Surface Electromyography-Driven Parameters for Representing Muscle Mass and Strength
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Bioimpedance Analysis
2.3. Ultrasound
2.4. Exercise Protocol
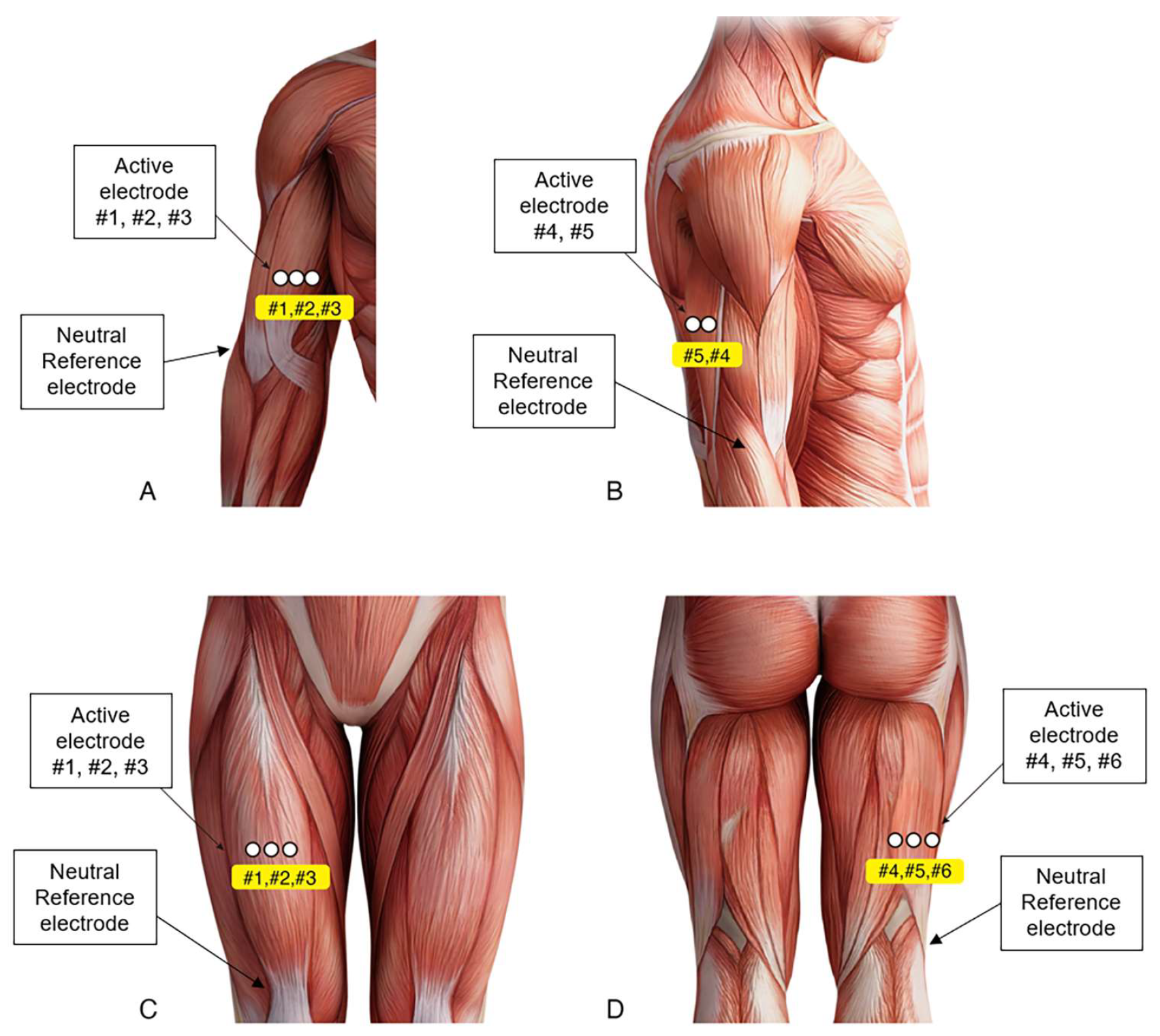
2.5. sEMG Activity Recording and Analysis
2.6. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. Correlation of sEMG with Other Parameters
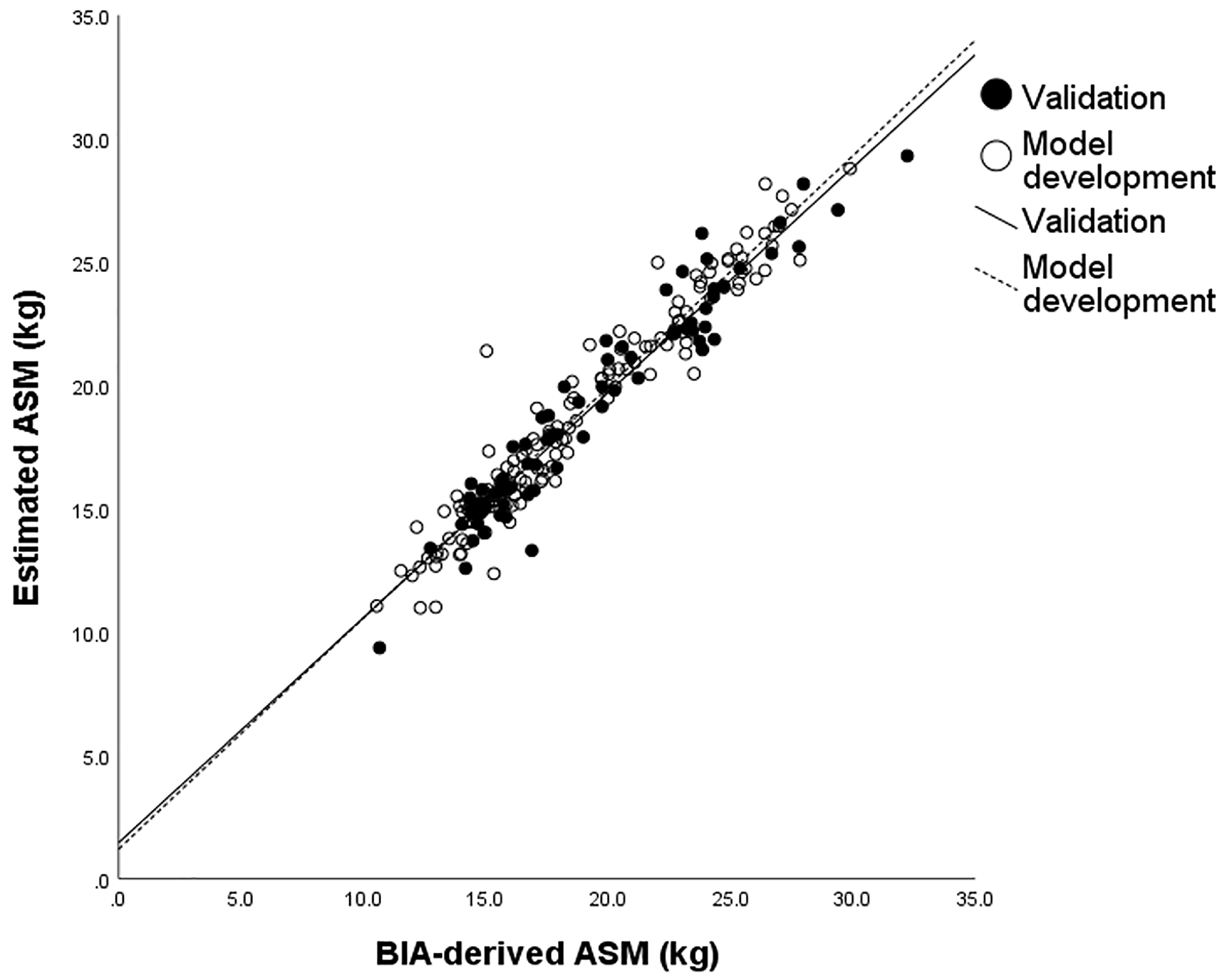
3.3. ASM Predicting Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef] [PubMed]
- Stringer, H.J.; Wilson, D. The Role of Ultrasound as a Diagnostic Tool for Sarcopenia. J Frailty Aging 2018, 7, 258–261. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.J. The use of surface electromyography in biomechanics. J. Appl. Biomech. 1997, 13, 135–163. [Google Scholar] [CrossRef]
- Habenicht, R.; Ebenbichler, G.; Bonato, P.; Kollmitzer, J.; Ziegelbecker, S.; Unterlerchner, L.; Mair, P.; Kienbacher, T. Age-specific differences in the time-frequency representation of surface electromyographic data recorded during a submaximal cyclic back extension exercise: A promising biomarker to detect early signs of sarcopenia. J. Neuroeng. Rehabil. 2020, 17, 8. [Google Scholar] [CrossRef]
- Leone, A.; Rescio, G.; Manni, A.; Siciliano, P.; Caroppo, A. Comparative Analysis of Supervised Classifiers for the Evaluation of Sarcopenia Using a sEMG-Based Platform. Sensors 2022, 22, 2721. [Google Scholar] [CrossRef]
- Park, J.W.; Baek, S.H.; Sung, J.H.; Kim, B.J. Predictors of Step Length from Surface Electromyography and Body Impedance Analysis Parameters. Sensors 2022, 22, 5686. [Google Scholar] [CrossRef] [PubMed]
- Biagetti, G.; Crippa, P.; Falaschetti, L.; Orcioni, S.; Turchetti, C. Human activity monitoring system based on wearable sEMG and accelerometer wireless sensor nodes. Biomed. Eng. Online 2018, 17, 132. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Sung, J.H.; Park, J.W.; Son, M.H.; Lee, J.H.; Kim, B.J. Usefulness of muscle ultrasound in appendicular skeletal muscle mass estimation for sarcopenia assessment. PLoS ONE 2023, 18, e0280202. [Google Scholar] [CrossRef] [PubMed]
- Güner Oytun, M.; Topuz, S.; Baş, A.O.; Çöteli, S.; Kahyaoğlu, Z.; Boğa, İ.; Ceylan, S.; Doğu, B.B.; Cankurtaran, M.; Halil, M. Relationships of Fall Risk With Frailty, Sarcopenia, and Balance Disturbances in Mild-to-Moderate Alzheimer’s Disease. J. Clin. Neurol. 2023, 19, 251–259. [Google Scholar] [CrossRef]
- Hirata, K.; Ito, M.; Nomura, Y.; Yoshida, T.; Yamada, Y.; Akagi, R. Can phase angle from bioelectrical impedance analysis associate with neuromuscular properties of the knee extensors? Front. Physiol. 2022, 13, 965827. [Google Scholar] [CrossRef] [PubMed]
- Sjøgaard, G.; Saltin, B. Extra- and intracellular water spaces in muscles of man at rest and with dynamic exercise. Am. J. Physiol. 1982, 243, R271–R280. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.C.; Heymsfield, S.B. Bioelectrical impedance analysis for diagnosing sarcopenia and cachexia: What are we really estimating? J. Cachexia Sarcopenia Muscle 2017, 8, 187–189. [Google Scholar] [CrossRef]
- McLester, C.N.; Nickerson, B.S.; Kliszczewicz, B.M.; McLester, J.R. Reliability and Agreement of Various InBody Body Composition Analyzers as Compared to Dual-Energy X-Ray Absorptiometry in Healthy Men and Women. J. Clin. Densitom. 2020, 23, 443–450. [Google Scholar] [CrossRef]
- SENIAM Management Group. Recommendations for Sensor Locations on Individual Muscles. Available online: http://seniam.org/sensor_location.htm (accessed on 12 July 2022).
- Abe, T.; Thiebaud, R.S.; Loenneke, J.P.; Young, K.C. Prediction and validation of DXA-derived appendicular lean soft tissue mass by ultrasound in older adults. Age 2015, 37, 114. [Google Scholar] [CrossRef] [PubMed]
- Staudenmann, D.; Roeleveld, K.; Stegeman, D.F.; van Dieën, J.H. Methodological aspects of SEMG recordings for force estimation--a tutorial and review. J. Electromyogr. Kinesiol. 2010, 20, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Vigotsky, A.D.; Halperin, I.; Lehman, G.J.; Trajano, G.S.; Vieira, T.M. Interpreting Signal Amplitudes in Surface Electromyography Studies in Sport and Rehabilitation Sciences. Front. Physiol. 2017, 8, 985. [Google Scholar] [CrossRef]
- Cavalcanti Garcia, M.A.; Vieira, T.M.M. Surface electromyography: Why, when and how to use it. Rev. Andal. Med. Deporte 2011, 4, 17–28. [Google Scholar]
- Blazevich, A.J.; Coleman, D.R.; Horne, S.; Cannavan, D. Anatomical predictors of maximum isometric and concentric knee extensor moment. Eur. J. Appl. Physiol. 2009, 105, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, T.; Miyatani, M.; Tachi, M.; Kouzaki, M.; Kawakami, Y.; Kanehisa, H. Muscle volume is a major determinant of joint torque in humans. Acta Physiol. Scand. 2001, 172, 249–255. [Google Scholar] [CrossRef]
- Narici, M.V.; Hoppeler, H.; Kayser, B.; Landoni, L.; Claassen, H.; Gavardi, C.; Conti, M.; Cerretelli, P. Human quadriceps cross-sectional area, torque and neural activation during 6 months strength training. Acta Physiol. Scand. 1996, 157, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, I.; Tanimoto, Y.; Takahashi, Y.; Kusabiraki, T.; Tamaki, J. Correlation between muscle strength and muscle mass, and their association with walking speed, in community-dwelling elderly Japanese individuals. PLoS ONE 2014, 9, e111810. [Google Scholar] [CrossRef] [PubMed]
- Freilich, R.J.; Kirsner, R.L.; Byrne, E. Isometric strength and thickness relationships in human quadriceps muscle. Neuromuscul. Disord. 1995, 5, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.H.; McGill, S.M. A comparison of ultrasound and electromyography measures of force and activation to examine the mechanics of abdominal wall contraction. Clin. Biomech. 2010, 25, 115–123. [Google Scholar] [CrossRef]
- Kim, C.Y.; Choi, J.D.; Kim, S.Y.; Oh, D.W.; Kim, J.K.; Park, J.W. Comparison between muscle activation measured by electromyography and muscle thickness measured using ultrasonography for effective muscle assessment. J. Electromyogr. Kinesiol. 2014, 24, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Trezise, J.; Collier, N.; Blazevich, A.J. Anatomical and neuromuscular variables strongly predict maximum knee extension torque in healthy men. Eur. J. Appl. Physiol. 2016, 116, 1159–1177. [Google Scholar] [CrossRef]
- Ruiz-Muñoz, M.; Cuesta-Vargas, A.I. Electromyography and sonomyography analysis of the tibialis anterior: A cross sectional study. J. Foot Ankle Res. 2014, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Monjo, H.; Fukumoto, Y.; Asai, T.; Shuntoh, H. Muscle Thickness and Echo Intensity of the Abdominal and Lower Extremity Muscles in Stroke Survivors. J. Clin. Neurol. 2018, 14, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Monjo, H.; Fukumoto, Y.; Asai, T.; Ohshima, K.; Kubo, H.; Tajitsu, H.; Koyama, S. Changes in Muscle Thickness and Echo Intensity in Chronic Stroke Survivors: A 2-Year Longitudinal Study. J. Clin. Neurol. 2022, 18, 308–314. [Google Scholar] [CrossRef]
- Walker, F.O.; Cartwright, M.S.; Alter, K.E.; Visser, L.H.; Hobson-Webb, L.D.; Padua, L.; Strakowski, J.A.; Preston, D.C.; Boon, A.J.; Axer, H.; et al. Indications for neuromuscular ultrasound: Expert opinion and review of the literature. Clin. Neurophysiol. 2018, 129, 2658–2679. [Google Scholar] [CrossRef] [PubMed]
- Sipilä, S.; Suominen, H. Ultrasound imaging of the quadriceps muscle in elderly athletes and untrained men. Muscle Nerve 1991, 14, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef]
- Kim, S.; Leng, X.I.; Kritchevsky, S.B. Body Composition and Physical Function in Older Adults with Various Comorbidities. Innov. Aging 2017, 1, igx008. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Carlson, C.L.; Visser, M.; Kelley, D.E.; Scherzinger, A.; Harris, T.B.; Stamm, E.; Newman, A.B. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J. Appl. Physiol. (1985) 2001, 90, 2157–2165. [Google Scholar] [CrossRef]
- Guo, J.Y.; Zheng, Y.P.; Xie, H.B.; Chen, X. Continuous monitoring of electromyography (EMG), mechanomyography (MMG), sonomyography (SMG) and torque output during ramp and step isometric contractions. Med. Eng. Phys. 2010, 32, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.W.; Pengel, L.H.; Herbert, R.D.; Gandevia, S.C. Measurement of muscle contraction with ultrasound imaging. Muscle Nerve 2003, 27, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zheng, Y.P.; Huang, Q.H.; Chen, X. Continuous monitoring of sonomyography, electromyography and torque generated by normal upper arm muscles during isometric contraction: Sonomyography assessment for arm muscles. IEEE Trans. Biomed. Eng. 2008, 55, 1191–1198. [Google Scholar] [CrossRef]
- Kiesel, K.B.; Uhl, T.L.; Underwood, F.B.; Rodd, D.W.; Nitz, A.J. Measurement of lumbar multifidus muscle contraction with rehabilitative ultrasound imaging. Man. Ther. 2007, 12, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Petrofsky, J. The effect of the subcutaneous fat on the transfer of current through skin and into muscle. Med. Eng. Phys. 2008, 30, 1168–1176. [Google Scholar] [CrossRef]
- Lanza, M.B.; Balshaw, T.G.; Massey, G.J.; Folland, J.P. Does normalization of voluntary EMG amplitude to M(MAX) account for the influence of electrode location and adiposity? Scand. J. Med. Sci. Sports 2018, 28, 2558–2566. [Google Scholar] [CrossRef] [PubMed]
- Lanza, M.B.; Ryan, A.S.; Gray, V.; Perez, W.J.; Addison, O. Intramuscular Fat Influences Neuromuscular Activation of the Gluteus Medius in Older Adults. Front. Physiol. 2020, 11, 614415. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Hirata, K.; Iida, N.; Kanda, A.; Shoji, M.; Yoshida, T.; Myachi, M.; Akagi, R. Membrane capacitance and characteristic frequency are associated with contractile properties of skeletal muscle. Med. Eng. Phys. 2022, 106, 103832. [Google Scholar] [CrossRef]
- Uemura, K.; Yamada, M.; Okamoto, H. Association of bioimpedance phase angle and prospective falls in older adults. Geriatr. Gerontol. Int. 2019, 19, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, Y.; Kusakabe, T.; Arai, H.; Yamamoto, Y.; Nakao, K.; Ikeue, K.; Ishihara, Y.; Tagami, T.; Yasoda, A.; Ishii, K.; et al. Phase angle from bioelectrical impedance analysis is a useful indicator of muscle quality. J. Cachexia Sarcopenia Muscle 2022, 13, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Sacco, A.M.; Valerio, G.; Alicante, P.; Di Gregorio, A.; Spera, R.; Ballarin, G.; Scalfi, L. Raw bioelectrical impedance analysis variables (phase angle and impedance ratio) are significant predictors of hand grip strength in adolescents and young adults. Nutrition 2021, 91–92, 111445. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, S.; Brau, F.; Forino, R.; Berti, A.; D’Ignazio, F.; Loreti, C.; Bellieni, A.; D’Angelo, E.; Di Caro, F.; Biscotti, L.; et al. Sarcopenia: Diagnosis and Management, State of the Art and Contribution of Ultrasound. J. Clin. Med. 2021, 10, 5552. [Google Scholar] [CrossRef] [PubMed]
- Ata, A.M.; Kara, M.; Kaymak, B.; Gürçay, E.; Çakır, B.; Ünlü, H.; Akıncı, A.; Özçakar, L. Regional and total muscle mass, muscle strength and physical performance: The potential use of ultrasound imaging for sarcopenia. Arch. Gerontol. Geriatr. 2019, 83, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, C.; Armijo-Olivo, S.; De la Fuente, C.; Fuentes, J.; Javier Chirosa, L. Absolute Reliability and Concurrent Validity of Hand Held Dynamometry and Isokinetic Dynamometry in the Hip, Knee and Ankle Joint: Systematic Review and Meta-analysis. Open Med. 2017, 12, 359–375. [Google Scholar] [CrossRef]
- Mentiplay, B.F.; Perraton, L.G.; Bower, K.J.; Adair, B.; Pua, Y.H.; Williams, G.P.; McGaw, R.; Clark, R.A. Assessment of Lower Limb Muscle Strength and Power Using Hand-Held and Fixed Dynamometry: A Reliability and Validity Study. PLoS ONE. 2015, 10, e0140822. [Google Scholar] [CrossRef]
- Abe, T.; Loenneke, J.P.; Thiebaud, R.S.; Fujita, E.; Akamine, T.; Loftin, M. Prediction and Validation of DXA-Derived Appendicular Fat-Free Adipose Tissue by a Single Ultrasound Image of the Forearm in Japanese Older Adults. J. Ultrasound Med. 2018, 37, 347–353. [Google Scholar] [CrossRef]
- Abe, T.; Loenneke, J.P.; Young, K.C.; Thiebaud, R.S.; Nahar, V.K.; Hollaway, K.M.; Stover, C.D.; Ford, M.A.; Bass, M.A.; Loftin, M. Validity of ultrasound prediction equations for total and regional muscularity in middle-aged and older men and women. Ultrasound Med. Biol. 2015, 41, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Takai, Y.; Ohta, M.; Akagi, R.; Kato, E.; Wakahara, T.; Kawakami, Y.; Fukunaga, T.; Kanehisa, H. Applicability of ultrasound muscle thickness measurements for predicting fat-free mass in elderly population. J. Nutr. Health Aging. 2014, 18, 579–585. [Google Scholar] [CrossRef] [PubMed]

| Total (n = 212) | Model Development (n = 137) | Cross-Validation (n = 75) | |
|---|---|---|---|
| Age | 59 (11) | 61 (10) | 57 (10) |
| Female (n, %) | 121 (56.5) | 78 (56.9) | 43 (57.3) |
| Height (m) | 1.62 (0.08) | 1.62 (0.09) | 1.63 (0.08) |
| Weight (kg) | 64.6 (11.2) | 64.5 (10.8) | 64.9 (12.0) |
| BMI (kg/m2) | 24.4 (3.0) | 24.4 (3.0) | 24.3 (3.1) |
| ASM (kg) | 18.95 (4.56) | 18.83 (4.56) | 19.18 (4.59) |
| BFM (kg) | 18.75 (5.84) | 18.85 (6.23) | 18.55 (5.10) |
| MVCstrength(EF) (kg) | 19.7 (6.2) | 19.6 (6.3) | 19.9 (6.2) |
| MVCstrength(EE) (kg) | 13.8 (4.3) | 13.9 (4.5) | 13.6 (4.0) |
| MVCstrength(KF) (kg) | 13.0 (4.3) | 12.8 (4.2) | 13.3 (4.3) |
| MVCstrength(KE) (kg) | 24.0 (8.2) | 24.2 (8.4) | 23.6 (7.9) |
| MeanRMS(EF) (mV) | 0.66 (0.27) | 0.66 (0.28) | 0.65 (0.24) |
| MeanRMS(EE) (mV) | 0.73 (0.30) | 0.74 (0.30) | 0.70 (0.30) |
| MeanRMS(KF) (mV) | 0.32 (0.13) | 0.32 (0.13) | 0.32 (0.13) |
| MeanRMS(KE) (mV) | 0.23 (0.10) | 0.23 (0.10) | 0.23 (0.10) |
| MaxRMS(EF) (mV) | 0.72 (0.30) | 0.72 (0.31) | 0.73 (0.28) |
| MaxRMS(EE) (mV) | 0.31 (0.13) | 0.30 (0.13) | 0.33 (0.14) |
| MaxRMS(KF) (mV) | 0.37 (0.16) | 0.37 (0.16) | 0.38 (0.16) |
| MaxRMS(KE) (mV) | 0.25 (0.11) | 0.25 (0.10) | 0.26 (0.11) |
| RatioRMS(EF) | 2.30 (0.69) | 2.36 (0.73) | 2.20 (0.61) |
| RatioRMS(EE) | 2.44 (0.42) | 1.44 (0.38) | 1.43 (0.48) |
| RatioRMS(KF) | 2.53 (0.98) | 2.57 (0.96) | 2.45 (1.01) |
| RatioRMS(KE) | 1.88 (0.63) | 1.87 (0.57) | 1.90 (0.71) |
| BB thickness (mm) | 13.91 (3.03) | 13.82 (2.84) | 14.08 (3.36) |
| TB thickness (mm) | 10.61 (3.51) | 10.52 (3.52) | 10.79 (3.51) |
| BF thickness (mm) | 19.09 (4.51) | 19.05 (4.35) | 19.15 (4.81) |
| RF thickness (mm) | 11.26 (2.51) | 11.11 (2.45) | 11.53 (2.61) |
| MeanRMS | MaxRMS | RatioRMS | SLM (kg) | SFM (kg) | Muscle Thickness (mm) | |
|---|---|---|---|---|---|---|
| MVCstrength(EF) (kg) | 0.671 ** | 0.679 ** | 0.412 ** | 0.747 ** | −0.279 ** | 0.719 ** |
| MVCstrength(EE) (kg) | 0.531 ** | 0.288 ** | 0.400 ** | 0.778 ** | −0.258 ** | 0.475 ** |
| MVCstrength(KF) (kg) | 0.529 ** | 0.505 ** | 0.266 ** | 0.618 ** | −0.177 * | 0.321 ** |
| MVCstrength(KE) (kg) | 0.506 ** | 0.480 ** | 0.390 ** | 0.595 ** | −0.195 * | 0.429 ** |
| SLM (kg) | SFM (kg) | Muscle Thickness (mm) | ASM (kg) | |
|---|---|---|---|---|
| MeanRMS(EF) (mV) | 0.432 ** | −0.347 ** | 0.457 ** | 0.428 ** |
| MeanRMS(EE) (mV) | 0.351 ** | −0.392 ** | 0.260 ** | 0.377 ** |
| MeanRMS(KF) (mV) | 0.270 ** | −0.393 ** | 0.151 | 0.235 ** |
| MeanRMS(KE) (mV) | 0.154 | −0.162 | 0.363 ** | 0.136 |
| MaxRMS(EF) (mV) | 0.447 ** | −0.357 ** | 0.472 ** | 0.441 ** |
| MaxRMS(EE) (mV) | 0.158 | −0.211 | 0.213 * | 0.170 * |
| MaxRMS(KF) (mV) | 0.229 ** | −0.416 ** | 0.146 | 0.203 * |
| MaxRMS(KE) (mV) | 0.129 | −0.166 | 0.375 ** | 0.114 |
| RatioRMS(EF) (mV) | 0.341 ** | −0.140 | 0.297 ** | 0.329 ** |
| RatioRMS(EE) (mV) | 0.274 ** | −0.249 ** | 0.164 | 0.297 ** |
| RatioRMS(KF) (mV) | 0.366 ** | −0.339 ** | 0.264 ** | 0.388 ** |
| RatioRMS(KE) (mV) | 0.220 ** | −0.133 | 0.324 ** | 0.218 * |
| Entered predictor variables | Equation: ASM = −26.04 + 20.345 × Height + 0.178 × weight − 2.065 × (1, if, female; 0, if male) + 0.327 × RatioRMS(KF) + 0.965 × MeanRMS(EE) | ||||
| Age, sex, height, weight, MeanRMS(EF, EE, KF, KE), MaxRMS(EF, EE, KF, KE), RatioRMS(EF, EE, KF, KE) | β | Standard error | VIF | p-value | |
| Constant | −26.040 | 3.155 | |||
| Height (m) | 20.345 | 2.058 | 3.162 | <0.001 | |
| Weight (kg) | 0.178 | 0.013 | 1.984 | <0.001 | |
| Sex (female) | −2.065 | 0.338 | 2.824 | <0.001 | |
| RatioRMS(KF) (mV) | 0.327 | 0.118 | 1.275 | 0.006 | |
| MeanRMS(EE) (mV) | 0.965 | 0.373 | 1.270 | 0.011 | |
| R2 | 0.937 | ||||
| Adjusted R2 | 0.934 | ||||
| SEE | 1.167 | ||||
| Durbin–Watson statistic | 1.844 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, J.H.; Baek, S.-H.; Park, J.-W.; Rho, J.H.; Kim, B.-J. Surface Electromyography-Driven Parameters for Representing Muscle Mass and Strength. Sensors 2023, 23, 5490. https://doi.org/10.3390/s23125490
Sung JH, Baek S-H, Park J-W, Rho JH, Kim B-J. Surface Electromyography-Driven Parameters for Representing Muscle Mass and Strength. Sensors. 2023; 23(12):5490. https://doi.org/10.3390/s23125490
Chicago/Turabian StyleSung, Joo Hye, Seol-Hee Baek, Jin-Woo Park, Jeong Hwa Rho, and Byung-Jo Kim. 2023. "Surface Electromyography-Driven Parameters for Representing Muscle Mass and Strength" Sensors 23, no. 12: 5490. https://doi.org/10.3390/s23125490
APA StyleSung, J. H., Baek, S.-H., Park, J.-W., Rho, J. H., & Kim, B.-J. (2023). Surface Electromyography-Driven Parameters for Representing Muscle Mass and Strength. Sensors, 23(12), 5490. https://doi.org/10.3390/s23125490





