Prediction of Diabetes Mellitus Progression Using Supervised Machine Learning
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Methods
- Pre-DM (PD),
- DM without peripheral neuropathy (D), and
- DM with peripheral neuropathy (DN).
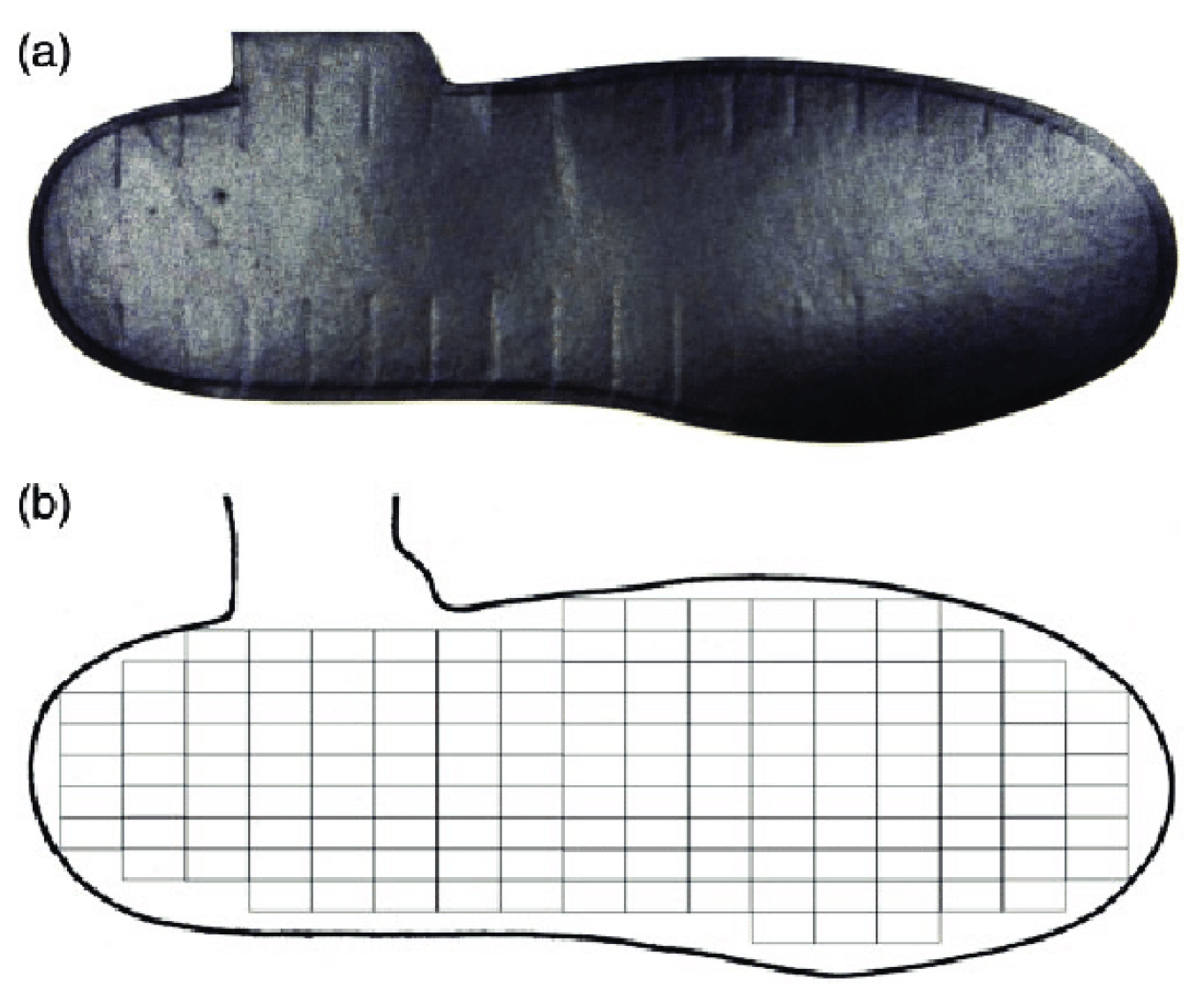
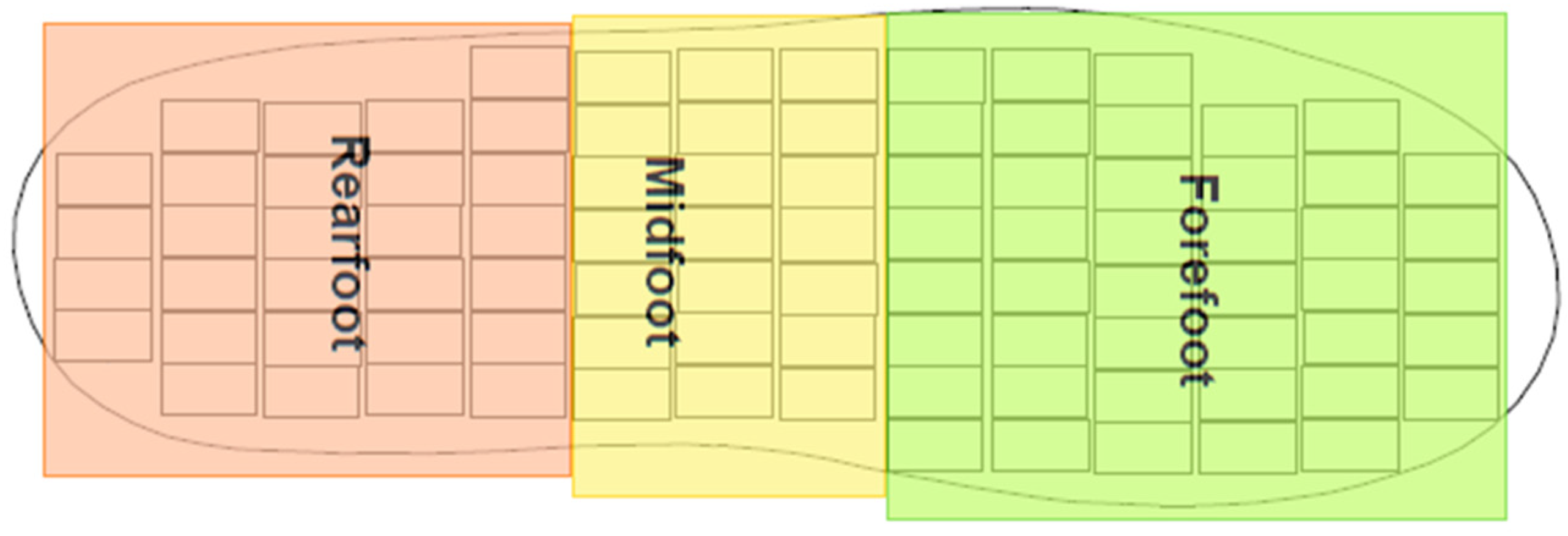
2.3. Preprocessing
- Rearfoot (RF),
- Midfoot (MF), and
- Forefoot (FF).
2.4. Feature Selection and Creation of Feature Subsets
2.5. Classification
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Algorithm | Hyperparameters |
|---|---|
| Fine Tree | Maximum number of splits: 100 Split Criterion: Gini’s diversity index Surrogate decision splits: Off |
| Medium Tree | Maximum number of splits: 20 Split Criterion: Gini’s diversity index Surrogate decision splits: Off |
| Coarse Tree | Maximum number of splits: 4 Split Criterion: Gini’s diversity index Surrogate decision splits: Off |
| Linear Discriminant | Covariance structure: Full |
| Quadratic Discriminant | Covariance structure: Full |
| Gaussian Naïve Bayes | Distribution name for numeric predictors: Gaussian Distribution name for categorical predictors: Not Applicable |
| Kernel Naïve Bayes | Distribution name for numeric predictors: Kernel Distribution name for categorical predictors: Not Applicable Kernel type: Gaussian Support: Unbounded |
| Linear SVM | Kernel function: Linear Kernel scale: Automatic Box constraint level: 1 Multiclass method: One-vs-One Standardize data: true |
| Quadratic SVM | Kernel function: Quadratic Kernel scale: Automatic Box constraint level: 1 Multiclass method: One-vs-One Standardize data: true |
| Cubic SVM | Kernel function: Cubic Kernel scale: Automatic Box constraint level: 1 Multiclass method: One-vs-One Standardize data: true |
| Fine Gaussian SVM | Kernel function: Gaussian Kernel scale: 1.2 Box constraint level: 1 Multiclass method: One-vs-One Standardize data: true |
| Medium Gaussian SVM | Kernel function: Gaussian Kernel scale: 4.8 Box constraint level: 1 Multiclass method: One-vs-One Standardize data: true |
| Coarse Gaussian SVM | Kernel function: Gaussian Kernel scale: 19 Box constraint level: 1 Multiclass method: One-vs-One Standardize data: true |
| Fine KNN | Number of neighbors: 1 Distance metric: Euclidean Distance weight: Equal Standardize data: true |
| Medium KNN | Number of neighbors: 10 Distance metric: Euclidean Distance weight: Equal Standardize data: true |
| Coarse KNN | Number of neighbors: 100 Distance metric: Euclidean Distance weight: Equal Standardize data: true |
| Cosine KNN | Number of neighbors: 10 Distance metric: Cosine Distance weight: Equal Standardize data: true |
| Cubic KNN | Number of neighbors: 10 Distance metric: Minkowski (cubic) Distance weight: Equal Standardize data: true |
| Weighted KNN | Number of neighbors: 10 Distance metric: Euclidean Distance weight: Squared inverse Standardize data: true |
| Boosted Trees | Ensemble method: AdaBoost Learner type: Decision tree Maximum number of splits: 20 Number of learners: 30 Learning rate: 0.1 Number of predictors to sample: Select All |
| Bagged Trees | Ensemble method: Bag Learner type: Decision tree Maximum number of splits: 1296 Number of learners: 30 Number of predictors to sample: Select All |
| Subspace Discriminant | Ensemble method: Subspace Learner type: Discriminant Number of learners: 30 Subspace dimension: 12 |
| Subspace KNN | Ensemble method: Subspace Learner type: Nearest neighbors Number of learners: 30 Subspace dimension: 12 |
| RUSBoosted Trees | Ensemble method: RUSBoost Learner type: Decision tree Maximum number of splits: 20 Number of learners: 30 Learning rate: 0.1 Number of predictors to sample: Select All |
| Narrow Neural Network | Number of fully connected layers: 1 First layer size: 10 Activation: ReLU Iteration limit: 1000 Regularization strength (Lambda): 0 Standardize data: Yes |
| Medium Neural Network | Number of fully connected layers: 1 First layer size: 25 Activation: ReLU Iteration limit: 1000 Regularization strength (Lambda): 0 Standardize data: Yes |
| Wide Neural Network | Number of fully connected layers: 1 First layer size: 100 Activation: ReLU Iteration limit: 1000 Regularization strength (Lambda): 0 Standardize data: Yes |
| Bilayered Neural Network | Number of fully connected layers: 2 First layer size: 10 Activation: ReLU Iteration limit: 1000 Regularization strength (Lambda): 0 Standardize data: Yes |
| Trilayered Neural Network | Number of fully connected layers: 3 First layer size: 10 Activation: ReLU Iteration limit: 1000 Regularization strength (Lambda): 0 Standardize data: Yes |
| SVM Kernel | Learner: SVM Number of expansion dimensions: Auto Regularization strength (Lambda): Auto Kernel scale: Auto Multiclass method: One-vs-One Iteration limit: 1000 |
| Logistic Regression Kernel | Learner: Logistic Regression Number of expansion dimensions: Auto Regularization strength (Lambda): Auto Kernel scale: Auto Multiclass method: One-vs-One Iteration limit: 1000 |
References
- American Diabetes Association. Common Terms. Available online: https://www.diabetes.org/resources/students/common-terms (accessed on 23 August 2022).
- Word Health Organization. Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 20 April 2023).
- Statistics About Diabetes: American Diabetes Association. Available online: http://www.diabetes.org/diabetes-basics/statistics/ (accessed on 20 April 2023).
- Centers for Disease Control and Prevention. Prevent Diabetes Complications. Available online: https://www.cdc.gov/diabetes/managing/problems.html#:~:text=Common%20diabetes%20health%20complications%20include,how%20to%20improve%20overall%20health (accessed on 23 August 2022).
- Cavanagh, P.R.; Simoneau, G.G.; Ulbrecht, J.S. Ulceration, unsteadiness, and uncertainty: The biomechanical consequences of diabetes mellitus. J. Biomech. 1993, 26 (Suppl. 1), 23–40. Available online: http://www.ncbi.nlm.nih.gov/pubmed/8505350 (accessed on 23 August 2022).
- Pecoraro, R.; Reiber, G.; Burgess, E. Pathways to Diabetic Basis for Prevention. Diabetes Care. 1990, 13, 513–521. [Google Scholar] [CrossRef]
- Boulton, A.; Vileikyte, L.; Ragnarson-Tennvall, G.; Apelqvist, J. The global burden of diabetic foot disease. Lancet 2005, 366, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.; Bech, F.; Hernandez-Boussard, T. National review of factors influencing disparities and types of major lower extremity amputations. Ann. Vasc. Surg. 2014, 28, 1157–1165. [Google Scholar] [CrossRef]
- Sheehan, T.; Gondo, G. Impact of Limb Loss in the United States. Phys. Med. Rehabil. Clin. N. Am. 2014, 25, 9–28. Available online: http://www.sciencedirect.com/science/article/pii/S1047965113000740 (accessed on 22 October 2014). [CrossRef] [PubMed]
- Cook, E.; Cook, J.; Labre, M.; Givens, H.; Di Resta, J. The Amputation Prevention Initiative. J. Am. Podiatr. Med. Assoc. 2014, 104, 1–10. Available online: http://www.japmaonline.org/doi/abs/10.7547/0003-0538-104.1.1 (accessed on 22 October 2014). [CrossRef] [PubMed]
- Dros, J.; Wewerinke, A.; Bindels, P.J.; Van Weert, H.C. Accuracy of monofilament testing to diagnose peripheral neuropathy: A systematic review. Ann. Fam. Med. 2009, 7, 555–558. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, J.; Yu, J.; Liu, S.; Zhang, R.; Ma, X.; Yang, Y.; Wang, P. Diagnostic Accuracy of Monofilament Tests for Detecting Diabetic Peripheral Neuropathy: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2017, 2017, 8787261. [Google Scholar] [CrossRef]
- Selvarajah, D.; Kar, D.; Khunti, K.; Davies, M.J.; Scott, A.R.; Walker, J.; Tesfaye, S. Diabetic peripheral neuropathy: Advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol. 2019, 7, 938–948. [Google Scholar] [CrossRef]
- Fuller, G. How to get the most out of nerve conduction studies and electromyography. Neurol. Pract. 2005, 76, ii41–ii46. [Google Scholar] [CrossRef]
- Papanas, N.; Boulton, A.J.M.; Malik, R.; Manes, C.; Schnell, O.; Spallone, V.; Tentolouris, N.; Tesfaye, S.; Valensi, P.; Ziegler, D.; et al. A simple new non-invasive sweat indicator test for the diagnosis of diabetic neuropathy. Diabet. Med. 2013, 30, 525–534. [Google Scholar] [CrossRef]
- Liu, C.; van der Heijden, F.; Klein, M.E.; van Baal, J.G.; Bus, S.A.; van Netten, J.J. Infrared dermal thermography on diabetic feet soles to predict ulcerations: A case study. SPIE BiOS. Int. Soc. Opt. Photonics 2013, 8572, 102–110. [Google Scholar] [CrossRef]
- Zou, D.; Mueller, M.J.; Lott, D.J. Effect of peak pressure and pressure gradient on subsurface shear stresses in the neuropathic foot. J. Biomech. 2007, 40, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, P.R.; Hewitt, F.; Perry, J. In-shoe plantar pressure measurement: A review. Foot 1992, 2, 185–194. [Google Scholar] [CrossRef]
- Perry, J.; Ulbrecht, J.; Derr, J.; Cavanagh, P. The use of running shoes to reduce plantar pressures in patients who have diabetes. J. Bone Jt. Surg. 1995, 77 Pt A, 1819–1828. Available online: http://jbjs.org/article.aspx?articleid=22857 (accessed on 3 September 2014).
- Maluf, K.; Mueller, M. Comparison of physical activity and cumulative plantar tissue stress among subjects with and without diabetes mellitus and a history of recurrent plantar ulcers. Clin. Biomech. 2003, 18, 567–575. [Google Scholar] [CrossRef]
- Acharya, U.R.; Tan, P.H.; Subramaniam, T.; Tamura, T.; Chua, K.C.; Goh, S.C.E.; Lim, C.M.; Goh, S.Y.D.; Chung, K.R.C.; Law, C.; et al. Automated Identification of Diabetic Type 2 Subjects with and without Neuropathy Using Wavelet Transform on Pedobarograph. J. Med. Syst. 2008, 32, 21–29. [Google Scholar] [CrossRef]
- Rahman, M.A.; Aziz, Z.; Acharya, U.R.; Ha, T.P.; Kannathal, N.; Ng, E.; Law, C.; Subramaniam, T.; Shuen, W.Y.; Fang, S.C. Analysis of plantar pressure in diabetic type 2 subjects with and without neuropathy. ITBM-RBM 2006, 27, 46–55. [Google Scholar] [CrossRef]
- Botros, F.S.; Taher, M.F.; Elsayed, N.M.; Fahmy, A.S. Prediction of diabetic foot ulceration using spatial and temporal dynamic plantar pressure. In Proceedings of the 2016 8th Cairo International Biomedical Engineering Conference (CIBEC), Cairo, Egypt, 15–17 December 2016; Volume 2016, pp. 43–47. [Google Scholar] [CrossRef]
- Robinson, C.C.; Balbinot, L.F.; Silva, M.F.; Achaval, M.; Zaro, M.A. Plantar Pressure Distribution Patterns of Individuals with Prediabetes. J. Diabetes Sci. Technol. 2013, 7, 1113–1121. [Google Scholar] [CrossRef]
- T&T Medilogic Medizintechnik GmbH. Manual: Medilogic Pressure Measurement; T&T Medilogic Medizintechnik GmbH: Schöenfeld, Germany, 2012; pp. 48–63. [Google Scholar]
- De Berardinis, J. The Development of a Viscoelastic Ellipsoidal Model for Use in Measuring Plantar Tissue Material Properties during Walking. Ph.D. Thesis, Department of Mechanical Engineering, UNLV, Las Vegas, NV, USA, 2019. Available online: https://www.proquest.com/docview/2312584828?pq-origsite=gscholar&fromopenview=true (accessed on 3 September 2022).
- De Berardinis, J.; Dufek, J.S.; Trabia, M.B.; Lidstone, D.E. Assessing the validity of pressure-measuring insoles in quantifying gait variables. J. Rehabil. Assist. Technol. Eng. 2018, 5, 2055668317752088. [Google Scholar] [CrossRef]
- Mueller, M.J.; Zou, D.; Lott, D.J. “Pressure gradient” as an indicator of plantar skin injury. Diabetes Care 2005, 28, 2908–2912. [Google Scholar] [CrossRef]
- Khan, Y.F.; Kaushik, B.; Chowdhary, C.L.; Srivastava, G. Ensemble Model for Diagnostic Classification of Alzheimer’s Disease Based on Brain Anatomical Magnetic Resonance Imaging. Diagnostics 2022, 12, 3193. [Google Scholar] [CrossRef]
- Hossin, M.; Sulaiman, M.N. A Review on Evaluation Metrics for Data Classification Evaluations. Int. J. Data Min. Knowl. Manag. Process 2015, 5, 1–12. [Google Scholar] [CrossRef]
- BuiltIn. A Step-by-Step Explanation of Principal Component Analysis (PCA). Available online: https://builtin.com/data-science/step-step-explanation-principal-component-analysis (accessed on 23 July 2021).
- Guiotto, A.; Bortolami, G.; Ciniglio, A.; Spolaor, F.; Guarneri, G.; Avogaro, A.; Cibin, F.; Silvestri, F.; Sawacha, Z. Machine learning approach to diabetic foot risk classification with biomechanics data. Gait Posture 2022, 97, 30–31. [Google Scholar] [CrossRef]
- Ji, X.; Akiyarna, Y.; Yamada, Y.; Okamoto, S.; Hayashi, H. Development of deep clustering model to stratify occurrence risk of diabetic foot ulcers based on foot pressure patterns and clinical indices. In Proceedings of the 2020 IEEE In-ternational Joint Conference on Biometrics (IJCB), Houston, TX, USA, 28 September–1 October 2020; pp. 1–8. [Google Scholar] [CrossRef]
- Haque, F.; Reaz, M.B.I.; Chowdhury, M.E.H.; Ezeddin, M.; Kiranyaz, S.; Alhatou, M.; Ali, S.H.M.; A Bakar, A.A.; Srivastava, G. Machine Learning-Based Diabetic Neuropathy and Previous Foot Ulceration Patients Detection Using Electromyography and Ground Reaction Forces during Gait. Sensors 2022, 22, 3507. [Google Scholar] [CrossRef] [PubMed]

| PD | D | DN | |
|---|---|---|---|
| No. of participants | 19 | 62 | 29 |
| Sex (M/F) | 9/10 | 29/33 | 14/15 |
| Age (Years) | 59.6 ± 11.4 | 58 ± 15.8 | 63.8 ± 10.2 |
| Body Mass (kg) | 93.7 ± 27.6 | 90.8 ± 26.2 | 96.9 ± 25.9 |
| Height (m) | 1.68 ± 0.09 | 1.66 ± 0.09 | 1.70 ± 0.10 |
| HbA1c (%) | 5.9 ± 0.4 | 7.6 ± 1.6 | 7.2 ± 1.3 |
| European Insole Size | Region | Number of Sensors |
|---|---|---|
| 35–36 | RF | 29 |
| MF | 29 | |
| FF | 35 | |
| 37–38 | RF | 38 |
| MF | 32 | |
| FF | 37 | |
| 39–40 | RF | 34 |
| MF | 32 | |
| FF | 50 | |
| 41–42 | RF | 45 |
| MF | 34 | |
| FF | 50 | |
| 43–44 | RF | 47 |
| MF | 38 | |
| FF | 66 | |
| 45–46 | RF | 48 |
| MF | 36 | |
| FF | 78 |
| Training Dataset | Description | No. of Features | Features |
|---|---|---|---|
| 1 | Pressure Features: PPP, PPG, PTI, Corresponding Asymmetry | 18 | |
| Non-Pressure Features | 4 | ||
| 2 | Pressure Features: PPP, PPG, PTI, Corresponding Asymmetry | 18 | |
| Non-Pressure Features | 3 | ||
| 3 | Pressure Features: PPP, PPG, Corresponding Asymmetry | 12 | |
| Non-Pressure Features | 4 | ||
| 4 | Pressure Features: PPP, PPG, Corresponding Asymmetry | 12 | |
| Non-Pressure Features | 3 | ||
| 5 | Pressure Features: PPP, PTI, Corresponding Asymmetry | 12 | |
| Non-Pressure Features | 4 | ||
| 6 | Pressure Features: PPP, PTI, Corresponding Asymmetry | 12 | |
| Non-Pressure Features | 3 | ||
| 7 | Pressure Features: PPG, PTI, Corresponding Asymmetry | 12 | |
| Non-Pressure Features | 4 | ||
| 8 | Pressure Features: PPG, PTI, Corresponding Asymmetry | 12 | |
| Non-Pressure Features | 3 | ||
| 9 | Pressure Features: PPP and Corresponding Asymmetry | 6 | |
| Non-Pressure Features | 4 | ||
| 10 | Pressure Features: PPP and Corresponding Asymmetry | 6 | |
| Non-Pressure Features | 3 | ||
| 11 | Pressure Features: PPG and Corresponding Asymmetry | 6 | |
| Non-Pressure Features | 4 | ||
| 12 | Pressure Features: PPG and Corresponding Asymmetry | 6 | |
| Non-Pressure Features | 3 | ||
| 13 | Pressure Features: PTI and Corresponding Asymmetry | 6 | |
| Non-Pressure Features | 4 | ||
| 14 | Pressure Features: PTI and Corresponding Asymmetry | 6 | |
| Non-Pressure Features | 3 | ||
| 15 | Non-Pressure Features | 4 | |
| 16 | Non-Pressure Features | 3 |
| Algorithm | Precision (%) | Recall (%) | F1 Score | False Negative Rate (%) |
|---|---|---|---|---|
| Cubic Support Vector Machine (SVM) | 97.9 | 98.5 | 98.2 | 0 |
| Subspace K-Nearest Neighbors (KNN) * | 96.9 | 98.5 | 97.7 | 1.9 |
| Bagged Trees * | 98.0 | 96.8 | 97.4 | 1.9 |
| Wide Neural Network | 96.4 | 98.4 | 97.4 | 1.9 |
| Boosted Trees | 97.6 | 96.4 | 97.0 | 1.9 |
| Fine KNN | 96.6 | 96.7 | 96.6 | 2.8 |
| Weighted KNN | 96.0 | 97.2 | 96.6 | 0.9 |
| Fine Tree | 96.3 | 95.4 | 95.9 | 1.9 |
| Medium Neural Network | 94.2 | 97.3 | 95.7 | 0 |
| Trilayered Neural Network | 94.9 | 95.6 | 95.2 | 2.8 |
| Quadratic SVM | 94.0 | 95.4 | 94.7 | 0 |
| RUSBoosted Trees * | 96.6 | 93.1 | 94.8 | 1.9 |
| Dataset | Best Performing Algorithm | Precision (%) | Recall (%) | F1 Score | False Negative Rate (%) |
|---|---|---|---|---|---|
| 1 | Cubic SVM | 97.9 | 98.5 | 98.2 | 0 |
| 2 | Subspace KNN * | 98.3 | 98.7 | 98.4 | 2.4 |
| 3 | Bagged Trees * | 98.4 | 99.3 | 98.7 | 4.7 |
| 4 | Bagged Trees * | 98.2 | 99.5 | 98.8 | 0 |
| 5 | Subspace KNN * | 99.0 | 99.2 | 99.1 | 2.3 |
| 6 | Subspace KNN * | 99.2 | 99.2 | 99.2 | 2.3 |
| 7 | Bagged Trees * | 98.5 | 98.5 | 98.5 | 3.5 |
| 8 | Subspace KNN * | 98.0 | 98.9 | 98.5 | 3.5 |
| 9 | Bagged Trees * | 100 | 100 | 100 | 0 |
| 10 | Subspace KNN * | 98.6 | 99.5 | 99.0 | 2.4 |
| 11 | Bagged Trees * | 99.2 | 99.4 | 99.3 | 0 |
| 12 | Bagged Trees * | 99.8 | 99.6 | 99.7 | 0 |
| 13 | Subspace KNN * | 100 | 100 | 100 | 0 |
| 14 | Subspace KNN * | 100 | 100 | 100 | 0 |
| Dataset | Best Performing Algorithm | Precision (%) | Recall (%) | F1 Score (%) | False Negative Rate (%) |
|---|---|---|---|---|---|
| 1 | Fine KNN | 91.2 | 92.3 | 91.7 | 0 |
| 3 | Fine KNN | 64.6 | 65.1 | 64.8 | 39.5 |
| 5 | Quadratic SVM | 92.7 | 93.4 | 93.1 | 0 |
| 7 | Medium Neural Network | 88.6 | 89.2 | 88.9 | 2.4 |
| 9 | Weighted KNN * | 60.9 | 64.1 | 62.5 | 52.3 |
| 11 | Bagged Trees * | 49.9 | 57.5 | 53.4 | 67.1 |
| 13 | Fine Tree | 82.9 | 88.6 | 85.7 | 2.4 |
| Dataset | Algorithm | Number of Components | Precision (%) | Recall (%) | F1 Score (%) | False Negative Rate (%) |
|---|---|---|---|---|---|---|
| 1 | Subspace KNN * | 9 | 93.1 | 95.5 | 94.2 | 4.7 |
| 2 | Subspace KNN * | 9 | 94.4 | 94.7 | 94.6 | 4.7 |
| 3 | Subspace KNN * | 8 | 93.0 | 93.8 | 93.4 | 5.8 |
| 4 | Subspace KNN * | 8 | 95.5 | 96.7 | 96.1 | 3.5 |
| 5 | Subspace KNN * | 6 | 96.5 | 97.1 | 96.8 | 8.1 |
| 6 | Subspace KNN * | 6 | 96.9 | 97.5 | 97.2 | 4.7 |
| 7 | Subspace KNN * | 7 | 94.8 | 95.6 | 95.2 | 5.9 |
| 8 | Bilayered Neural Network | 6 | 97.3 | 96.2 | 96.8 | 1.2 |
| 9 | Subspace KNN * | 6 | 97.9 | 98.0 | 98.0 | 1.2 |
| 10 | Bagged Trees * | 6 | 95.5 | 97.4 | 96.4 | 3.5 |
| 11 | Bilayered Neural Network | 6 | 96.1 | 97.1 | 96.6 | 8.2 |
| 12 | Subspace KNN * | 6 | 94.8 | 95.5 | 95.2 | 1.2 |
| 13 | Subspace KNN * | 5 | 100 | 100 | 100 | 0 |
| 14 | Subspace KNN * | 5 | 100 | 100 | 100 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chauhan, A.S.; Varre, M.S.; Izuora, K.; Trabia, M.B.; Dufek, J.S. Prediction of Diabetes Mellitus Progression Using Supervised Machine Learning. Sensors 2023, 23, 4658. https://doi.org/10.3390/s23104658
Chauhan AS, Varre MS, Izuora K, Trabia MB, Dufek JS. Prediction of Diabetes Mellitus Progression Using Supervised Machine Learning. Sensors. 2023; 23(10):4658. https://doi.org/10.3390/s23104658
Chicago/Turabian StyleChauhan, Apoorva S., Mathew S. Varre, Kenneth Izuora, Mohamed B. Trabia, and Janet S. Dufek. 2023. "Prediction of Diabetes Mellitus Progression Using Supervised Machine Learning" Sensors 23, no. 10: 4658. https://doi.org/10.3390/s23104658
APA StyleChauhan, A. S., Varre, M. S., Izuora, K., Trabia, M. B., & Dufek, J. S. (2023). Prediction of Diabetes Mellitus Progression Using Supervised Machine Learning. Sensors, 23(10), 4658. https://doi.org/10.3390/s23104658












