Biocompatible Piezoelectric PVDF/HA/AgNO3 Thin Film Prepared by the Solvent Casting Method
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials and Instruments
2.2. Synthesis of HA
2.3. Preparation of Composites
3. Results and Discussion
3.1. Study of X-ray Diffraction
3.2. Study of FTIR
3.3. SEM Analysis
3.4. Investigation of EDX Analysis
3.5. Study of Electrical Potential
3.6. Study of Hydrophilicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Wu, J.; Chen, W.; Fan, M.; Zhang, D.; Zuo, X.; Pan, L. Study on Influence of Heat Treatment on Interfacial Adhesion of CF/PI Composites. Mater. Today Commun. 2022, 33, 104745. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Zhu, L.; Li, Y.; Yan, Z.; Song, Y.; Cheng, X. Investigation of Interfacial Structure and Dynamic Mechanical Behavior of Titanium Alloy Laminated Composites. J. Mater. Res. Technol. 2022, 21, 5111–5120. [Google Scholar] [CrossRef]
- Wu, C.; Tang, Y.; Mao, B.; Zhao, K.; Cao, S.; Wu, Z. Rapid Apatite Induction of Polarized Hydrophilic HA/PVDF Bio-Piezoelectric Coating on Titanium Surface. Surf. Coatings Technol. 2021, 405, 126510. [Google Scholar] [CrossRef]
- Ribeiro, A.A.; Vaz, L.G.; Guastaldi, A.C.; Campos, J.S.C. Adhesion Strength Characterization of PVDF/HA Coating on Cp Ti Surface Modified by Laser Beam Irradiation. Appl. Surf. Sci. 2012, 258, 10110–10114. [Google Scholar] [CrossRef]
- Saxena, P.; Shukla, P. A Comprehensive Review on Fundamental Properties and Applications of Poly(Vinylidene Fluoride) (PVDF). Adv. Compos. Hybrid Mater. 2021, 4, 8–26. [Google Scholar] [CrossRef]
- Tobolsky, A.V. Book Reviews: Thermal Degradation of Organic. Polymers. Sam Uel L. Madorsky. New York, Interscience Publishers, 1964. 309 Pages, $12.50. Text. Res. J. 2016, 35, 289–290. [Google Scholar] [CrossRef]
- Luo, Z.; Zhu, D.; Shi, J.; Beeby, S.; Zhang, C.; Proynov, P.; Stark, B. Energy Harvesting Study on Single and Multilayer Ferroelectret Foams under Compressive Force. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 1360–1368. [Google Scholar] [CrossRef]
- Sajkiewicz, P.; Wasiak, A.; Goclowski, Z. Phase Transitions during Stretching of Poly(Vinylidene Fluoride). Eur. Polym. J. 1999, 35, 423–429. [Google Scholar] [CrossRef]
- Ribeiro, A.A.; Marques, R.F.C.; Guastaldi, A.C.; De Carvalho Campos, J.S. Hydroxyapatite Deposition Study through Polymeric Process on Commercially Pure Ti Surfaces Modified by Laser Beam Irradiation. J. Mater. Sci. 2009, 44, 4056–4061. [Google Scholar] [CrossRef]
- Xie, L.; Wang, G.; Jiang, C.; Yu, F.; Zhao, X. Properties and Applications of Flexible Poly(Vinylidene Fluoride)-Based Piezoelectric Materials. Crystals 2021, 11, 644. [Google Scholar] [CrossRef]
- Sahoo, R.; Mishra, S.; Ramadoss, A.; Mohanty, S.; Mahapatra, S.; Nayak, S.K. An Approach towards the Fabrication of Energy Harvesting Device Using Ca-Doped ZnO/ PVDF-TrFE Composite Film. Polymer 2020, 205, 122869. [Google Scholar] [CrossRef]
- Soin, N.; Shah, T.H.; Anand, S.C.; Geng, J.; Pornwannachai, W.; Mandal, P.; Reid, D.; Sharma, S.; Hadimani, R.L.; Bayramol, D.V.; et al. Novel “3-D Spacer” All Fibre Piezoelectric Textiles for Energy Harvesting Applications. Energy Environ. Sci. 2014, 7, 1670–1679. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Zinadini, S.; Vatanpour, V. A New Approach to Improve Antifouling Property of PVDF Membrane Using in Situ Polymerization of PAA Functionalized TiO2 Nanoparticles. J. Memb. Sci. 2011, 380, 155–162. [Google Scholar] [CrossRef]
- Jung, B. Preparation of Hydrophilic Polyacrylonitrile Blend Membranes for Ultrafiltration. J. Memb. Sci. 2004, 229, 129–136. [Google Scholar] [CrossRef]
- Rabiei, M.; Palevicius, A.; Monshi, A.; Nasiri, S.; Vilkauskas, A.; Janusas, G. Comparing Methods for Calculating Nano Crystal Size of Natural Hydroxyapatite Using X-Ray Diffraction. Nanomaterials 2020, 10, 1627. [Google Scholar] [CrossRef]
- Xie, C.M.; Lu, X.; Wang, K.F.; Meng, F.Z.; Jiang, O.; Zhang, H.P.; Zhi, W.; Fang, L.M. Silver Nanoparticles and Growth Factors Incorporated Hydroxyapatite Coatings on Metallic Implant Surfaces for Enhancement of Osteoinductivity and Antibacterial Properties. ACS Appl. Mater. Interfaces 2014, 6, 8580–8589. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.; Xu, Z.; Yeung, K.W.K.; Wu, S. Dopamine Modified Organic-Inorganic Hybrid Coating for Antimicrobial and Osteogenesis. ACS Appl. Mater. Interfaces 2016, 8, 33972–33981. [Google Scholar] [CrossRef]
- Pon-On, W.; Meejoo, S.; Tang, I.M. Incorporation of iron into nano hydroxyapatite particles synthesized by the microwave process. Int. J. Nanosci. 2011, 6, 9–16. [Google Scholar] [CrossRef]
- Kanchana, P.; Sekar, C. Influence of Sodium Fluoride on the Synthesis of Hydroxyapatite by Gel Method. J. Cryst. Growth 2010, 312, 808–816. [Google Scholar] [CrossRef]
- Wang, M.; Wang, K.; Yang, Y.; Liu, Y.; Yu, D.G. Electrospun Environment Remediation Nanofibers Using Unspinnable Liquids as the Sheath Fluids: A Review. Polymers 2020, 12, 103. [Google Scholar] [CrossRef]
- Tankhiwale, R.; Bajpai, S.K. Silver-Nanoparticle-Loaded Chitosan Lactate Films with Fair Antibacterial Properties. J. Appl. Polym. Sci. 2010, 115, 1894–1900. [Google Scholar] [CrossRef]
- Stejskal, J. Conducting Polymer-Silver Composites. Chem. Pap. 2013, 67, 814–848. [Google Scholar] [CrossRef]
- Corrosion Protection of 316L Stainless Steel by …—Library of Science. Available online: https://bibliotekanauki.pl/articles/2128151 (accessed on 27 November 2022).
- Zhao, Q.; Lu, H.; Meng, L.; Yu, S.L. The Adsorption Performance of Hybrid PVDF Membrane by HA. Appl. Mech. Mater. 2015, 700, 298–301. [Google Scholar] [CrossRef]
- Braga, F.J.C.; Rogero, S.O.; Couto, A.A.; Marques, R.F.C.; Ribeiro, A.A.; Campos, J.S., de C. Characterization of PVDF/HAP Composites for Medical Applications. Mater. Res. 2007, 10, 247–251. [Google Scholar] [CrossRef]
- Zamin, H.; Yabutsuka, T.; Takai, S. Bioactivity Assessment of Apatite Nuclei-PVDF Composite Thin Films. Key Eng. Mater. 2018, 782, 78–83. [Google Scholar] [CrossRef]
- Agrawal, K.; Singh, G.; Puri, D.; Prakash, S. Synthesis and Characterization of Hydroxyapatite Powder by Sol-Gel Method for Biomedical Application. J. Miner. Mater. Charact. Eng. 2011, 10, 727–734. [Google Scholar] [CrossRef]
- Kim, I.S.; Kumta, P.N. Sol-Gel Synthesis and Characterization of Nanostructured Hydroxyapatite Powder. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2004, 111, 232–236. [Google Scholar] [CrossRef]
- Marino, T.; Galiano, F.; Simone, S.; Figoli, A. DMSO EVOLTM as Novel Non-Toxic Solvent for Polyethersulfone Membrane Preparation. Environ. Sci. Pollut. Res. 2019, 26, 14774–14785. [Google Scholar] [CrossRef]
- Figoli, A.; Marino, T.; Simone, S.; Di Nicolò, E.; Li, X.M.; He, T.; Tornaghi, S.; Drioli, E. Towards Non-Toxic Solvents for Membrane Preparation: A Review. Green Chem. 2014, 16, 4034–4059. [Google Scholar] [CrossRef]
- Enayatzadeh, M.; Mohammadi, T. Morphology and Performance of Poly(Vinylidene Fluoride) Flat Sheet Membranes: Thermodynamic and Kinetic Aspects. J. Appl. Polym. Sci. 2018, 135, 46419. [Google Scholar] [CrossRef]
- Arefi-Oskoui, S.; Khataee, A.; Vatanpour, V. Effect of Solvent Type on the Physicochemical Properties and Performance of NLDH/PVDF Nanocomposite Ultrafiltration Membranes. Sep. Purif. Technol. 2017, 184, 97–118. [Google Scholar] [CrossRef]
- Hai, C.; Li, S.; Zhou, Y.; Zeng, J.; Ren, X.; Li, X. Roles of Ethylene Glycol Solvent and Polymers in Preparing Uniformly Distributed MgO Nanoparticles. J. Asian Ceram. Soc. 2018, 5, 176–182. [Google Scholar] [CrossRef]
- Nasiri, S.; Nasr-Esfahani, M. Bioactive Organic-Inorganic Composite Monolith Derived from Poly Vinyl Trimethoxy Silane Using Sol- Gel Process. Plast. Polym. Technol. 2013, 2, 63–67. [Google Scholar]
- Cai, X.; Lei, T.; Sun, D.; Lin, L. A Critical Analysis of the a, b and g Phases in Poly(Vinylidene Fluoride) Using FTIR. RSC Adv. 2017, 7, 15382–15389. [Google Scholar] [CrossRef]
- Newman, B.A.; Yoon, C.H.; Pae, K.D.; Scheinbeim, J.I. Piezoelectric Activity and Field-induced Crystal Structure Transitions in Poled Poly(Vinylidene Fluoride) Films. J. Appl. Phys. 2008, 50, 6095. [Google Scholar] [CrossRef]
- Muralidhar, C.; Pillai, P.K.C. XRD Studies on Barium Titanate (BaTiO3)/Polyvinylidene Fluoride (PVDF) Composites. J. Mater. Sci. 1988, 23, 410–414. [Google Scholar] [CrossRef]
- Abdullah, I.Y.; Yahaya, M.; Jumali, M.H.H.; Shanshool, H.M. Effect of Annealing Process on the Phase Formation in Poly(Vinylidene Fluoride) Thin Films. AIP Conf. Proc. 2015, 1614, 147. [Google Scholar] [CrossRef]
- Satapathy, S.; Pawar, S.; Gupta, P.K.; RVarma, K.B. Effect of Annealing on Phase Transition in Poly(Vinylidene Fluoride) Films Prepared Using Polar Solvent. Bull. Mater. Sci. 2011, 34, 727–733. [Google Scholar] [CrossRef]
- Bhatti, I.; Banerjee, M.; Bhatti, I.N. Effect of Annealing and Time of Crystallization on Structural and Optical Properties of PVDF Thin Film Using Acetone as Solvent. IOSR J. Appl. Phys. 2013, 4, 42–47. [Google Scholar] [CrossRef]
- Nangia, A.; Desiraju, G.R. Pseudopolymorphism: Occurrences of Hydrogen Bonding Organic Solvents in Molecular Crystals. Chem. Commun. 1998, 7, 605–606. [Google Scholar] [CrossRef]
- Benz, M.; Euler, W.B.; Gregory, O.J. The Influence of Preparation Conditions on the Surface Morphology of Poly(Vinylidene Fluoride) Films. Langmuir 2001, 17, 239–243. [Google Scholar] [CrossRef]
- Ince-Gunduz, B.S.; Alpern, R.; Amare, D.; Crawford, J.; Dolan, B.; Jones, S.; Kobylarz, R.; Reveley, M.; Cebe, P. Impact of Nanosilicates on Poly(Vinylidene Fluoride) Crystal Polymorphism: Part 1. Melt-Crystallization at High Supercooling. Polymer 2010, 51, 1485–1493. [Google Scholar] [CrossRef]
- Silva, L.M.; Bonadio, T.G.M.; Rosso, J.M.; Dias, G.S.; Cótica, L.F.; Weinand, W.R.; Miyahara, R.Y.; Santos, I.A.; Freitas, V.F. On the Synthesis and Characterization of (Bio)Ferroelectrically Active PVDF-BCP Composites. Ferroelectrics 2019, 533, 63–71. [Google Scholar] [CrossRef]
- Rabiei, M.; Palevicius, A.; Ebrahimi-Kahrizsangi, R.; Nasiri, S.; Vilkauskas, A.; Janusas, G. New Approach for Preparing In Vitro Bioactive Scaffold Consisted of Ag-Doped Hydroxyapatite + Polyvinyltrimethoxysilane. Polymers 2021, 13, 1695. [Google Scholar] [CrossRef]
- Arami, H.; Mohajerani, M.; Mazloumi, M.; Khalifehzadeh, R.; Lak, A.; Sadrnezhaad, S.K. Rapid Formation of Hydroxyapatite Nanostrips via Microwave Irradiation. J. Alloys Compd. 2009, 469, 391–394. [Google Scholar] [CrossRef]
- Fern, H.W.; Salimi, M.N. Hydroxyapatite Nanoparticles Produced by Direct Precipitation Method: Optimization and Characterization Studies. AIP Conf. Proc. 2021, 2339, 020215. [Google Scholar] [CrossRef]
- Hwang, S.W.; Umar, A.; Dar, G.N.; Kim, S.H.; Badran, R.I. Synthesis and Characterization of Iron Oxide Nanoparticles for Phenyl Hydrazine Sensor Applications. Sens. Lett. 2014, 12, 97–101. [Google Scholar] [CrossRef]
- Ashok, M.; Meenakshi Sundaram, N.; Narayana Kalkura, S. Crystallization of Hydroxyapatite at Physiological Temperature. Mater. Lett. 2003, 57, 2066–2070. [Google Scholar] [CrossRef]
- dos Santos, G.G.; Malherbi, M.S.; de Souza, N.S.; César, G.B.; Tominaga, T.T.; Miyahara, R.Y.; de Mendonça, P.d.S.B.; Faria, D.R.; Rosso, J.M.; Freitas, V.F.; et al. 4th Generation Biomaterials Based on PVDF-Hydroxyapatite Composites Produced by Electrospinning: Processing and Characterization. Polymers 2022, 14, 4190. [Google Scholar] [CrossRef]
- Tandon, B.; Kamble, P.; Olsson, R.T.; Blaker, J.J.; Cartmell, S.H. Fabrication and Characterisation of Stimuli Responsive Piezoelectric PVDF and Hydroxyapatite-Filled PVDF Fibrous Membranes. Molecules 2019, 24, 1903. [Google Scholar] [CrossRef]
- Salimi, A.; Yousefi, A.A. Conformational Changes and Phase Transformation Mechanisms in PVDF Solution-Cast Films. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 3487–3495. [Google Scholar] [CrossRef]
- Chen, Q.; Natale, D.; Neese, B.; Ren, K.; Lin, M.; Zhang, Q.M.; Pattom, M.; Wang, K.W.; Fang, H.; Im, E. Piezoelectric Polymers Actuators for Precise Shape Control of Large Scale Space Antennas. Electroact. Polym. Actuators Devices (EAPAD) 2007, 6524, 491–501. [Google Scholar] [CrossRef]
- Garetz, B.A.; Matic, J.; Myerson, A.S. Polarization Switching of Crystal Structure in the Nonphotochemical Light-Induced Nucleation of Supersaturated Aqueous Glycine Solutions. Phys. Rev. Lett. 2002, 89, 175501. [Google Scholar] [CrossRef] [PubMed]
- Chinaglia, D.L.; Gregorio, R.; Stefanello, J.C.; Altafim, R.A.P.; Wirges, W.; Wang, F.; Gerhard, R. Influence of the Solvent Evaporation Rate on the Crystalline Phases of Solution-Cast Poly(Vinylidene Fluoride) Films. J. Appl. Polym. Sci. 2010, 116, 785–791. [Google Scholar] [CrossRef]
- Kato, S.; Furukawa, S.; Aoki, D.; Goseki, R.; Oikawa, K.; Tsuchiya, K.; Shimada, N.; Maruyama, A.; Numata, K.; Otsuka, H. Crystallization-Induced Mechanofluorescence for Visualization of Polymer Crystallization. Nat. Commun. 2021 121 2021, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Scorrano, S.; Mergola, L.; Di Bello, M.P.; Lazzoi, M.R.; Vasapollo, G.; Del Sole, R. Molecularly Imprinted Composite Membranes for Selective Detection of 2-Deoxyadenosine in Urine Samples. Int. J. Mol. Sci. 2015, 16, 13746–13759. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Motelica-Heino, M.; Guegan, R.; Buton, N. Evaluation of Antibacterial Activity of Zinc-Doped Hydroxyapatite Colloids and Dispersion Stability Using Ultrasounds. Nanomaterials 2019, 9, 515. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Iconaru, S.L.; Chifiriuc, M.C.; Costescu, A.; Le Coustumer, P.; Predoi, D. Synthesis and Antimicrobial Activity of Silver-Doped Hydroxyapatite Nanoparticles. Biomed Res. Int. 2013, 2013, 916218. [Google Scholar] [CrossRef]
- Biologically Compatible Lead-Free Piezoelectric Composite for Acoustophoresis Based Particle Manipulation Techniques—Google Search. Available online: https://www.google.com/search?q=Biologically+Compatible+Lead-Free+Piezoelectric+Composite+for+Acoustophoresis+Based+Particle+Manipulation+Techniques&oq=Biologically+Compatible+Lead-Free+Piezoelectric+Composite+for+Acoustophoresis+Based+Particle+Manipulation+Techniques&aqs=chrome..69i57j69i61l2.1167j0j4&sourceid=chrome&ie=UTF-8 (accessed on 14 December 2021).
- Tofail, S.A.M.; Haverty, D.; Stanton, K.T.; McMonagle, J.B. Structural Order and Dielectric Behaviour of Hydroxyapatite. Ferroelectrics 2011, 319, 117–123. [Google Scholar] [CrossRef]
- Nasiri, S.; Hosseinnezhad, M.; Rabiei, M.; Palevicius, A.; Janusas, G. The Effect of Calcination Temperature on the Photophysical and Mechanical Properties of Copper Iodide (5 Mol%)–Doped Hydroxyapatite. Opt. Mater. 2021, 121, 111559. [Google Scholar] [CrossRef]
- Lang, S.B.; Tofail, S.A.M.; Kholkin, A.L.; Wojtas, M.; Gregor, M.; Gandhi, A.A.; Wang, Y.; Bauer, S.; Krause, M.; Plecenik, A. Ferroelectric Polarization in Nanocrystalline Hydroxyapatite Thin Films on Silicon. Sci. Reports 2013, 3, 2215. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Rutherford, G.N.; Sharma, A.P.; Pradhan, S.K.; Bonner, C.E.; Bahoura, M.J. Surface Modification and Charge Injection in a Nanocomposite Of Metal Nanoparticles and Semiconductor Oxide Nanostructures. Sci. Reports 2020, 10, 4743. [Google Scholar] [CrossRef] [PubMed]
- Giridhar, G.; Manepalli, R.K.N.R.; Apparao, G. Contact Angle Measurement Techniques for Nanomaterials. Therm. Rheol. Meas. Tech. Nanomater. Charact. 2017, 3, 173–195. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, J.; Li, W.; Tang, J.; Peng, J.; Zhang, J.; Li, X. Investigation of the Characteristic of Solution-Processed Tetraphenyldibenzoperiflanthene (DBP) Film and Its Application on Organic Photovoltaic Cells. Phys. Status Solidi 2021, 218, 2100232. [Google Scholar] [CrossRef]
- Huhtamäki, T.; Tian, X.; Korhonen, J.T.; Ras, R.H.A. Surface-Wetting Characterization Using Contact-Angle Measurements. Nat. Protoc. 2018, 13, 1521–1538. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.; Lopes, A.C.; Botelho, G.; Neves, I.C.; Lanceros-Mendez, S. Influence of Solvent Properties on the Electrical Response of Poly(Vinylidene Fluoride)/NaY Composites. J. Polym. Res. 2013, 20, 143. [Google Scholar] [CrossRef]
- Dashtizad, S.; Alizadeh, P.; Yourdkhani, A. Improving Piezoelectric Properties of PVDF Fibers by Compositing with BaTiO3-Ag Particles Prepared by Sol-Gel Method and Photochemical Reaction. J. Alloys Compd. 2021, 883, 160810. [Google Scholar] [CrossRef]
- Sedlarik, V.; Galya, T.; Sedlarikova, J.; Valasek, P.; Saha, P. The Effect of Preparation Temperature on the Mechanical and Antibacterial Properties of Poly(Vinyl Alcohol)/Silver Nitrate Films. Polym. Degrad. Stab. 2010, 95, 399–404. [Google Scholar] [CrossRef]
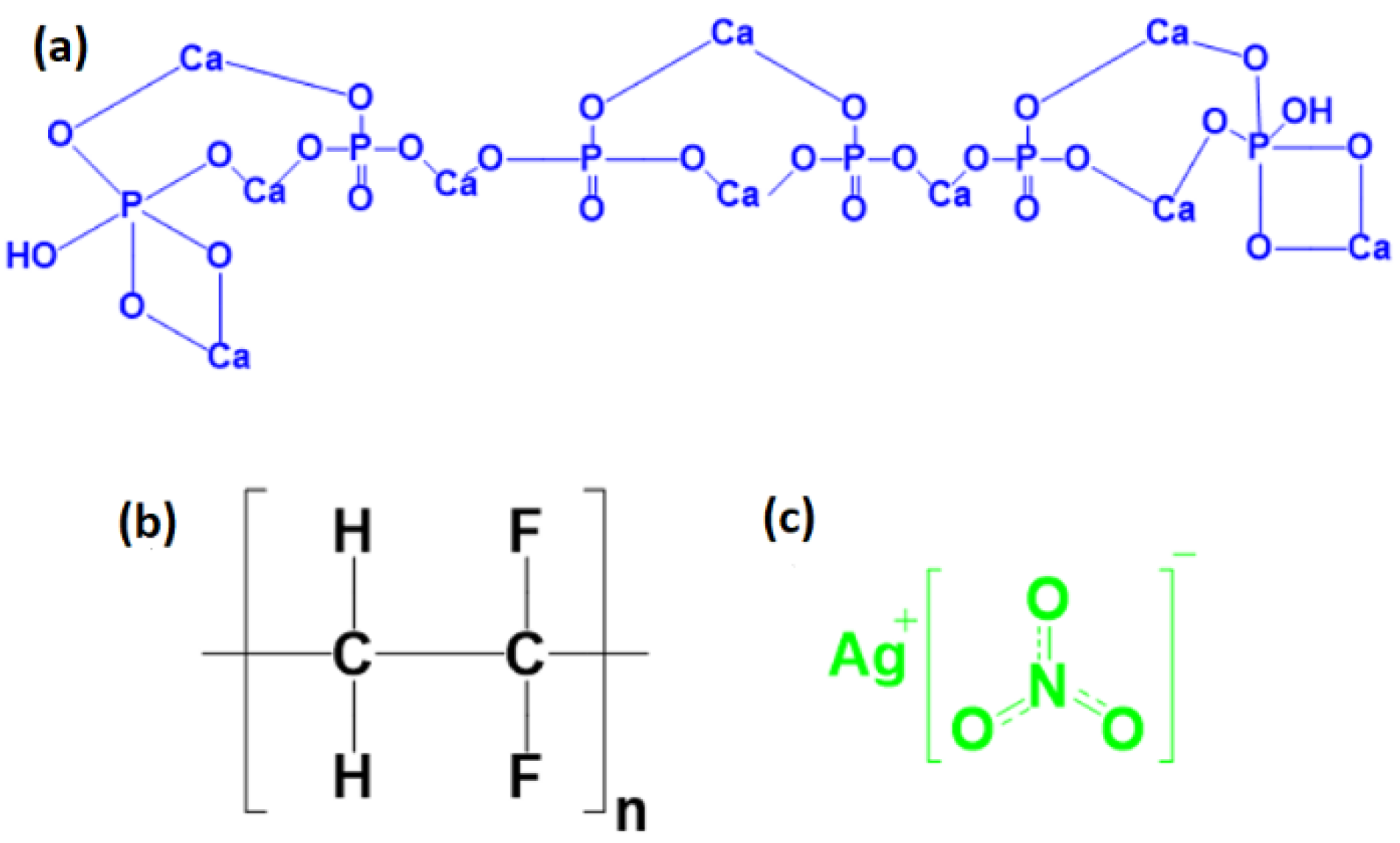
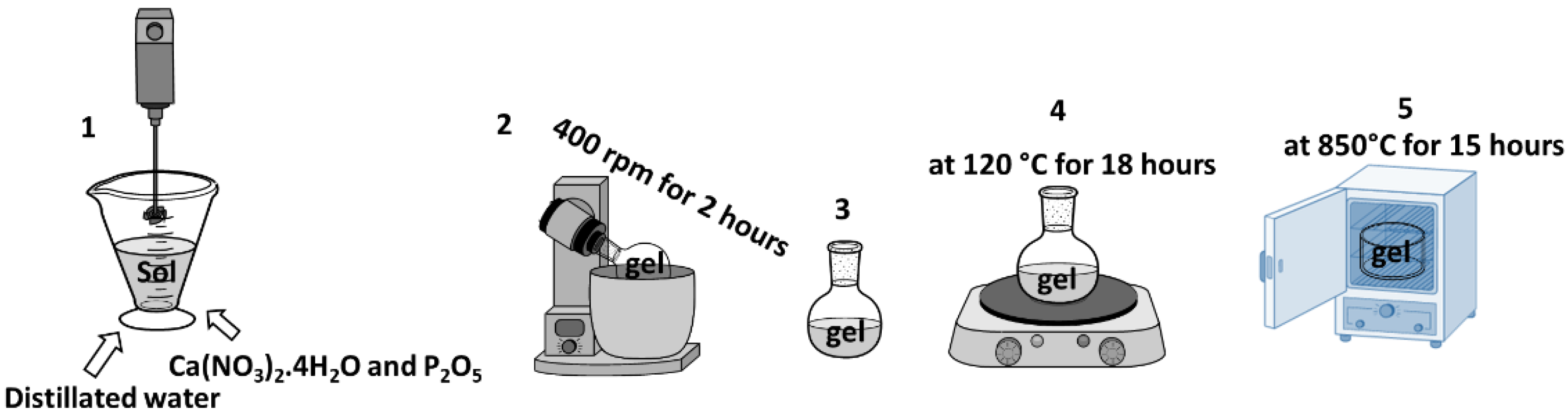
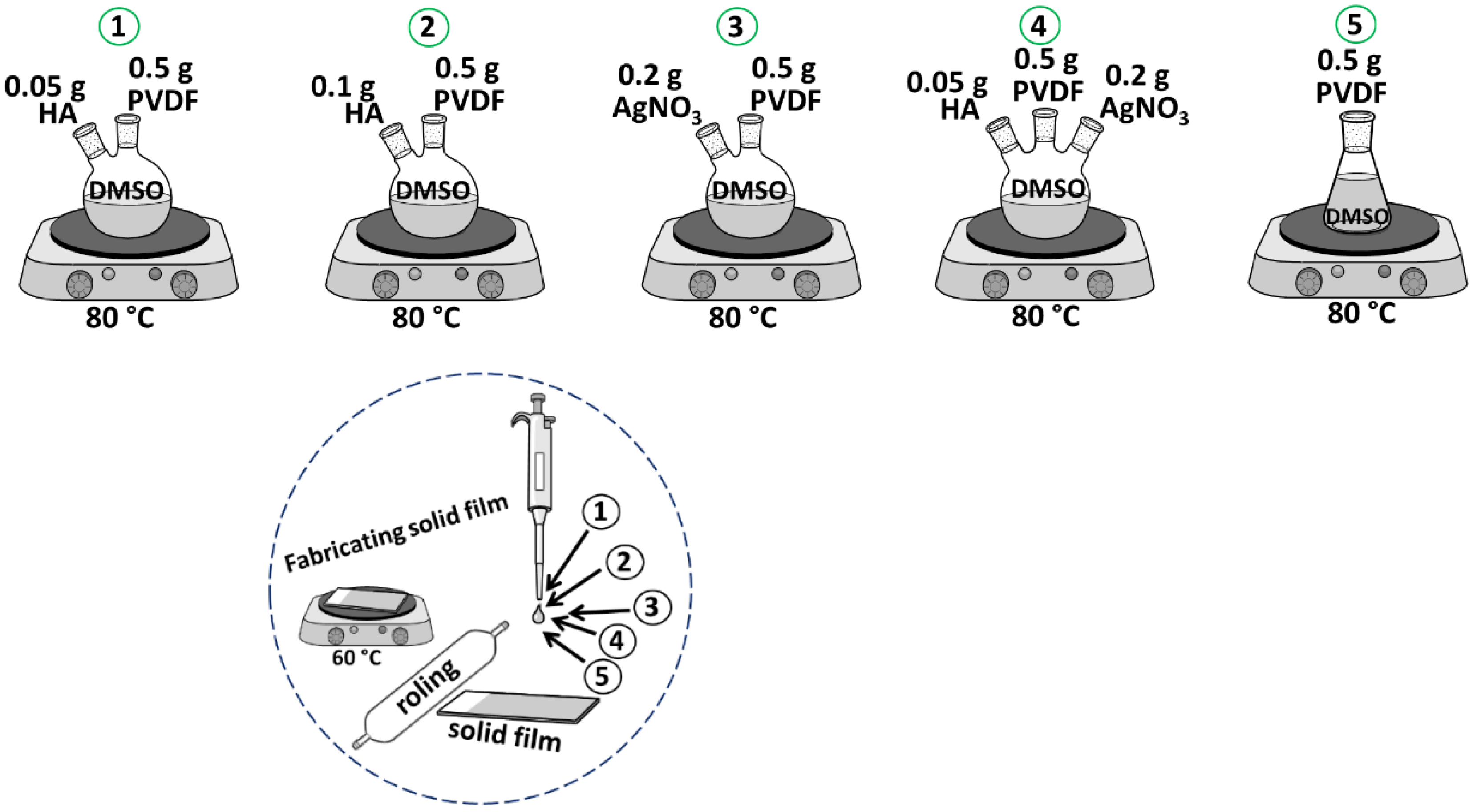
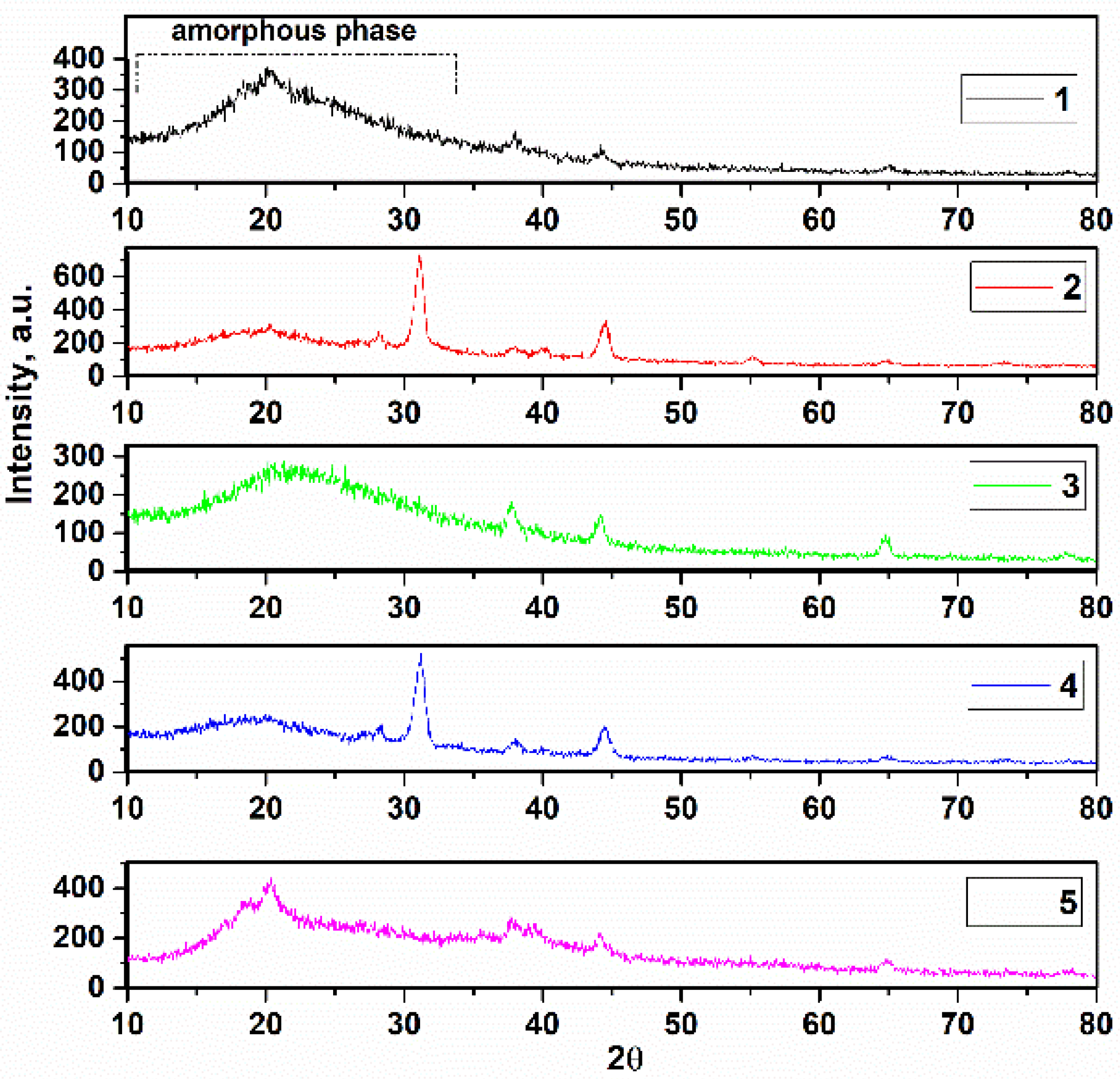
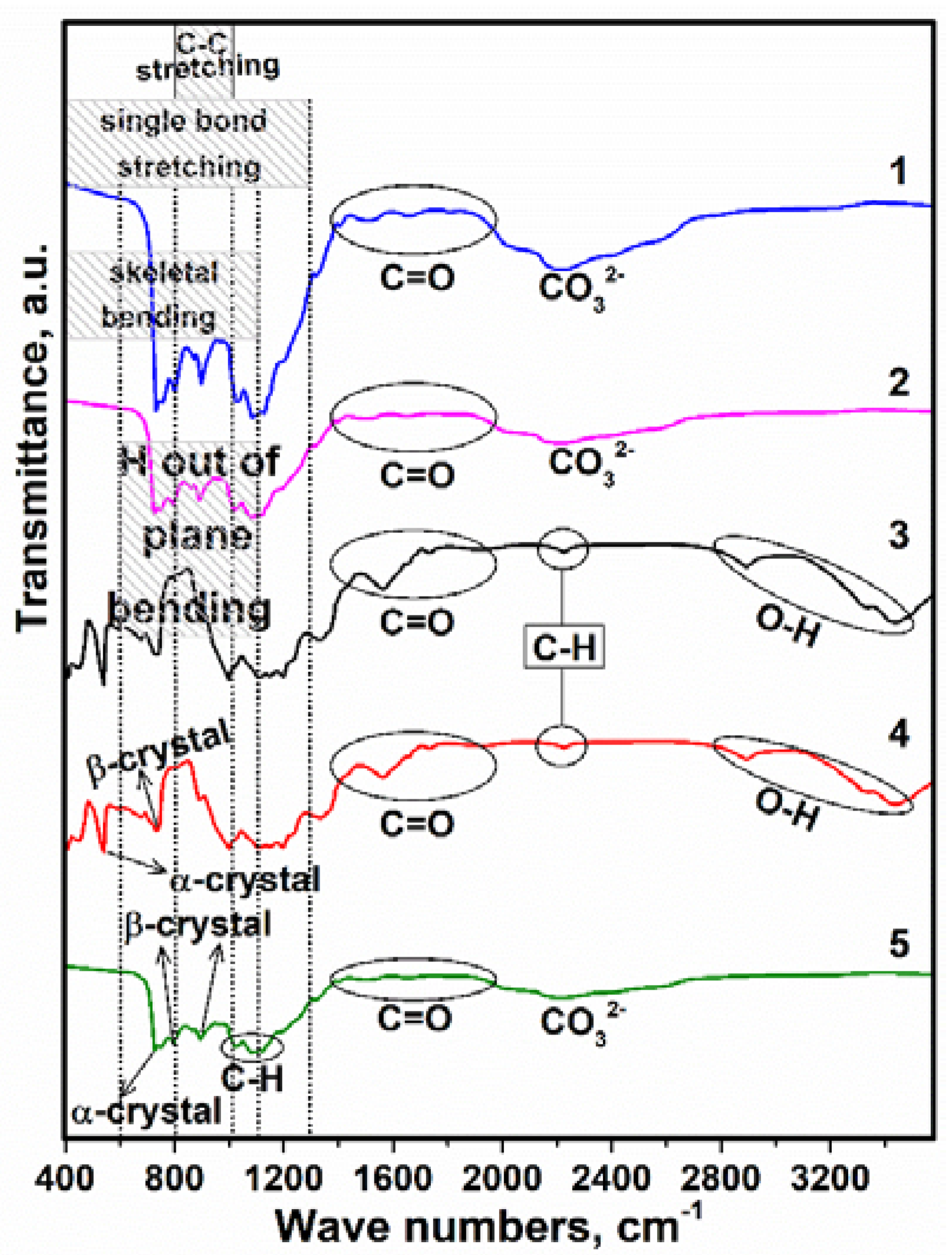

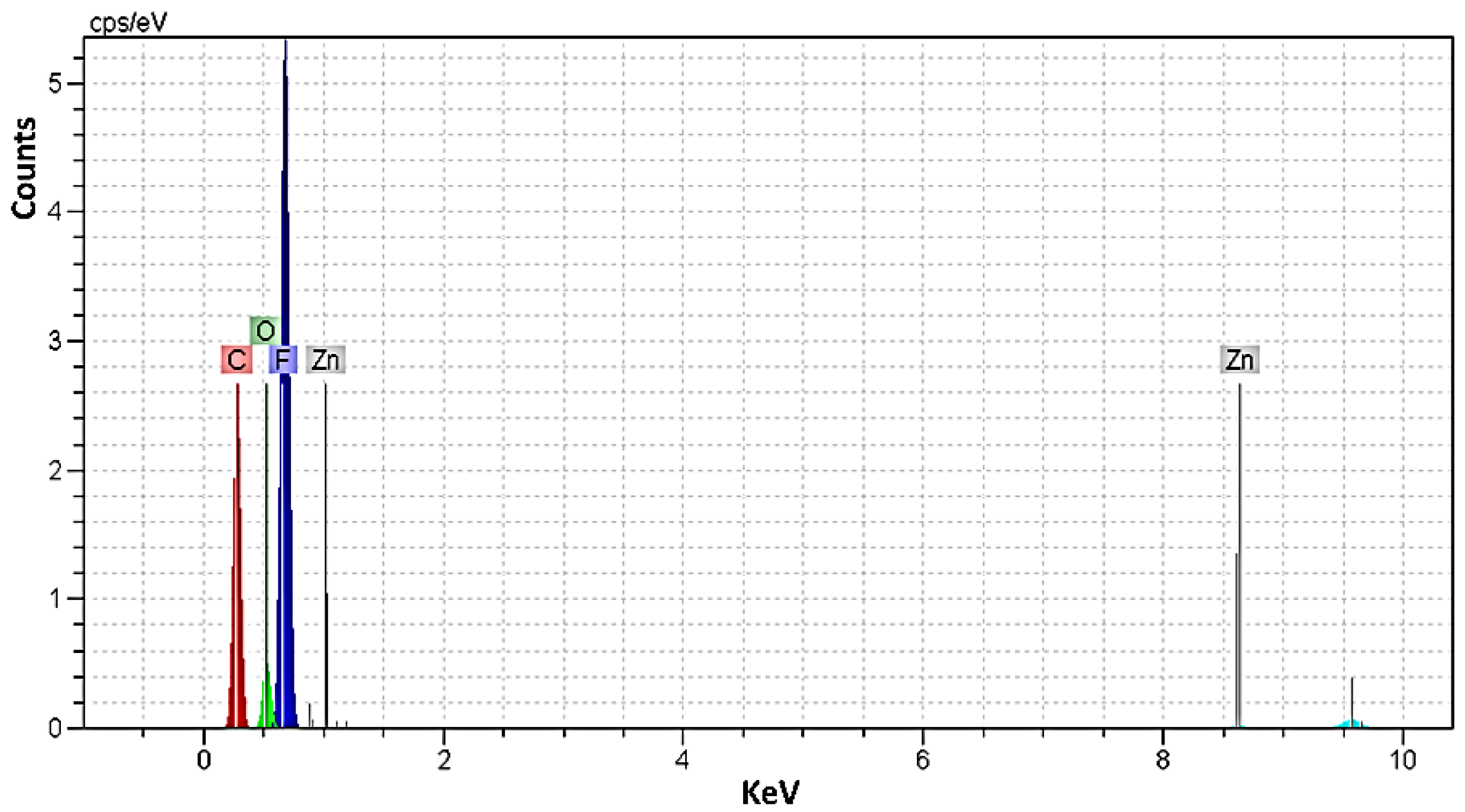
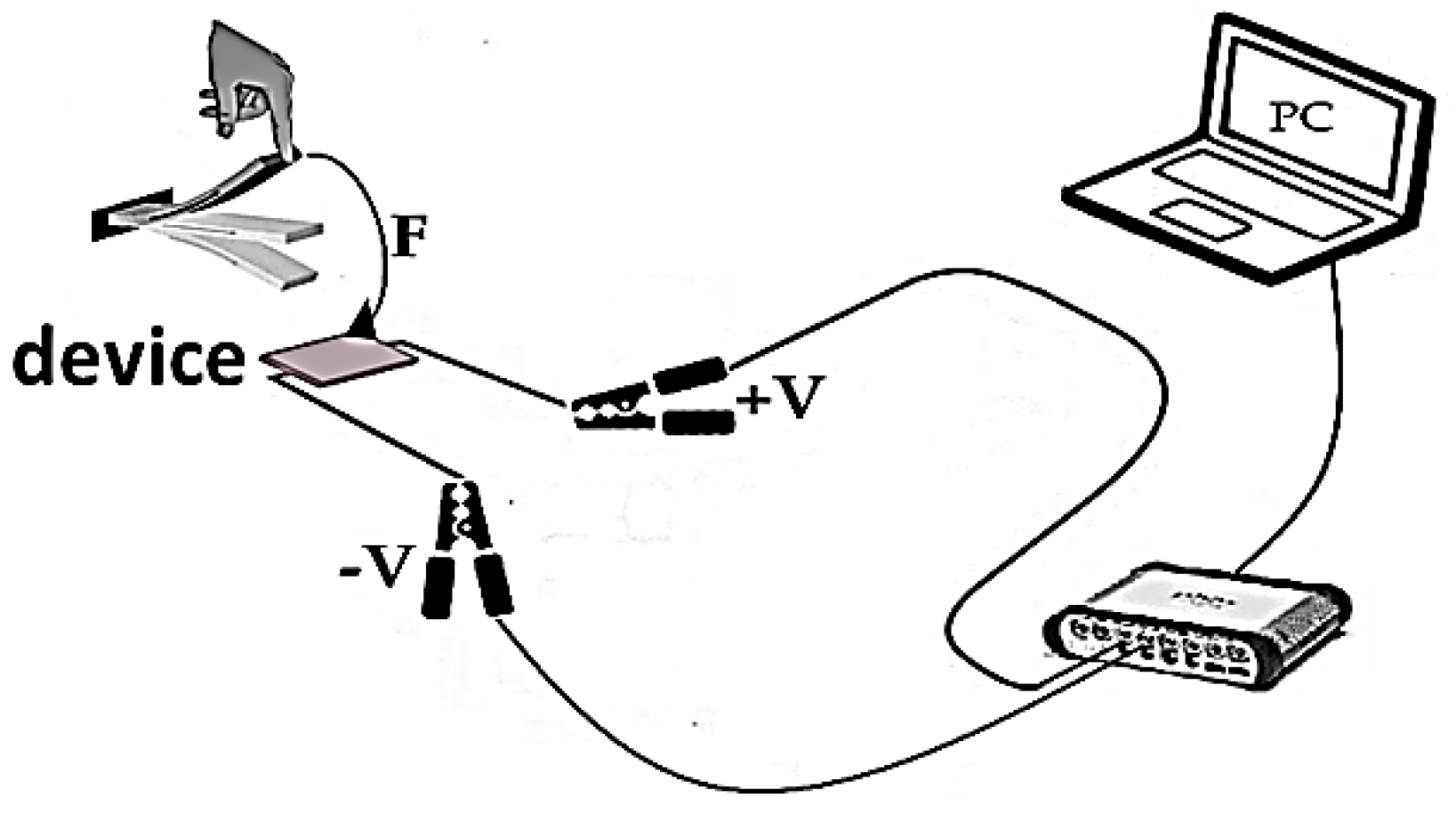
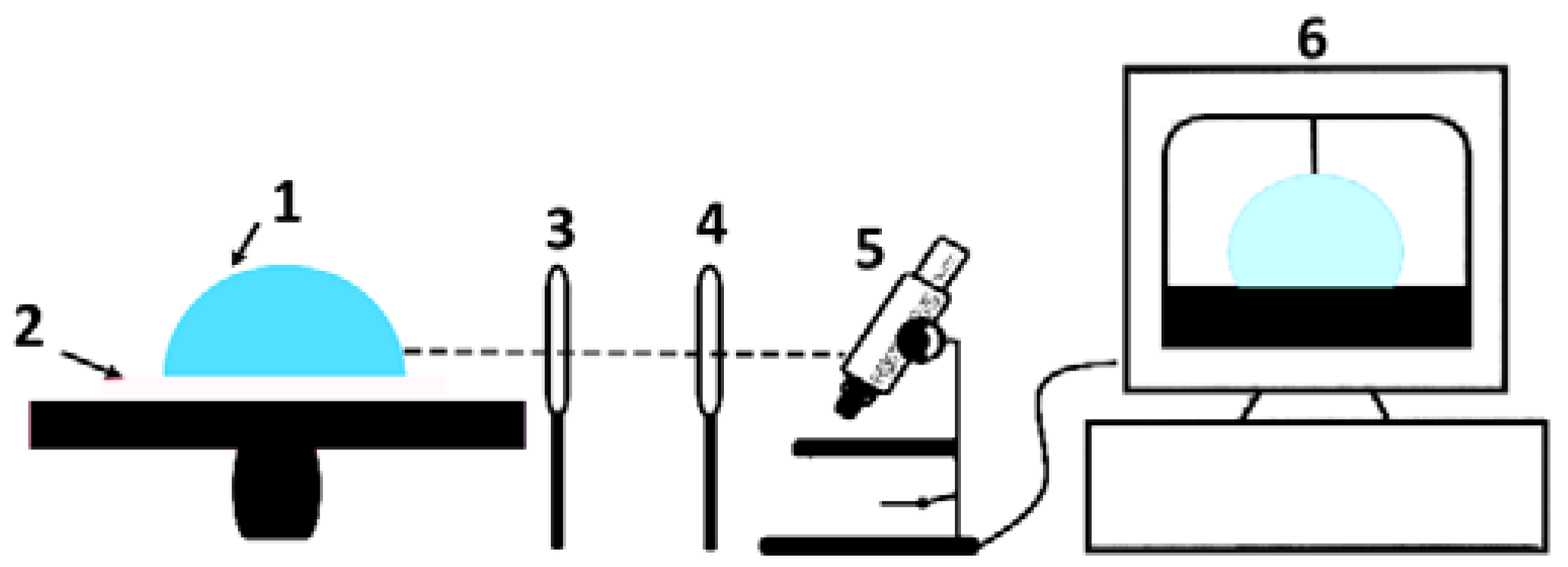

| Sample | PVDF (g) | HA (g) | AgNO3 (g) |
|---|---|---|---|
| (1) | 0.5 | 0.05 | - |
| (2) | 0.5 | 0.10 | - |
| (3) | 0.5 | - | 0.2 |
| (4) | 0.5 | 0.05 | 0.2 |
| (5) | 0.5 | - | - |
| Element | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Weight, % | |||||
| Carbon | 17.37 | 19.16 | 17.04 | 15.04 | 22.01 |
| Oxygen | 5.30 | 5.18 | 8.53 | 8.73 | 6.29 |
| Fluorine | 53.01 | 47.37 | 65.61 | 47.60 | 69.54 |
| Calcium | 13.91 | 16.41 | - | 12.94 | - |
| Silver | - | - | 6.79 | 6.01 | - |
| Phosphorus | 8.09 | 9.83 | - | 7.70 | - |
| Zinc | 2.32 | 2.05 | 2.03 | 1.98 | 2.16 |
| Sample | |||||
|---|---|---|---|---|---|
| Energy (W/A) | 1 | 2 | 3 | 4 | 5 |
| Sample | Short Explanation | Ref. |
|---|---|---|
| PVDF/HA (60:40) | The stress was reported 459.2 ± 4.1 MPa, and deformation was 0.23 mm | [4] |
| PVDF/NaYzeolite composites | Solvent such as DMSO and DMF were compared and the important difference was related to dielectric constant | [68] |
| PVDF in DMSO solvent | The phase characterization was done and maximum β-phase was appeared in the films when PVDF was annealed at 90 °C for 5 h | [39] |
| PVDF-BaTiO3-Ag | Fibers showed an increase in the output voltage (1.78 (12) mV) compared to pristine PVDF and PVDF-BaTiO3 composite fibers (1.48(26) mV) upon applying 1.2 N force at 5 Hz frequency | [69] |
| PVDF in DMSO solvent | PVDF films with high content of β-phase up to 98.8% was obtained by using DMSO as the solvent at optimized crystallizing temperature of 60 °C | [17] |
| HA/PVDF | The HA content from [0% to 15%] reduced the d33 constant from 2.61 pC/N to 1.08 pC/N, while the HA content increased further to 20%, the d33 value increased to 1.53 pC/N. The addition of HA changed the amount of β-PVDF, which determined the piezoelectric performance of the HA/PVDF composite | [3] |
| PVA/AgNO3 | The high antibacterial activity was found to series of the samples dried at 25 °C. The antibacterial activity of the investigated samples was ascribed to silver ions formed during the samples dissolution in the presence of water | [70] |
| PVDF/HA/AgNO3 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markuniene, I.; Rabiei, M.; Nasiri, S.; Urbaite, S.; Palevicius, A.; Janusas, G. Biocompatible Piezoelectric PVDF/HA/AgNO3 Thin Film Prepared by the Solvent Casting Method. Sensors 2023, 23, 289. https://doi.org/10.3390/s23010289
Markuniene I, Rabiei M, Nasiri S, Urbaite S, Palevicius A, Janusas G. Biocompatible Piezoelectric PVDF/HA/AgNO3 Thin Film Prepared by the Solvent Casting Method. Sensors. 2023; 23(1):289. https://doi.org/10.3390/s23010289
Chicago/Turabian StyleMarkuniene, Ieva, Marzieh Rabiei, Sohrab Nasiri, Sigita Urbaite, Arvydas Palevicius, and Giedrius Janusas. 2023. "Biocompatible Piezoelectric PVDF/HA/AgNO3 Thin Film Prepared by the Solvent Casting Method" Sensors 23, no. 1: 289. https://doi.org/10.3390/s23010289
APA StyleMarkuniene, I., Rabiei, M., Nasiri, S., Urbaite, S., Palevicius, A., & Janusas, G. (2023). Biocompatible Piezoelectric PVDF/HA/AgNO3 Thin Film Prepared by the Solvent Casting Method. Sensors, 23(1), 289. https://doi.org/10.3390/s23010289







