Dynamic Seat Assessment for Enabled Restlessness of Children with Learning Difficulties
Abstract
:1. Introduction
Enabling Restlessness in School
2. Materials and Methods
2.1. Participants
2.2. Instrumentation
2.2.1. Seats
2.2.2. Inertial Measurement Unit
2.2.3. SenseWear Sensor
2.2.4. Thermal Images and Cameras
2.2.5. Cognitive Tests
2.3. Measures
2.3.1. Movement Intensity
2.3.2. In-Seat Behaviour
2.3.3. Cognitive Tests Solving Performance
2.3.4. Electrodermal Activity
2.3.5. Facial Temperature
2.3.6. Subjective Assessment
2.3.7. Statistical Analysis
2.3.8. Protocol
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barkley, R.A. Attention-Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment, 2nd ed.; The Guilford Press: New York, NY, USA, 1998. [Google Scholar]
- Association, A.P. Desk Reference to the Diagnostic Criteria from DSM-5; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Halperin, J.M.; Schulz, K.P. Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychol. Bull. 2006, 132, 560. [Google Scholar] [CrossRef] [PubMed]
- Cherkasova, M.; Sulla, E.M.; Dalena, K.L.; Pondé, M.P.; Hechtman, L. Developmental course of attention deficit hyperactivity disorder and its predictors. J. Can. Acad. Child Adolesc. Psychiatry 2013, 22, 47–54. [Google Scholar] [PubMed]
- Sergeant, J.A.; Geurts, H.; Oosterlaan, J. How specific is a deficit of executive functioning for attention-deficit/hyperactivity disorder? Behav. Brain Res. 2002, 130, 3–28. [Google Scholar] [CrossRef]
- Boonstra, M.; Oosterlaan, J.; Sergeant, J.; Buitelaar, J. Executive functioning in adult ADHD: A meta-analytic review. Psychol. Med. 2005, 35, 1097–1108. [Google Scholar] [CrossRef] [Green Version]
- Fischer, M.; Barkley, R.A.; Smallish, L.; Fletcher, K. Executive functioning in hyperactive children as young adults: Attention, inhibition, response perseveration, and the impact of comorbidity. Dev. Neuropsychol. 2005, 27, 107–133. [Google Scholar] [CrossRef]
- DuPaul, G.J.; Gormley, M.J.; Laracy, S.D. Comorbidity of LD and ADHD: Implications of DSM-5 for assessment and treatment. J. Learn. Disabil. 2013, 46, 43–51. [Google Scholar] [CrossRef]
- Boada, R.; Willcutt, E.G.; Pennington, B.F. Understanding the comorbidity between dyslexia and attention-deficit/hyperactivity disorder. Top. Lang. Disord. 2012, 32, 264–284. [Google Scholar] [CrossRef] [Green Version]
- Barkley, R.A. Managing ADHD In School: The Best Evidence-Based Methods for Teachers; PESI Publishing Media: Eau Claire, WI, USA, 2016. [Google Scholar]
- DuPaul, G.J. School-based interventions for students with attention deficit hyperactivity disorder: Current status and future directions. Sch. Psychol. Rev. 2007, 36, 183–194. [Google Scholar] [CrossRef]
- Zentall, S.S. Theory-and evidence-based strategies for children with attentional problems. Psychol. Sch. 2005, 42, 821–836. [Google Scholar] [CrossRef]
- Mulligan, S. Classroom strategies used by teachers of students with attention deficit hyperactivity disorder. Phys. Occup. Ther. Pediatr. 2001, 20, 25–44. [Google Scholar] [CrossRef]
- Bellato, A.; Arora, I.; Hollis, C.; Groom, M.J. Is autonomic nervous system function atypical in attention deficit hyperactivity disorder (ADHD)? A systematic review of the evidence. Neurosci. Biobehav. Rev. 2020, 108, 182–206. [Google Scholar] [CrossRef] [PubMed]
- Yerkes, R.M.; Dodson, J.D. The relation of strength of stimulus to rapidity of habit-formation. J. Comp. Neurol. Psychol. 1908, 18, 459–482. [Google Scholar] [CrossRef] [Green Version]
- Boucsein, W. Electrodermal Activity; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Geršak, G. Electrodermal activity—A beginner’s guide. Elektrotehniski Vestn. 2020, 87, 175–182. [Google Scholar]
- Storm, H. Development of emotional sweating in preterms measured by skin conductance changes. Early Hum. Dev. 2001, 62, 149–158. [Google Scholar] [CrossRef]
- Harker, M. Psychological sweating: A systematic review focused on aetiology and cutaneous response. Ski. Pharmacol. Physiol. 2013, 26, 92–100. [Google Scholar] [CrossRef]
- Ioannou, S.; Gallese, V.; Merla, A. Thermal infrared imaging in psychophysiology: Potentialities and limits. Psychophysiology 2014, 51, 951–963. [Google Scholar] [CrossRef] [Green Version]
- Charkoudian, N. Skin blood flow in adult human thermoregulation: How it works, when it does not, and why. Mayo Clin. Proc. 2003, 78, 603–612. [Google Scholar] [CrossRef] [Green Version]
- Abdelrahman, Y.; Velloso, E.; Dingler, T.; Schmidt, A.; Vetere, F. Cognitive heat: Exploring the usage of thermal imaging to unobtrusively estimate cognitive load. ACM Interact. Mob. Wearable Ubiquitous Technol. 2017, 1, 1–20. [Google Scholar] [CrossRef]
- Cabanac, M.; Brinnel, H. Blood flow in the emissary veins of the human head during hyperthermia. Eur. J. Appl. Physiol. Occup. Physiol. 1985, 54, 172–176. [Google Scholar] [CrossRef]
- Moliné, A.; Dominguez, E.; Salazar-López, E.; Gálvez-García, G.; Fernández-Gómez, J.; De la Fuente, J.; Iborra, O.; Tornay, F.; Gómez Milán, E. The mental nose and the Pinocchio effect: Thermography, planning, anxiety, and lies. J. Investig. Psychol. Offender Profiling 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Or, C.K.; Duffy, V.G. Development of a facial skin temperature-based methodology for non-intrusive mental workload measurement. Occup. Ergon. 2007, 7, 83–94. [Google Scholar] [CrossRef]
- Engert, V.; Merla, A.; Grant, J.A.; Cardone, D.; Tusche, A.; Singer, T. Exploring the use of thermal infrared imaging in human stress research. PLoS ONE 2014, 9, e90782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quesada, J.I.P.; Guillamón, N.M.; de Anda, R.M.C.O.; Psikuta, A.; Annaheim, S.; Rossi, R.M.; Salvador, J.M.C.; Pérez-Soriano, P.; Palmer, R.S. Effect of perspiration on skin temperature measurements by infrared thermography and contact thermometry during aerobic cycling. Infrared Phys. Technol. 2015, 72, 68–76. [Google Scholar] [CrossRef]
- Halperin, J.M.; Matier, K.; Bedi, G.; Sharma, V.; Newcorn, J.H. Specificity of inattention, impulsivity, and hyperactivity to the diagnosis of attention-deficit hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 1992, 31, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Zentall, S.S.; Zentall, T.R. Optimal stimulation: A model of disordered activity and performance in normal and deviant children. Psychol. Bull. 1983, 94, 446. [Google Scholar] [CrossRef]
- Rapport, M.D.; Bolden, J.; Kofler, M.J.; Sarver, D.E.; Raiker, J.S.; Alderson, R.M. Hyperactivity in Boys with Attention-Deficit/Hyperactivity Disorder (ADHD): A Ubiquitous Core Symptom or Manifestation of Working Memory Deficits? J. Abnorm. Child Psychol. 2009, 37, 521–534. [Google Scholar] [CrossRef]
- Hartanto, T.; Krafft, C.; Iosif, A.M.; Schweitzer, J.B. A trial-by-trial analysis reveals more intense physical activity is associated with better cognitive control performance in attention-deficit/hyperactivity disorder. Child Neuropsychol. 2016, 22, 618–626. [Google Scholar] [CrossRef] [Green Version]
- Hudec, K.L.; Alderson, R.M.; Kasper, L.J.; Patros, C.H. Working memory contributes to elevated motor activity in adults with ADHD: An examination of the role of central executive and storage/rehearsal processes. J. Atten. Disord. 2014, 18, 357–368. [Google Scholar] [CrossRef] [Green Version]
- Sarver, D.E.; Rapport, M.D.; Kofler, M.J.; Raiker, J.S.; Friedman, L.M. Hyperactivity in attention-deficit/hyperactivity disorder (ADHD): Impairing deficit or compensatory behavior? J. Abnorm. Child Psychol. 2015, 43, 1219–1232. [Google Scholar] [CrossRef]
- Song, J.H. The role of attention in motor control and learning. Curr. Opin. Psychol. 2019, 29, 261–265. [Google Scholar] [CrossRef]
- Song, J.H.; Bédard, P. Paradoxical benefits of dual-task contexts for visuomotor memory. Psychol. Sci. 2015, 26, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, M.; Taylor, E. The neuropsychology of attention development. In Child Neuropsychology: Concepts, Theory, and Practice; Wiley-Blackwell: Chichester, UK; Malden, MA, USA, 2008; pp. 233–263. [Google Scholar]
- Hamilton, M.T.; Healy, G.N.; Dunstan, D.W.; Zderic, T.W.; Owen, N. Too little exercise and too much sitting: Inactivity physiology and the need for new recommendations on sedentary behavior. Curr. Cardiovasc. Risk Rep. 2008, 2, 292–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proper, K.I.; Singh, A.S.; Van Mechelen, W.; Chinapaw, M.J. Sedentary behaviors and health outcomes among adults: A systematic review of prospective studies. Am. J. Prev. Med. 2011, 40, 174–182. [Google Scholar] [CrossRef] [PubMed]
- da Costa, B.G.; da Silva, K.S.; George, A.M.; de Assis, M.A.A. Sedentary behavior during school-time: Sociodemographic, weight status, physical education class, and school performance correlates in Brazilian schoolchildren. J. Sci. Med. Sport 2017, 20, 70–74. [Google Scholar] [CrossRef] [PubMed]
- del Pozo-Cruz, J.; García-Hermoso, A.; Alfonso-Rosa, R.M.; Alvarez-Barbosa, F.; Owen, N.; Chastin, S.; del Pozo-Cruz, B. Replacing sedentary time: Meta-analysis of objective-assessment studies. Am. J. Prev. Med. 2018, 55, 395–402. [Google Scholar] [CrossRef]
- Triglav, J.; Howe, E.; Cheema, J.; Dube, B.; Fenske, M.J.; Strzalkowski, N.; Bent, L. Physiological and cognitive measures during prolonged sitting: Comparisons between a standard and multi-axial office chair. Appl. Ergon. 2019, 78, 176–183. [Google Scholar] [CrossRef]
- Kett, A.R.; Sichting, F. Sedentary behaviour at work increases muscle stiffness of the back: Why roller massage has potential as an active break intervention. Appl. Ergon. 2020, 82, 102947. [Google Scholar] [CrossRef]
- Schilling, D.L.; Washington, K.; Billingsley, F.F.; Deitz, J. Classroom seating for children with attention deficit hyperactivity disorder: Therapy balls versus chairs. Am. J. Occup. Ther. 2003, 57, 534–541. [Google Scholar] [CrossRef] [Green Version]
- Fedewa, A.L.; Erwin, H.E. Stability balls and students with attention and hyperactivity concerns: Implications for on-task and in-seat behavior. Am. J. Occup. Ther. 2011, 65, 393–399. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.L.; Wang, C.C.; Chen, C.H.; Lai, C.L.; Yang, P.C.; Guo, L.Y. Influence of therapy ball seats on attentional ability in children with attention deficit/hyperactivity disorder. J. Phys. Ther. Sci. 2012, 24, 1177–1182. [Google Scholar] [CrossRef] [Green Version]
- Taipalus, A.C.; Hixson, M.D.; Kanouse, S.K.; Wyse, R.D.; Fursa, S. Effects of therapy balls on children diagnosed with attention deficit hyperactivity disorder. Behav. Interv. 2017, 32, 418–426. [Google Scholar] [CrossRef]
- Macphee, F.L.; Merrill, B.M.; Altszuler, A.R.; Ramos, M.C.; Gnagy, E.M.; Greiner, A.R.; Coxe, S.; Raiker, J.S.; Coles, E.; Burger, L.; et al. The effect of weighted vests and stability balls with and without psychostimulant medication on classroom outcomes for children with ADHD. Sch. Psychol. Rev. 2019, 48, 276–289. [Google Scholar] [CrossRef]
- Panagiotopoulou, G.; Christoulas, K.; Papanckolaou, A.; Mandroukas, K. Classroom furniture dimensions and anthropometric measures in primary school. Appl. Ergon. 2004, 35, 121–128. [Google Scholar] [CrossRef]
- Gouvali, M.K.; Boudolos, K. Match between school furniture dimensions and children’s anthropometry. Appl. Ergon. 2006, 37, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Castellucci, H.; Arezes, P.; Molenbroek, J. Applying different equations to evaluate the level of mismatch between students and school furniture. Appl. Ergon. 2014, 45, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Beravs, T.; Podobnik, J.; Munih, M. Three-axial accelerometer calibration using Kalman filter covariance matrix for online estimation of optimal sensor orientation. IEEE Trans. Instrum. Meas. 2012, 61, 2501–2511. [Google Scholar] [CrossRef]
- Repnik, E.; Puh, U.; Goljar, N.; Munih, M.; Mihelj, M. Using inertial measurement units and electromyography to quantify movement during action research arm test execution. Sensors 2018, 18, 2767. [Google Scholar] [CrossRef] [Green Version]
- BodyMedia, Inc. BodyMedia FIT Armband and Display Manual; BodyMedia, Inc.: Pittsburgh, PA, USA, 2011. [Google Scholar]
- Baddeley, A. Working memory: Looking back and looking forward. Nat. Rev. Neurosci. 2003, 4, 829–839. [Google Scholar] [CrossRef]
- Reitan, R.M. Validity of the Trail Making Test as an indicator of organic brain damage. Percept. Mot. Ski. 1958, 8, 271–276. [Google Scholar] [CrossRef]
- Shallice, T. Specific impairments of planning. Philos. Trans. R. Soc. London. Biol. Sci. 1982, 298, 199–209. [Google Scholar]
- Geršak, G.; Gržinič Frelih, N.; Drnovšek, J. Measurement anxiety in physiological measurements. In Proceedings of the Twentieth International Electrotechnical and Computer Science Conference ERK 2011, Portorož, Slovenia, 21–22 September 2011; pp. 482–485. [Google Scholar]
- Rapport, M.D.; Alderson, R.M.; Kofler, M.J.; Sarver, D.E.; Bolden, J.; Sims, V. Working memory deficits in boys with attention-deficit/hyperactivity disorder (ADHD): The contribution of central executive and subsystem processes. J. Abnorm. Child Psychol. 2008, 36, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.C.; Keenan, J.M.; Betjemann, R.S.; Willcutt, E.G.; Pennington, B.F.; Olson, R.K. Reading comprehension in children with ADHD: Cognitive underpinnings of the centrality deficit. J. Abnorm. Child Psychol. 2013, 41, 473–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cepeda, N.J.; Cepeda, M.L.; Kramer, A.F. Task switching and attention deficit hyperactivity disorder. J. Abnorm. Child Psychol. 2000, 28, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.J.; Robertson, M.M.; Baker, N.A. The effect of sit-stand desks on office worker behavioral and health outcomes: A scoping review. Appl. Ergon. 2019, 78, 37–53. [Google Scholar] [CrossRef]
- Sherry, A.P.; Pearson, N.; Clemes, S.A. The effects of standing desks within the school classroom: A systematic review. Prev. Med. Rep. 2016, 3, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Finch, L.E.; Tomiyama, A.J.; Ward, A. Taking a stand: The effects of standing desks on task performance and engagement. Int. J. Environ. Res. Public Health 2017, 14, 939. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Ruiz, N.; Taib, R.; Choi, E.; Chen, F. Galvanic skin response (GSR) as an index of cognitive load. In Proceedings of the CHI ’07 Extended Abstracts on Human Factors in Computing Systems, San Jose, CA, USA, 28 April–3 May 2007; pp. 2651–2656. [Google Scholar]
- Ikehara, C.S.; Crosby, M.E. Assessing cognitive load with physiological sensors. In Proceedings of the 38th Annual Hawaii International Conference on System Sciences, Big Island, HI, USA, 3–6 January 2005; p. 295a. [Google Scholar]
- Kreibig, S.D. Autonomic nervous system activity in emotion: A review. Biol. Psychol. 2010, 84, 394–421. [Google Scholar] [CrossRef]
- Collet, C.; Vernet-Maury, E.; Delhomme, G.; Dittmar, A. Autonomic nervous system response patterns specificity to basic emotions. J. Auton. Nerv. Syst. 1997, 62, 45–57. [Google Scholar] [CrossRef]
- Fernández, C.; Pascual, J.C.; Soler, J.; Elices, M.; Portella, M.J.; Fernández-Abascal, E. Physiological responses induced by emotion-eliciting films. Appl. Psychophysiol. Biofeedback 2012, 37, 73–79. [Google Scholar] [CrossRef]
- Cruz-Albarran, I.A.; Benitez-Rangel, J.P.; Osornio-Rios, R.A.; Morales-Hernandez, L.A. Human emotions detection based on a smart-thermal system of thermographic images. Infrared Phys. Technol. 2017, 81, 250–261. [Google Scholar] [CrossRef]
- Salazar-López, E.; Domínguez, E.; Ramos, V.J.; De la Fuente, J.; Meins, A.; Iborra, O.; Gálvez, G.; Rodríguez-Artacho, M.; Gómez-Milán, E. The mental and subjective skin: Emotion, empathy, feelings and thermography. Conscious. Cogn. 2015, 34, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Goulart, C.; Valad ao, C.; Delisle-Rodriguez, D.; Caldeira, E.; Bastos, T. Emotion analysis in children through facial emissivity of infrared thermal imaging. PLoS ONE 2019, 14, e0212928. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Participant | Comorbid Disorders |
|---|---|
| ES01 | / |
| ES02 | Specific learning disabilities |
| ES03 | Dyslexia |
| ES04 | Dyslexia |
| ES05 | Dyslexia |
| ES06 | Specific learning disabilities |
| ES07 | Speech and language disorder, Specific learning disabilities |
| ES08 | Developmental co-ordination disorder (dyspraxia) |
| ES09 | / |
| ES10 | Dyslexia |
| ES11 | Specific learning disabilities |
| Seating Order | Participants |
|---|---|
| A-B-C | ES01, KS01, ES07, KS07 |
| A-C-B | ES02, KS02, ES08, KS08 |
| B-A-C | ES03, KS03, ES09, KS09 |
| B-C-A | ES04, KS04, ES10, KS10 |
| C-A-B | ES05, KS05, ES11, KS11 |
| C-B-A | ES06, KS06, KS12 |
| Children with ADHD [s] | Children without ADHD [s] | ||
|---|---|---|---|
| A | 88.0 | 92.1 | |
| SWM | B | 93.5 | 88.9 |
| C | 59.6 | 97.6 | |
| A | 193.3 | 224.5 | |
| VWM | B | 177.3 | 206.0 |
| C | 174.8 | 201.2 | |
| A | 189.8 | 136.1 | |
| TMT-AD | B | 184.0 | 135.6 |
| C | 153.9 | 130.4 | |
| A | 743.1 | 852.8 | |
| RC | B | 707.3 | 825.7 |
| C | 680.6 | 840.3 | |
| A | 315.0 | 261.2 | |
| ToL-AD | B | 265.3 | 251.1 |
| C | 256.4 | 245.1 |
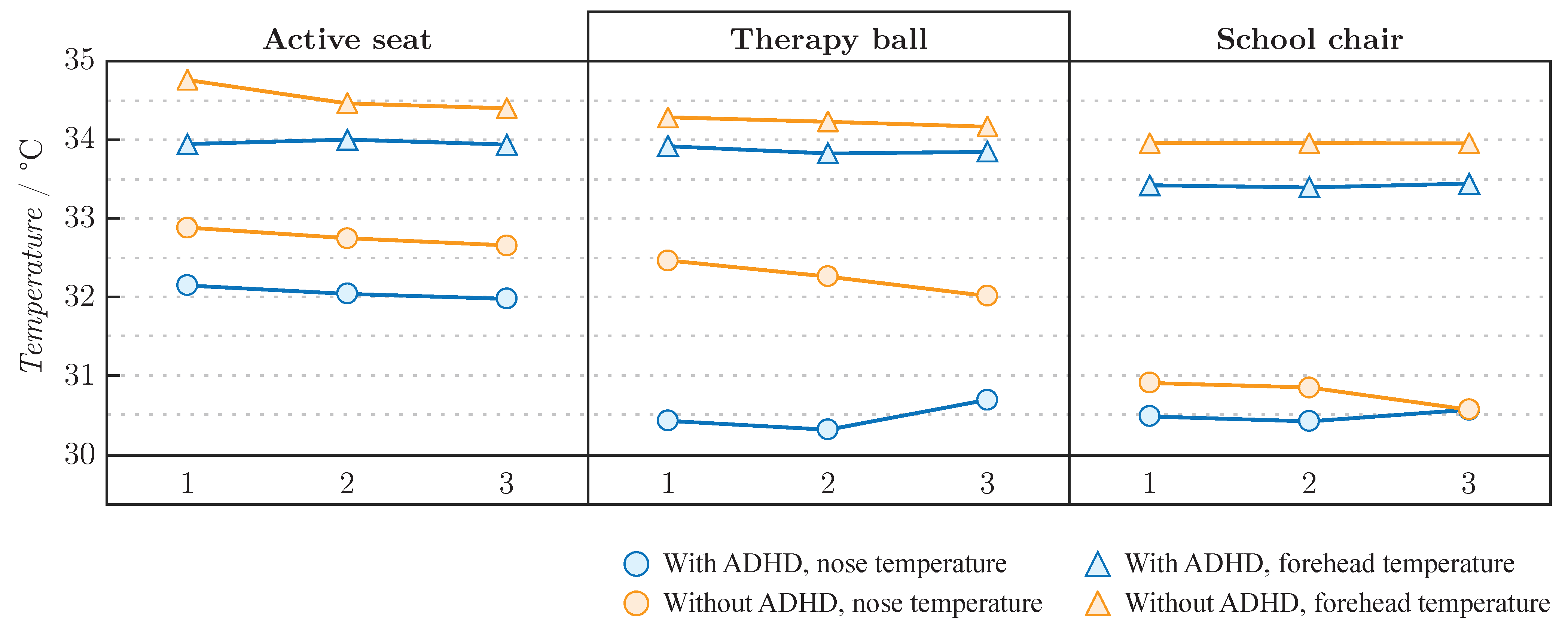
| A [°C] | B [°C] | C [°C] | ||
|---|---|---|---|---|
| Forehead | 34.0 | 33.9 | 33.4 | |
| Children with ADHD | Nose | 32.1 | 30.5 | 30.5 |
| Difference | 1.9 | 3.4 | 2.9 | |
| Forehead | 34.5 | 34.2 | 34.0 | |
| Children without ADHD | Nose | 32.8 | 32.2 | 30.8 |
| Difference | 1.8 | 2.0 | 3.2 |
| Source of Variation | SS | df | MS | F | p | |
|---|---|---|---|---|---|---|
| Movement Intensity | ||||||
| Group | 0.441 | 1 | 0.441 | 4.049 | 0.057 | 0.162 |
| Error(Group) | 2.286 | 21 | 0.109 | |||
| Seat | 0.576 | 2 | 0.288 | 12.014 | <0.001 | 0.364 |
| Seat × Group | 0.137 | 2 | 0.068 | 2.850 | 0.069 | 0.120 |
| Error(Seat) | 1.007 | 42 | 0.024 | |||
| In-seat Behaviour | ||||||
| Group | 2.396 | 1 | 2.396 | 1.688 | 0.208 | 0.074 |
| Error(Group) | 29.813 | 21 | 1.420 | |||
| Seat 1 | 16.899 | 1.600 | 10.562 | 11.838 | <0.001 | 0.361 |
| Seat × Group 1 | 1.213 | 1.600 | 0.758 | 0.850 | 0.414 | 0.039 |
| Error(Seat) 1 | 29.977 | 33.600 | 0.892 | |||
| Cognitive Tests Solving Performance | ||||||
| Group | 656.628 | 1 | 656.628 | 1.587 | 0.222 | 0.070 |
| Error(Group) | 8688.643 | 21 | 413.745 | |||
| Seat | 12.238 | 2 | 6.119 | 0.123 | 0.885 | 0.006 |
| Seat × Group | 27.126 | 2 | 13.563 | 0.273 | 0.763 | 0.013 |
| Error(Seat) | 2088.831 | 42 | 49.734 | |||
| Electrodermal Activity | ||||||
| Group | 565.688 | 1 | 565.688 | 1.622 | 0.218 | 0.079 |
| Error(Group) | 6628.284 | 19 | 348.857 | |||
| Seat | 93.566 | 2 | 46.783 | 0.190 | 0.828 | 0.010 |
| Seat × Group | 742.876 | 2 | 371.438 | 1.505 | 0.235 | 0.073 |
| Error(Seat) | 9378.676 | 38 | 246.807 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanić, V.; Žnidarič, T.; Repovš, G.; Geršak, G. Dynamic Seat Assessment for Enabled Restlessness of Children with Learning Difficulties. Sensors 2022, 22, 3170. https://doi.org/10.3390/s22093170
Stanić V, Žnidarič T, Repovš G, Geršak G. Dynamic Seat Assessment for Enabled Restlessness of Children with Learning Difficulties. Sensors. 2022; 22(9):3170. https://doi.org/10.3390/s22093170
Chicago/Turabian StyleStanić, Valentina, Taja Žnidarič, Grega Repovš, and Gregor Geršak. 2022. "Dynamic Seat Assessment for Enabled Restlessness of Children with Learning Difficulties" Sensors 22, no. 9: 3170. https://doi.org/10.3390/s22093170
APA StyleStanić, V., Žnidarič, T., Repovš, G., & Geršak, G. (2022). Dynamic Seat Assessment for Enabled Restlessness of Children with Learning Difficulties. Sensors, 22(9), 3170. https://doi.org/10.3390/s22093170






