed. Anal. 2021, 196, 113906. [Google Scholar] [CrossRef]
- Tewari, B.B. Paper electrophoretic determination of the stability constants of binary and ternary complexes of copper(II) and cobalt(II) with nitrilotriacetate and cysteine. J. Chromatogr. A 2006, 1103, 139–144. [Google Scholar] [CrossRef]
- Mu, X.; Tu, R.; Wang, H.; Li, M.-J.; Fu, F. Amino group-driven distinguishing homocysteine from cysteine and glutathione in photoluminesecent signal of the iridium(III) complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 263, 120167. [Google Scholar] [CrossRef]

| Probe | λex/nm | λem/nm | LOD/µM | Linear Range/μM | Literature |
|---|---|---|---|---|---|
| HDFC | 390 | 500 | 0.19 | 10–70 | [1] |
| BC | 400 | 452 | 0.366 | 0–100 | [2] |
| Cy-NB | 560 | 640 | 0.2 | 35–100 | [3] |
| Probe 1 | 612 | 690 | 0.18 | 0–25 | [4] |
| NR-NBD | 670 | 716 | 0.027 | 0.5–25 | [6] |
| NN | 510 | 559 | 0.05 | - | [7] |
| YF | 418 | 496 | 2.5 | 0–30 | [19] |
| NBD-O-1 | - | 550 | 0.061 | 0–27 | [20] |
| MBI-CuNPs | 200 | 415 | 0.052 | 0.05–65 | This work |
| Sample | Cys (µM) | Fund Cys (µM) | Absolute Recovery Rate (%) | RSD (%) |
|---|---|---|---|---|
| Serum sample | 0 | 3.30 | - | - |
| 5 | 8.15 | 97.00 | 0.41 | |
| 10 | 12.60 | 93.07 | 0.97 | |
| 20 | 21.34 | 90.23 | 0.49 | |
| 30 | 32.04 | 95.83 | 0.99 | |
| 40 | 39.97 | 91.69 | 2.73 | |
| 50 | 48.89 | 91.19 | 1.56 | |
| 65 | 63.76 | 93.02 | 1.86 | |
| 65 a | 64.28 a | 93.80 a | 1.96 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Dou, X.; Zhang, H. 2-Mercaptobenzimidazole Functionalized Copper Nanoparticles Fluorescence Probe for Sensitivity and Selectivity Detection of Cys in Serum. Sensors 2023, 23, 5814. https://doi.org/10.3390/s23135814
Liu J, Dou X, Zhang H. 2-Mercaptobenzimidazole Functionalized Copper Nanoparticles Fluorescence Probe for Sensitivity and Selectivity Detection of Cys in Serum. Sensors. 2023; 23(13):5814. https://doi.org/10.3390/s23135814
Chicago/Turabian StyleLiu, Jing, Xiaozong Dou, and Hongyan Zhang. 2023. "2-Mercaptobenzimidazole Functionalized Copper Nanoparticles Fluorescence Probe for Sensitivity and Selectivity Detection of Cys in Serum" Sensors 23, no. 13: 5814. https://doi.org/10.3390/s23135814
APA StyleLiu, J., Dou, X., & Zhang, H. (2023). 2-Mercaptobenzimidazole Functionalized Copper Nanoparticles Fluorescence Probe for Sensitivity and Selectivity Detection of Cys in Serum. Sensors, 23(13), 5814. https://doi.org/10.3390/s23135814







