Detection and Reconstruction of Poor-Quality Channels in High-Density EMG Array Measurements
Abstract
1. Introduction
2. Materials and Methods
2.1. EMG Database
2.2. Poor-Quality Channel Ground Truth
2.3. Simulated Poor-Quality Channels
2.4. Detection of Poor-Quality EMG Channels
2.4.1. Interpolation-Based Detection Method
2.4.2. RMS Detection Method
2.4.3. NMI Detection Method
2.5. Reconstruction of Poor-Quality EMG Channels
- Linear interpolation: The target channel is estimated with linear interpolation over two dimensions (i.e., bilinear interpolation) [18];
- Cubic interpolation: A cubic polynomial is fit on each edge of a Delaunay triangulation [18];
- Nearest-neighbour interpolation: The target channel is estimated as equivalent to the closest channel perpendicular to the muscle fibres.
2.6. Evaluation
2.6.1. Simulated Data
2.6.2. Real Data
3. Results
3.1. Detection Results: Simulation
3.2. Detection Results: Real Data
3.2.1. Rater Agreement
3.2.2. Detection
3.3. Reconstruction Results
4. Discussion
4.1. Detection: Simulated Data
4.1.1. SNR of Noisy Channels
4.1.2. Number of Noisy Channels
4.1.3. Location of Noisy Channels
4.2. Real Data
4.3. Reconstruction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| EMG | Electromyography |
| HD-EMG | High-density electromyography |
| LDOF | Local distance-based outlier factor |
| MAV | Mean absolute value |
| NMI | Normalized mutual information |
| PRD | Percent residual difference |
| RMS | Root mean square |
| SNR | Signal-to-noise ratio |
| WGN | White Gaussian noise |
References
- Bao, T.; Zaidi, S.A.R.; Xie, S.; Yang, P.; Zhang, Z.Q. A CNN-LSTM Hybrid Model for Wrist Kinematics Estimation Using Surface Electromyography. IEEE Trans. Instrum. Meas. 2021, 70, 2503809. [Google Scholar] [CrossRef]
- Wang, C.; Sivan, M.; Wang, D.; Zhang, Z.Q.; Li, G.Q.; Bao, T.; Xie, S.Q. Quantitative Elbow Spasticity Measurement Based on Muscle Activation Estimation Using Maximal Voluntary Contraction. IEEE Trans. Instrum. Meas. 2022, 71, 1–11. [Google Scholar] [CrossRef]
- Borghetti, M.; Sardini, E.; Serpelloni, M. Sensorized Glove for Measuring Hand Finger Flexion for Rehabilitation Purposes. IEEE Trans. Instrum. Meas. 2013, 62, 3308–3314. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, X.; Fan, J.; Ye, X.; Dai, C.; Clancy, E.A.; Chen, W. Measuring Neuromuscular Electrophysiological Activities to Decode HD-sEMG Biometrics for Cross-Application Discrepant Personal Identification with Unknown Identities. IEEE Trans. Instrum. Meas. 2022, 71, 4009515. [Google Scholar] [CrossRef]
- Fraser, G.D.; Chan, A.D.C.; Green, J.R.; MacIsaac, D.T. Automated Biosignal Quality Analysis for Electromyography Using a One-Class Support Vector Machine. IEEE Trans. Instrum. Meas. 2014, 63, 2919–2930. [Google Scholar] [CrossRef]
- Thongpanja, S.; Phinyomark, A.; Quaine, F.; Laurillau, Y.; Limsakul, C.; Phukpattaranont, P. Probability Density Functions of Stationary Surface EMG Signals in Noisy Environments. IEEE Trans. Instrum. Meas. 2016, 65, 1547–1557. [Google Scholar] [CrossRef]
- Farago, E.; Macisaac, D.; Suk, M.; Chan, A.D.C. A Review of Techniques for Surface Electromyography Signal Quality Analysis. IEEE Rev. Biomed. Eng. 2022, 16, 472–486. [Google Scholar] [CrossRef] [PubMed]
- Marateb, H.R.; Rojas-Martínez, M.; Mansourian, M.; Merletti, R.; Villanueva, M.A. Outlier detection in high-density surface electromyographic signals. Med. Biol. Eng. Comput. 2012, 50, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Tallam Puranam Raghu, S.; MacIsaac, D.; Chan, A.D. Automated Biomedical Signal Quality Assessment of Electromyograms: Current Challenges and Future Prospects. IEEE Instrum. Meas. Mag. 2022, 25, 12–19. [Google Scholar] [CrossRef]
- Marateb, H.R.; Rojas-Martínez, M.; Mañanas Villanueva, M.A.; Merletti, R. Robust outlier detection in high-density surface electromyographic signals. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 4850–4853. [Google Scholar] [CrossRef]
- Rojas-Martínez, M.; Mañanas, M.A.; Alonso, J.F. High-density surface EMG maps from upper-arm and forearm muscles. J. Neuroeng. Rehabil. 2012, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bingham, A.; Jelfs, B.; Arjunan, S.P.; Kumar, D.K. Identifying Noisy Electrodes in High Density Surface Electromyography Recordings Through Analysis of Spatial Similarities. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 2325–2328. [Google Scholar] [CrossRef]
- Farago, E.; Chan, A.D.C. Evaluation of interpolation methods for EMG arrays. In Proceedings of the 2022 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Ottawa, ON, Canada, 16–19 May 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Afsharipour, B.; Soedirdjo, S.; Merletti, R. Two-dimensional surface EMG: The effects of electrode size, interelectrode distance and image truncation. Biomed. Signal Process. Control. 2019, 49, 298–307. [Google Scholar] [CrossRef]
- Rojas-Martínez, M.; Mañanas, M.A.; Alonso, J.F.; Merletti, R. Identification of isometric contractions based on High Density EMG maps. J. Electromyogr. Kinesiol. 2013, 23, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Martínez, M.; Serna, L.Y.; Jordanic, M.; Marateb, H.R.; Merletti, R.; Mañanas, M.A. High-density surface electromyography signals during isometric contractions of elbow muscles of healthy humans. Sci. Data 2020, 7, 397. [Google Scholar] [CrossRef] [PubMed]
- Bingham, A. Applications of Normalised Mutual Information in High Density Surface Electromyography. Ph.D. Thesis, RMIT University, Melbourne, VIC, Australia, 2019. [Google Scholar]
- Kreyszig, E. Advanced Engineering Mathematics, 9th ed.; John Wiley & Sons Inc.: Singapore, 2006. [Google Scholar]
- Segeth, K. Polyharmonic splines generated by multivariate smooth interpolation. Comput. Math. Appl. 2019, 78, 3067–3076. [Google Scholar] [CrossRef]
- Sandwell, D.T. Biharmonic spline interpolation of GEOS-3 and SEASAT altimeter data. Geophys. Res. Lett. 1987, 14, 139–142. [Google Scholar] [CrossRef]
- Powers, D.M. Evaluation: From precision, recall and F-measure to ROC, informedness, markedness and correlation. arXiv 2020, arXiv:2010.16061. [Google Scholar]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Medica 2012, 22, 276–282. [Google Scholar] [CrossRef]

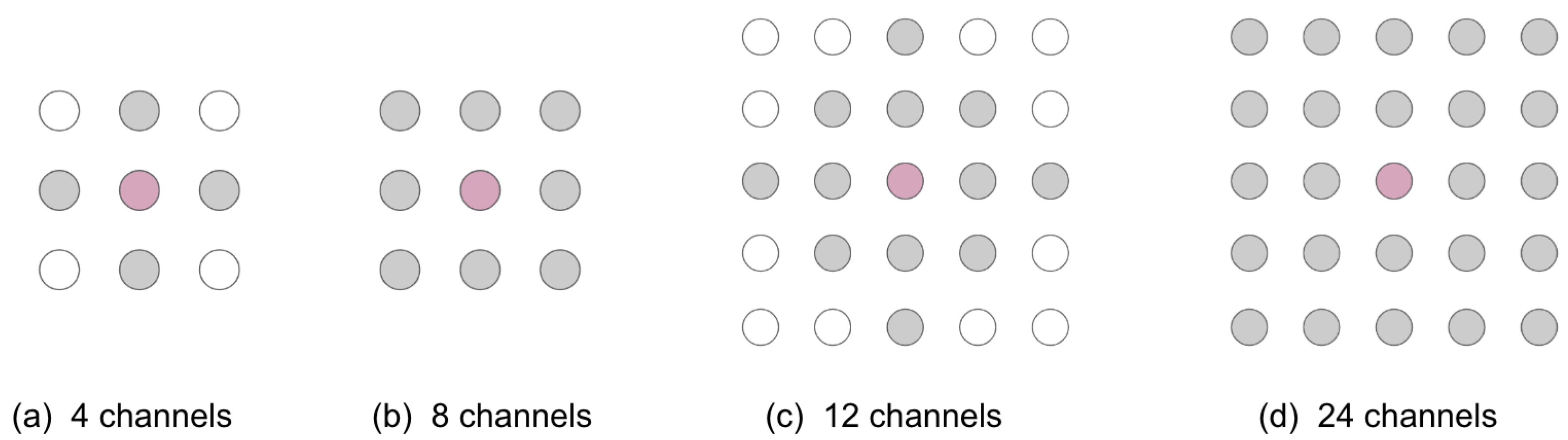


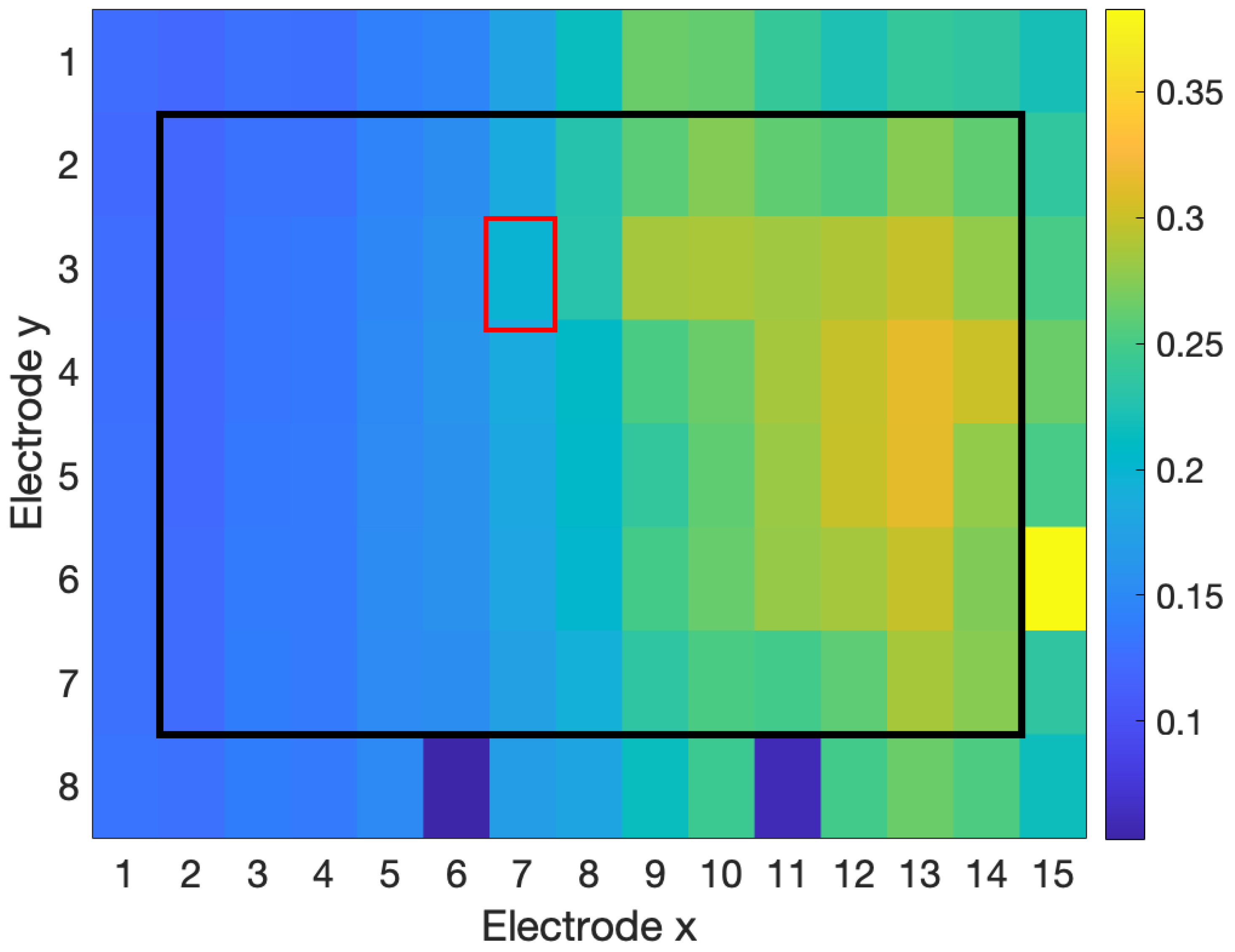

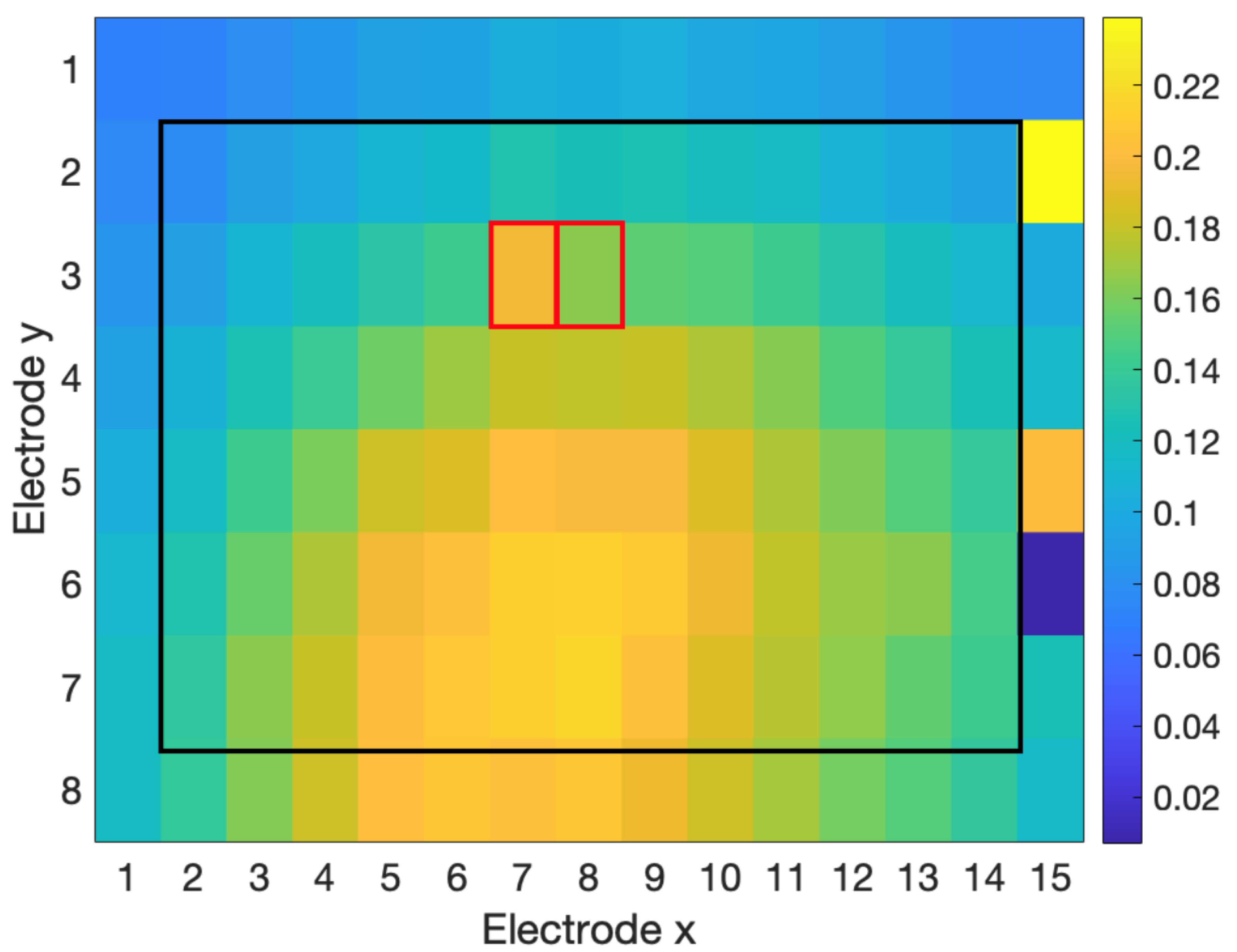
| Interpolation Method | 4 Channels | 8 Channels | 12 Channels | 24 Channels |
|---|---|---|---|---|
| Linear | 21.6 ± 15.6 | 21.7 ± 15.7 | 21.9 ± 15.8 | 21.9 ± 16.0 |
| Triangular Cubic | 21.6 ± 15.6 | 19.8 ± 14.9 | 19.2 ± 15.2 | 19.3 ± 15.3 |
| Biharmonic Spline | 16.9 ± 12.1 | 17.0 ± 12.4 | 17.9 ± 10.8 | 15.5 ± 12.1 |
| Nearest Neighbour | 28.4 ± 31.9 | 28.4 ± 14.1 | 28.5 ± 14.2 | 28.5 ± 14.6 |
| SNR (db) | Interpolation | RMS | NMI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P | R | F | P | R | F | P | R | F | |
| −20 | 100 | 100 | 100 | 100 | 100 | 100 | 68.8 | 100 | 81.5 |
| −15 | 100 | 100 | 100 | 100 | 100 | 100 | 68.8 | 100 | 81.5 |
| −10 | 100 | 100 | 100 | 100 | 96.1 | 98.0 | 68.8 | 100 | 81.5 |
| −5 | 100 | 100 | 100 | 100 | 71.3 | 83.3 | 68.8 | 100 | 81.5 |
| 0 | 100 | 98.2 | 99.1 | 100 | 24.8 | 39.7 | 61.1 | 100 | 75.9 |
| 5 | 100 | 19.1 | 32.1 | 100 | 0.30 | 0.60 | 41.7 | 45.5 | 43.5 |
| 10 | – | 0 | – | – | 0 | – | 25.0 | 27.3 | 26.1 |
| 15 | – | 0 | – | – | 0 | – | 10.0 | 9.1 | 9.5 |
| SNR (db) | Interpolation | RMS | NMI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P | R | F | P | R | F | P | R | F | |
| −20 | 100 | 100 | 100 | 100 | 100 | 100 | 84.6 | 100 | 91.7 |
| −15 | 100 | 100 | 100 | 100 | 100 | 100 | 84.6 | 100 | 91.7 |
| −10 | 100 | 100 | 100 | 100 | 95.7 | 97.8 | 88.0 | 100 | 93.6 |
| −5 | 100 | 100 | 100 | 100 | 68.9 | 81.6 | 88.0 | 100 | 93.6 |
| 0 | 100 | 97.8 | 98.9 | 100 | 22.8 | 37.1 | 84.6 | 100 | 91.7 |
| 5 | 100 | 6.0 | 11.3 | 100 | 0.30 | 0.59 | 66.7 | 45.5 | 54.1 |
| 10 | 100 | 0.45 | 0.89 | – | 0 | – | 41.7 | 22.7 | 29.4 |
| 15 | – | 0 | – | – | 0 | – | 18.2 | 9.1 | 12.1 |
| SNR (db) | Interpolation | RMS | NMI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P | R | F | P | R | F | P | R | F | |
| −20 | 100 | 100 | 100 | 100 | 99.8 | 99.9 | 93.0 | 90.9 | 92.0 |
| −15 | 100 | 100 | 100 | 100 | 99.8 | 99.9 | 95.7 | 100 | 97.8 |
| −10 | 100 | 100 | 100 | 100 | 96.2 | 98.1 | 95.7 | 100 | 97.8 |
| −5 | 100 | 100 | 100 | 100 | 67.6 | 80.6 | 95.7 | 100 | 97.8 |
| 0 | 100 | 99.0 | 99.5 | 100 | 15.6 | 27.0 | 95.3 | 93.2 | 94.3 |
| 5 | 100 | 13.5 | 24.0 | 100 | 0.30 | 0.59 | 88.0 | 50.0 | 63.8 |
| 10 | 100 | 0.52 | 1.0 | – | 0 | – | 75.0 | 27.3 | 40.0 |
| 15 | – | 0 | – | – | 0 | – | 41.7 | 11.4 | 17.9 |
| SNR (db) | Interpolation | RMS | NMI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P | R | F | P | R | F | P | R | F | |
| −20 | 100 | 100 | 100 | 100 | 96.9 | 98.4 | 97.0 | 73.9 | 83.9 |
| −15 | 100 | 100 | 100 | 100 | 95.1 | 97.5 | 98.8 | 93.2 | 95.9 |
| −10 | 100 | 100 | 100 | 100 | 87.1 | 93.1 | 98.9 | 100 | 99.4 |
| −5 | 100 | 100 | 100 | 100 | 55.7 | 71.6 | 98.9 | 100 | 99.4 |
| 0 | 100 | 99.9 | 99.9 | 100 | 6.8 | 12.7 | 98.7 | 88.6 | 93.4 |
| 5 | 100 | 19.8 | 33.1 | 100 | 0.22 | 0.44 | 96.1 | 55.7 | 70.5 |
| 10 | 100 | 0.33 | 0.66 | – | 0 | – | 93.8 | 34.1 | 50.0 |
| 15 | – | 0 | – | – | 0 | – | 70.0 | 15.9 | 24.9 |
| SNR (db) | Interpolation | RMS | NMI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P | R | F | P | R | F | P | R | F | |
| −20 | 100 | 100 | 100 | 100 | 99.9 | 99.9 | 83.3 | 90.0 | 87.0 |
| −15 | 100 | 100 | 100 | 100 | 100 | 100 | 84.0 | 100 | 91.3 |
| −10 | 100 | 100 | 100 | 100 | 94.4 | 97.1 | 88.5 | 100 | 93.9 |
| −5 | 100 | 100 | 100 | 100 | 69.4 | 82.0 | 85.2 | 100 | 92.0 |
| 0 | 100 | 98.7 | 99.3 | 100 | 19.4 | 32.4 | 85.2 | 100 | 92.0 |
| 5 | 100 | 3.5 | 6.7 | 100 | 0.14 | 0.29 | 80.0 | 72.7 | 76.2 |
| 10 | 100 | 0.15 | 0.30 | – | 0 | – | 53.8 | 31.8 | 40.0 |
| 15 | – | 0 | – | – | 0 | – | 27.3 | 12.5 | 17.1 |
| SNR (db) | Interpolation | RMS | NMI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P | R | F | P | R | F | P | R | F | |
| −20 | 100 | 100 | 100 | 100 | 98.8 | 99.4 | 87.9 | 64.4 | 74.4 |
| −15 | 99.9 | 100 | 100 | 100 | 97.5 | 98.7 | 93.0 | 85.1 | 88.9 |
| −10 | 99.9 | 100 | 100 | 100 | 89.3 | 94.4 | 92.6 | 100 | 96.7 |
| −5 | 99.9 | 100 | 99.9 | 100 | 63.5 | 77.6 | 93.8 | 100 | 96.7 |
| 0 | 100 | 98.0 | 99.0 | 100 | 18.3 | 31.0 | 94.0 | 100 | 96.9 |
| 5 | 100 | 3.8 | 7.4 | 100 | 0.37 | 0.73 | 90.6 | 63.0 | 74.4 |
| 10 | 100 | 0.07 | 0.14 | – | 0 | – | 75.0 | 32.6 | 45.5 |
| 15 | – | 0 | – | – | 0 | – | 41.7 | 10.4 | 15.7 |
| SNR (db) | Interpolation | RMS | NMI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P | R | F | P | R | F | P | R | F | |
| −20 | 99.9 | 100 | 100 | 100 | 89.9 | 94.7 | 98.1 | 57.3 | 72.3 |
| −15 | 99.7 | 100 | 99.9 | 100 | 87.0 | 93.0 | 97.4 | 82.4 | 80.3 |
| −10 | 99.9 | 100 | 99.9 | 100 | 78.6 | 88.0 | 97.9 | 100 | 98.9 |
| −5 | 99.9 | 100 | 99.9 | 100 | 55.1 | 71.0 | 98.9 | 100 | 99.4 |
| 0 | 99.9 | 97.6 | 98.8 | 100 | 13.3 | 23.4 | 97.6 | 89.9 | 92.6 |
| 5 | 98.3 | 2.2 | 4.2 | 100 | 0.18 | 0.37 | 96.0 | 52.2 | 67.6 |
| 10 | 100 | 0.26 | 0.51 | – | 0 | – | 92.9 | 28.0 | 43.0 |
| 15 | – | 0 | – | – | 0 | – | 71.4 | 10.8 | 18.7 |
| Interpolation | RMS | NMI | ||||||
|---|---|---|---|---|---|---|---|---|
| P | R | F | P | R | F | P | R | F |
| 94.7 | 94.7 | 94.7 | 83.3 | 52.6 | 64.5 | 35.6 | 84.2 | 50.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farago, E.; Chan, A.D.C. Detection and Reconstruction of Poor-Quality Channels in High-Density EMG Array Measurements. Sensors 2023, 23, 4759. https://doi.org/10.3390/s23104759
Farago E, Chan ADC. Detection and Reconstruction of Poor-Quality Channels in High-Density EMG Array Measurements. Sensors. 2023; 23(10):4759. https://doi.org/10.3390/s23104759
Chicago/Turabian StyleFarago, Emma, and Adrian D. C. Chan. 2023. "Detection and Reconstruction of Poor-Quality Channels in High-Density EMG Array Measurements" Sensors 23, no. 10: 4759. https://doi.org/10.3390/s23104759
APA StyleFarago, E., & Chan, A. D. C. (2023). Detection and Reconstruction of Poor-Quality Channels in High-Density EMG Array Measurements. Sensors, 23(10), 4759. https://doi.org/10.3390/s23104759






