Abstract
Vibrotactile sensory augmentation (SA) decreases postural sway during real-time use; however, limited studies have investigated the long-term effects of training with SA. This study assessed the retention effects of long-term balance training with and without vibrotactile SA among community-dwelling healthy older adults, and explored brain-related changes due to training with SA. Sixteen participants were randomly assigned to the experimental group (EG) or control group (CG), and trained in their homes for eight weeks using smart-phone balance trainers. The EG received vibrotactile SA. Balance performance was assessed before, and one week, one month, and six months after training. Functional MRI (fMRI) was recorded before and one week after training for four participants who received vestibular stimulation. Both groups demonstrated significant improvement of SOT composite and MiniBESTest scores, and increased vestibular reliance. Only the EG maintained a minimal detectable change of 8 points in SOT scores six months post-training and greater improvements than the CG in MiniBESTest scores one month post-training. The fMRI results revealed a shift from activation in the vestibular cortex pre-training to increased activity in the brainstem and cerebellum post-training. These findings showed that additional balance improvements were maintained for up to six months post-training with vibrotactile SA for community-dwelling healthy older adults.
1. Introduction
Medical costs associated with age-related falls exceed $50 billion per year [1]. Exercise programs with targeted balance and strength training have been shown to improve balance and reduce falls among community-dwelling older adults [2,3,4]. Supervised training programs are typically individually tailored and lead to better outcomes as compared to class-based balance programs but are costly and not universally accessible [5]. While independent in-home programs may address these issues, the lack of clinician guidance results in fewer clinical improvements than supervised programs [6]. Telerehabilitation technologies may address the need for intensive, accessible, in-home semi-supervised balance training. Technologies, such as the Wii-fit [7], Kinect [8], and wearables that provide feedback [9] or incentives [10], have been investigated in combination with balance training. Sustained quality of life improvements and fewer fall incidents require retention of balance improvements.
Vibrotactile sensory augmentation (SA) systems have been used in balance-related research studies to estimate body motion and provide postural corrective cues in the form of vibration to the user. Multiple studies (although many were uncontrolled) have demonstrated short-term retentive (or possibly habituation or context-specific adaptation) effects of training with vibrotactile SA [11,12,13,14,15,16]. For example, Basta et al. showed that participants with balance deficits reduced their trunk sway after two weeks of training with vibrotactile SA and retained the effects of training for three months [12]. Kingma et al. reported improved mobility and balance scores in a small group of participants with bilateral vestibular loss who wore a vibrotactile belt daily for one month while standing and moving during activities of daily living [15].
A limited number of controlled studies have examined retention and/or carryover effects following longer-term training with SA. In a randomized controlled study, people with Parkinson’s disease participated in 12 sessions of clinical balance training [17]. The authors compared the effects of virtual reality (VR) augmented balance training using a dynamic balance board (VR group) to conventional balance training [17]. The VR group improved significantly on the Computerized Dynamic Posturography (CDP) Sensory Organization Test (SOT) condition 6 (unreliable vision and somatosensory inputs) as assessed within seven days after training; however, this finding was not significant at the four-week follow-up, suggesting limited retention effects. In another study involving people with Parkinson’s disease, improvements in SOT scores were retained and falls were reduced three months after 10 training sessions with vibrotactile SA over a two-week period [18]. Although this study did not have a control group, the SOT scores of the participants in this study showed greater improvement compared with 10 participants with Parkinson’s disease previously trained using CDP in a different study.
In a study involving older adults, balance training with vibrotactile SA three times per week for two weeks had minimal additional effects on both immediate and longer-term balance outcomes compared to a control group that performed balance training without vibrotactile SA [9]. However, in this study, balance tasks were not customized on an individual participant basis and the limited training period did not follow the recommended FITT principles of frequency, intensity, type, and time [19].
In a six-week study (totaling 18 balance training sessions) involving vestibular rehabilitation exercises, participants with vestibular deficits, regardless of group, demonstrated improvements in a subset of clinical and balance metrics immediately following completion of the balance training protocol [20]. However, the experimental group that trained with vibrotactile SA showed significantly greater improvements than the control group who trained without vibrotactile SA on the Activities-specific Balance Confidence Scale and postural stability during two standing balance exercises with head movements.
Our prior work investigated the effects of long-term (eight-week home-based balance training program) balance training with and without vibrotactile SA on clinical outcome measures for community-dwelling older adults [21]. Participants who completed training with vibrotactile SA had greater improvements in SOT scores, Mini Balance Evaluation Systems Test scores, and Five Times Sit to Stand Test duration compared with the control group who trained without vibrotactile SA. Both groups also demonstrated increased vestibular reliance as measured by a ratio of SOT scores [21].
Beyond the lack of studies on retention effects, a limited number of studies have been performed to investigate the changes in the sensorimotor brain regions during and following balance training with SA. Multiple hypotheses have been posited to explain the potential mechanisms underlying balance improvements during and following training with SA devices, including sensory reweighting and context-specific adaptation [14,22]. Among the most relevant, a previous study reported that SA coupled with navigation training was associated with changes in brain activity in the sensorimotor and navigation (hippocampus, caudate) brain regions [23]. However, it is unknown whether brain-related changes occur after balance training with SA.
Given the lack of long-term training studies that explore the retention effects of training with SA and the lack of studies that have evaluated potential brain-related changes, there is a need for additional research that investigates the retention of training effects following longer-term training with vibrotactile SA. The two main purposes of this study were to (1) understand the retention effects of balance improvements by examining the balance performance following completion of an eight-week in-home balance training program with wearable vibrotactile sensory augmentation (SA) [21]; and (2) further the understanding of mechanisms underlying the balance improvements by examining brain changes in processing vestibular stimulation from pre- to post-training with SA. This study is one of the first of its kind to assess balance improvements and retention effects on a battery of clinical outcome measures after a long-term customized balance training program with and without SA.
2. Materials and Methods
2.1. Participants
In total, 16 healthy older adults (5 M, 75.4 ± 4.7 years) were recruited from the community. Participants were eligible if they were 65–85 years old, in good general health, and had self-reported balance concerns [21]. Participants were randomly allocated to either a control group (CG, n = 8) or experimental group (EG, n = 8). One participant was withdrawn due to an unrelated orthopedic condition, and four were lost to the six-month follow-up (Figure 1). All participants gave written informed consent, and the study protocol was approved by the University of Michigan Institutional Review Board (HUM00086479) and adhered to the Declaration of Helsinki.
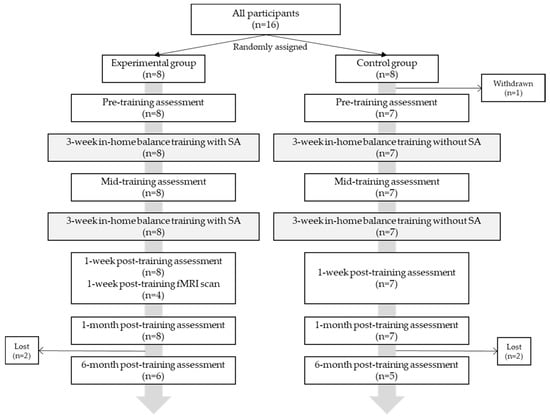
Figure 1.
Participant allocation and experimental protocol.
2.2. Protocol
Participants performed eight weeks of in-home balance training (24 sessions, n = 15) and five clinical balance testing (CBT) sessions throughout the study: pre—(n = 15), mid—(n = 15), one week post—(n = 15), one month post—(n = 15), and six months post-training (n = 11) (Figure 1). CBT was performed by a licensed physical therapist blinded to the group and included: Computerized Dynamic Posturography (CDP) (Sensory Organization Tests (SOT)), Activity-specific Balance Confidence (ABC) Scale, Mini Balance Evaluations Systems Test (Mini-BESTest28 and Mini-BESTest32), Five Times Sit to Stand Test (5xSST), Four-Square Step Test (FSST), Functional Reach Test (FRT), 10-m walk test (self-selected and fast gait speeds), Timed Up and Go (TUG), and Timed up and Go–Cognitive (TUG-COG). Somatosensory, visual, and vestibular reliance were calculated as ratios of individual SOT scores [21,24]. The minimal detectable change (MDC) was determined for functional measures. MDC is an estimate of the smallest change in an outcome score that is correlated with a change in ability [25].
All participants wore a smartphone-based balance trainer comprising two Apple iPods (6th generation iPod touch, 2015), an elastic belt, and a customized tactor accessory during training, which has been described in detail in a prior publication (Figure 2) [21]. One of the two iPods that served as the sensing unit was attached to the elastic belt, which was worn around the torso at approximately the L4/L5 level to measure trunk sway; the second iPod served as the user interface unit attached to a lanyard and was worn around the neck. Participants in the EG received vibrotactile cues on their navel, spine, and left and right sides of their torso when their trunk motion (combination of angular position and angular velocity) exceeded preset thresholds. The preset thresholds for each exercise type (details below) were informed by the study team expertise and the thresholds used in previously published studies [20,21,26]. Participants were instructed to “move away from the vibration”. The gravitational outputs (Class CoreMotion, Apple Inc., Cupertino, CA, USA) from the torso-mounted iPod’s (i.e., sensing unit) accelerometers were used to estimate angular displacements (tilt angles) in the anterior-posterior and medial-lateral directions based on an algorithm developed by Lee et al. [27]. Angular velocities were measured from the sensing unit’s gyroscopes; both accelerometers and gyroscopes were sampled at 50 Hz. The customized tactor accessory included four tactors (Precision Microdrives™, 310–101 vibration motors encased in plastic housings [27]) that interfaced with a PCB-designed controller board and were powered by a 3.7 V battery. The controller board analyzed audio signals provided by the sensing unit and activated the corresponding tactor to provide vibrotactile cues.
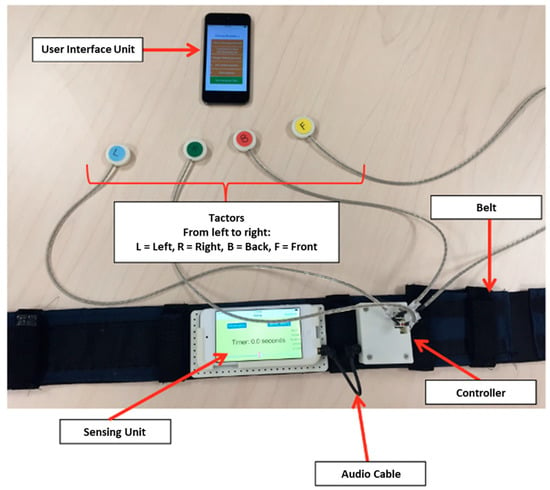
Figure 2.
Smartphone-based balance trainer.
Each hour-long balance training session consisted of six repetitions of six types of balance exercises from the following five exercise categories: static standing on firm and compliant surfaces, weight shifting, modified center of gravity (arm raises), and gait [21,28]. Other important exercise variables included eyes open/closed conditions and the addition of head movements. Vibrotactile cues were not provided for gait-based exercises. Participants were progressed through each category remotely by a physical therapist using the participants’ reported perceived stability scores [21], with the goal of providing a continuum of moderately challenging exercises using a progression protocol [28].
Functional magnetic resonance imaging (fMRI) was acquired from four EG participants using a 3.0 T MRI scanner (GE DISCOVERY MR750) one week post-training to investigate changes in brain function due to balance training. First, a whole brain structural image was acquired using a T1-weighted interleaved echo-planar imaging (EPI) sequence (TR = 12.2 s, TE = 5.1 ms, FA = 15°, matrix size = 256 × 256, FOV = 260 × 260 mm, slice thickness = 1 mm). Next, a gradient-echo spiral-pulse sequence (FOV = 220 mm, TR = 2 s, TE = 30 ms, number of slices = 43, voxel size = 3.4375 × 3.4375 mm) was used to acquire functional images. The participants’ head movements inside the scanner were minimized by a Velcro strap placed over their foreheads and padding placed around the sides of their heads. Participants’ physiological responses were collected using a pulse oximeter placed on their index fingers, and a respirometer wrapped around their abdomens. Low-force skull taps were applied over participants’ lateral cheekbones to stimulate the vestibular system using a pneumatic pulse system (Pneumatic Tactile Pulse System, Engineering Acoustics, Inc., Casselberry, FL, USA). Our prior work has shown that this system activates the vestibular cortical region and that resulting brain activity is correlated with balance under a variety of conditions [29,30]. A block design was implemented for the duration of each stimulation run (4 min) to include five alternating periods of rest (20 s) and stimulation (24 s) [29,30].
2.3. Analysis
All CBT outcome measures are shown as group mean values with standard errors of the means. Differences from pre- to post-training (one week post-, one month post-, six months post-training) were analyzed using a linear mixed model with time and the interaction between groups (EG vs. CG) as the main effects. Significance was set to 0.05.
The fMRI data preprocessing analyses were performed using spm8 software (The Wellcome Centre for Human Neuroimaging, London, UK) [31]. The raw data were examined for excessive motion as the skull vibration induced by the pneumatic taps could be a potential source of motion artifacts. Cut-off thresholds of >3 mm translation or >5° rotation of the head were implemented for head motion correction. The physiological responses (i.e., cardiac and respiration data) were regressed out of the functional data using the RETROICOR algorithm [32]. The first 10 volumes in each run were discarded to ensure the steady state of the MR signal at the beginning of the stimulation runs. Next, the functional images were realigned to the first functional volume of the run and the anatomical image. Both functional and anatomical images were then normalized to the Montreal Neurological Institute (MNI) template [33]. The cerebellum, however, was normalized to the Spatially Unbiased Atlas Template [32,34,35,36]. The normalized functional images were spatially smoothed with a Gaussian kernel function (8,8,8 mm). The smoothed functional images were then used to design the first-level analysis to compare brain activity during stimulation to rest. Next, a paired t-test was applied to compare brain activity pre- to post-training. A threshold of p ≤ 0.001 (unc.) and a minimum cluster size of 10 voxels (voxel size = 2 × 2 × 2 mm) were implemented for the results. The significant coordinates were localized using the MNI atlas [33] for the whole brain analyses, and the SUIT atlas [34] for the cerebellar coordinates. A gray matter inclusive mask was also applied using Automated Anatomical Labeling [37] to filter out activity in the white matter. To examine activity in regions previously identified as the vestibular nuclei (x = −16/16, y = −36, z = −32) [38], a small volume correction was applied, and the deep cerebellar nuclei were identified using the SUIT probabilistic atlas for deep cerebellar nuclei [35].
3. Results
3.1. Clinical Balance Testing Results
SOT composite scores were significantly improved one week, one month, and six months post-training (p < 0.01, 0.01, and 0.01, respectively) regardless of group (Figure 3a). The EG demonstrated a mean minimal detectable change (MDC) of at least 8 points (healthy population [39]) for their SOT composite scores one week and six months post balance training (∆ = 8.1 ± 4.5, 9.2 ± 3.7 points, respectively) while the CG demonstrated a MDC one month post-training (∆ = 8.7 ± 4.2). There were no significant changes in the visual reliance scores, but vestibular reliance increased significantly one week, one month, and six months post-training (p < 0.001, 0.001, and 0.001, respectively), with no effect of group (Figure 3b).
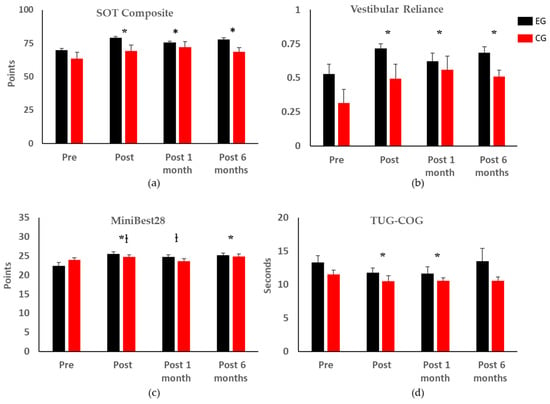
Figure 3.
Statistical analysis of clinical balance test outcome metrics (* indicates significant differences with respect to the pre-training assessment regardless of group; † indicates significant differences between the two groups). Error bars represent the standard errors of the means. Only the clinical balance test outcome metrics with significant changes are shown; the complete results can be found in Appendix A. (a) SOT Composite: Sensory Organization Test Composite Score; (b) Vestibular Reliance calculated from the SOT; (c) Mini-BESTest28: Mini Balance Evaluations Systems Test with total score of 28; (d) TUG-COG: Timed up and Go with Cognitive Task.
All participants had improved Mini-BESTest28 and Mini-BESTest32 scores one week and six months post-training (Mini-BESTest28: p < 0.001 and 0.01, respectively; Mini-BESTest32: p < 0.01 and 0.03, respectively); the EG had significantly better scores than the CG one week and one month post-training for the MiniBest28 (p = 0.04 and 0.03, respectively) and MiniBest32 (p = 0.01 and 0.05, respectively) (Figure 3c).
There were no changes in the TUG score, ABC score, self-selected, or fast gait speeds due to time or group. There was a significant improvement in TUG-COG one week and one month post-training (p = 0.04 and 0.03, respectively), with no difference between groups (Figure 3d). There was a significant improvement in the 5XSTS times one month post-training (p = 0.02), with no difference between groups. An MDC of 2.5 s (geriatric population) [40] was found in the EG one month post-training. There was a significant difference in the FSST six months post-training (p = 0.01), but no difference between groups. For the FRT, forward reach was reduced by 1.23 cm six months post-training (p = 0.01), with no difference between groups. Detailed data can be found in Appendix A.
3.2. fMRI Results
Pilot data from the four participants who underwent pre- and post-training fMRI of vestibular processing revealed a shift in activation from the vestibular cortex to the vestibular nucleus [41] in the brain stem and cerebellum immediately following the balance training with vibrotactile SA (Figure 4, Table 1).
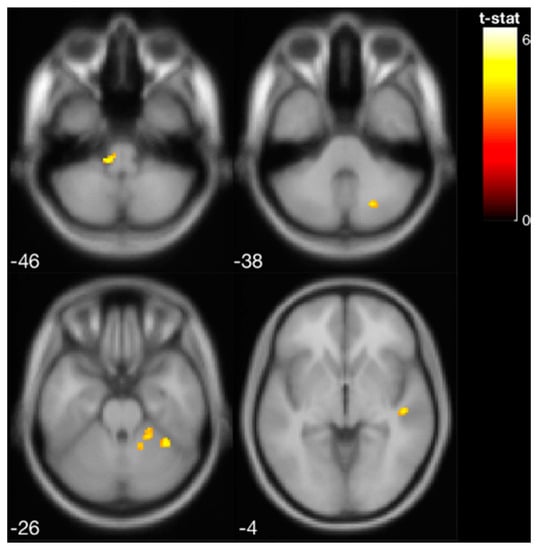
Figure 4.
Increased brain activity in the brainstem and cerebellar cortex following balance training with vibrotactile SA (i.e., increased activation from pre- to post-training with vibrotactile SA) for the four EG participants that were scanned.

Table 1.
Activated brain regions in response to vestibular stimulation at pre-training, post-training, and pre- vs. post-training (i.e., increased activation from pre- to post-training). Following balance training, the brain activity shifted from the vestibular cortex to the cerebellum and vestibular nucleus in the brainstem. The results are shown at p < 0.001 (unc.). MNI: Montreal Neurological Institute.
4. Discussion
This study presents retention effects of an 8-week in-home balance training program with SA for healthy older adults. The results demonstrate improvements due to balance training regardless of group, with retention effects observed up to six months after completing the balancing training. However, only the EG demonstrated a minimal detectable change in SOT composite scores and 5XSTS scores. The EG also maintained a significantly higher improvement than the CG for the MiniBEST scores one month post-training. There were no significant changes in the TUG score, self-selected, or fast gait speeds, which may be due to ceiling effects or because SA was not provided during gait training tasks.
Balance training works by challenging the somatosensory, visual, and vestibular systems individually and by integrating multiple system inputs [42,43,44]. A subset of four EG participants underwent fMRI scans, and the results suggest functional reorganization of sensory processing and integration. We found that the pattern of brain activity in response to vestibular stimulation changed following training, exhibiting greater involvement of the brainstem and cerebellar regions. These findings suggest that SA modulates neural processing of vestibular stimulation, resulting in a shift from cortical to more sub-cortical regions, supporting the sensory reweighting mechanism theory [14]. This finding is comparable to that of Wildenberg et al., who reported an increase in activation of the brainstem and cerebellum after tongue-based electrotactile SA [45]. Moreover, a series of prior experiments by Wildenberg et al. investigated brain changes associated with long-term balance training with tongue electrotactile SA and found that balance-impaired individuals exhibited overactivation prior to training in comparison to controls in the occipital lobe and cerebellar vermis; these activity patterns were normalized following nine training sessions [46,47]. Evidence suggests that cerebellar processing of vestibular information contributes to self-motion perceptions [48,49,50]. It may be that SA coupled with balance training results in improved self-motion detection, linked to the increasing cerebellar activity we observed here. These results from a small subset of our participants should be followed up in future larger controlled fMRI studies.
It is compelling that both the brain and behavioral changes (an increase in vestibular reliance for both groups) suggest shifts in sensory reliance and integration with training [50]; participants increased their reliance upon vestibular inputs for balance following training. Older adults are generally more reliant on visual and somatosensory inputs and this work supports previous research indicating that progressive balance training may lead to sensory reweighting, resulting in increased vestibular reliance scores [51].
Given the small sample size, additional research is required to elucidate the improved functional balance outcomes with and without SA, and to correlate these with brain changes.
Balance training programs should incorporate different sensory conditions to promote the use of visual, vestibular, and proprioceptive inputs to target the individual’s deficits, but further research is needed on the optimal dosage and intensity by varying the training duration, training frequency, and SA activation thresholds. The data presented here indicate that while SA may provide some added benefit, the frequency and intensity via progressive customized balance training are important components of an in-home balance program, i.e., the FITT principle [52,53]. Retention effects were apparent six months post-training, but scores were lower than one week and one month post-training, indicating post intervention improvement wash-out. Progressively challenging balance training should be incorporated into an ongoing exercise program and lifestyle change to promote healthy aging and delay age-related balance declines.
5. Conclusions
Retention of balance training effects is imperative for fall prevention in the older adult population. Both groups (experimental group that received vibrotactile SA during balance training and the control group that completed balance training without vibrotactile SA) demonstrated significant improvements in their SOT composite and MiniBESTest scores, and increased vestibular reliance as determined by their SOT performance. However, only the group that trained with vibrotactile SA maintained a minimal detectable change of 8 points in their SOT scores six months following completion of the balance training protocol and greater improvements in their MiniBESTest scores one month following completion of the balance training protocol compared with the control group. Preliminary research suggests that SA may stimulate areas of the brain that facilitate the use of vestibular inputs for postural control. This sensory reweighting mechanism may be a critical component to consider when designing fall prevention programs.
Author Contributions
Conceptualization, K.H.S., S.L.W. and R.D.S.; methodology, K.H.S., W.J.C., R.D.S., C.K., G.P. and T.B.; software, T.B.; data collection, C.K., W.J.C., F.N., T.B. and V.J.B.; formal analysis, C.K., F.N. and T.B.; writing—original draft preparation, C.K., T.B., W.J.C., R.D.S. and K.H.S.; visualization, T.B., F.N. and C.K.; writing—review and editing, all authors; supervision, R.D.S. and K.H.S.; funding acquisition, K.H.S. and R.D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Foundation (GARDE 1159635 and CAREER RAPD/GARDE 0846471), National Institutes of Health (5R21DC012410–02), and the University of Michigan (MiBrain Initiative Working Group Grant).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of the University of Michigan (HUM00086479).
Informed Consent Statement
Informed consent was obtained from all participants involved in the study.
Data Availability Statement
The de-identified datasets generated and analyzed are available upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Clinical balance testing results.
Table A1.
Clinical balance testing results.
| Subject | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 16 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | |||||||||||||||||
| Gender | M | F | F | F | M | M | M | F | F | F | F | M | F | F | F | ||
| Age | 83 | 83 | 70 | 72 | 70 | 80 | 73 | 70 | 82 | 78 | 74 | 74 | 74 | 73 | 75 | ||
| Group | CG | EG | EG | CG | CG | EG | CG | EG | EG | CG | CG | EG | EG | EG | CG | ||
| SOT | Pre | 71 | 63 | 78 | 49 | 76 | 68 | 60 | 83 | 74 | 83 | 68 | 58 | 50 | 74 | 43 | |
| Post 1wk | 81 | 83 | 76 | 46 | 73 | 86 | 65 | 85 | 79 | 85 | 76 | 60 | 79 | 65 | 67 | ||
| Post 1mo | 83 | 76 | 78 | 49 | 77 | 78 | 65 | 85 | 77 | 85 | 77 | 65 | 73 | 62 | 75 | ||
| Post 6mo | 80 | 77 | - | 56 | 75 | 76 | 69 | 85 | 76 | - | 71 | 61 | 76 | - | - | ||
| MiniBESTest28 | Pre | 21 | 22 | 20 | 25 | 26 | 23 | 24 | 24 | 25 | 23 | 24 | 24 | 19 | 24 | 25 | |
| Post 1wk | 25 | 25 | 27 | 24 | 22 | 23 | 25 | 27 | 26 | 25 | 24 | 27 | 26 | 25 | 26 | ||
| Post 1mo | 21 | 26 | 24 | 23 | 22 | 23 | 27 | 26 | 27 | 22 | 24 | 25 | 24 | 23 | 25 | ||
| Post 6mo | 26 | 24 | - | 23 | 25 | 24 | 27 | 27 | 25 | - | 22 | 26 | 26 | - | - | ||
| MiniBESTest32 | Pre | 24 | 26 | 23 | 28 | 30 | 25 | 26 | 27 | 28 | 26 | 27 | 28 | 21 | 28 | 29 | |
| Post 1wk | 27 | 29 | 31 | 27 | 24 | 26 | 28 | 31 | 29 | 27 | 26 | 31 | 30 | 29 | 30 | ||
| Post 1mo | 25 | 29 | 27 | 26 | 25 | 25 | 31 | 30 | 30 | 26 | 26 | 29 | 27 | 28 | 29 | ||
| Post 6mo | 30 | 26 | - | 27 | 29 | 26 | 30 | 30 | 28 | - | 24 | 30 | 30 | - | - | ||
| ABC | Pre | 95 | 92 | 94 | 95 | 95 | 94 | 98 | 86 | 93 | 89 | 92 | 94 | 87 | 98 | 96 | |
| Post 1wk | 92 | 82 | 89 | - | 93 | 87 | 99 | 98 | 97 | 91 | 94 | 83 | 95 | 98 | 93 | ||
| Post 1mo | 89 | 88 | 88 | - | 92 | 81 | 99 | 99 | 96 | 91 | 96 | 93 | 96 | 98 | 96 | ||
| Post 6mo | 93 | 91 | - | 88 | 93 | 86 | 99 | 99 | 98 | - | 91 | 88 | 94 | - | - | ||
| TUG | Pre | 9.9 | 8.4 | 9.3 | 9.3 | 9.3 | 13 | 8.6 | 9.8 | 13.6 | 10.5 | 12.4 | 10.6 | 10.7 | 10.1 | 10.5 | |
| Post 1wk | 9.8 | 9.9 | 10.4 | 8.8 | 9.5 | 12.8 | 9.7 | 8.9 | 11.4 | 12 | 9.3 | 10.3 | 9.6 | 9.7 | 13.2 | ||
| Post 1mo | 9.3 | 10.3 | 9.1 | 8.2 | 9.9 | 12.2 | 8.4 | 7.2 | 13.5 | 10.5 | 10.1 | 13.4 | 8.5 | 8.4 | 13.7 | ||
| Post 6mo | 9.8 | 9.3 | - | 10.1 | 10.2 | 15.5 | 8.7 | 8.1 | 9.6 | - | 11.2 | 10 | 9.1 | - | - | ||
| TUG-COG | Pre | 12.2 | 10 | 11.6 | 8.3 | 8.8 | 16.9 | 8.7 | 10.8 | 14.2 | 12.4 | 14.8 | 14.2 | 16.4 | 13.5 | 13 | |
| Post 1wk | 7.8 | 10.3 | 11.2 | 8.4 | 11.4 | 15 | 8.9 | 9.1 | 12.6 | 11.6 | 10.3 | 11.8 | 12.7 | 11.6 | 13.8 | ||
| Post 1mo | 9.1 | 9.4 | 13.1 | 9.1 | 12 | 15.2 | 10.1 | 7.7 | 13.4 | 11 | 12.5 | 8.7 | 11.9 | 10.4 | 12.2 | ||
| Post 6mo | 8.9 | 10.5 | - | 10.5 | 12 | 19.9 | 9.2 | 8.1 | 11 | - | 11.8 | 10.8 | 18.0 | - | - | ||
| 5TSTS | Pre | 11 | 12 | 10.1 | 7.4 | 9.7 | 14.5 | 8.8 | 12.2 | 17.8 | 13.7 | 17.7 | 8.9 | 14.6 | 9.7 | 16.9 | |
| Post 1wk | 14.6 | 11 | 8.7 | 11 | 11.4 | 12.3 | 8.3 | 6.6 | 15 | 9.9 | 9.5 | 14.7 | 10.6 | 9.4 | 12.9 | ||
| Post 1mo | 10.4 | 11.7 | 9.1 | 7.2 | 10 | 13.5 | 9.3 | 7.1 | 13.3 | 9.3 | 8.9 | 12.8 | 9.6 | 10.7 | 14.6 | ||
| Post 6mo | 14.4 | 10.3 | - | 8.4 | 10.3 | 14.4 | 7.1 | 6.5 | 16.8 | - | 11.1 | 12.7 | 10.6 | - | - | ||
| FSST | Pre | 13 | 10.4 | 10.2 | 11 | 8.3 | 10.7 | 8.5 | 6.5 | 13 | 10.7 | 11.7 | 10.9 | 8.3 | 11.3 | 10.8 | |
| Post 1wk | 14.4 | 10.2 | 10.2 | 9.9 | 8.9 | 11 | 8.1 | 6.2 | 11.8 | 9.8 | 9.7 | 12.5 | 7.8 | 11.8 | 12.3 | ||
| Post 1mo | 12.7 | 10.6 | 10.1 | 10.6 | 9.5 | 10.7 | 9 | 5.6 | 12.6 | 8.5 | 9.2 | 11.2 | 8.2 | 8.4 | 10.8 | ||
| Post 6mo | 11.2 | 10.3 | - | 12.1 | 10.1 | 11.7 | 9.1 | 5.8 | 11.5 | - | 10.2 | 10 | 7.8 | - | - | ||
| FRT | Pre | 9.2 | 10.7 | 15.8 | 14.2 | 12.8 | 9.8 | 14.8 | 13.7 | 15.7 | 11.8 | 12.7 | 12 | 13.8 | 15.5 | 12 | |
| Post 1wk | 12 | 11.3 | 13.5 | 14.3 | 14.8 | 10.7 | 13.5 | 10.3 | 14.8 | 13.3 | 14.7 | 7.8 | 13.5 | 13.7 | 12 | ||
| Post 1mo | 11.8 | 9.3 | 13.3 | 13.5 | 10.8 | 14.5 | 13.3 | 13 | 12.8 | 12.5 | 10 | 10.7 | 16 | 15.5 | 12.2 | ||
| Post 6mo | 10.2 | 13.2 | - | 11.8 | 12 | 10.7 | 11.7 | 12.3 | 12.2 | - | 11.2 | 7.7 | 12.8 | - | - | ||
| fMRI Scan | Yes | Yes | Yes | Yes | Yes | ||||||||||||
References
- Florence, C.S.; Bergen, G.; Atherly, A.; Burns, E.; Stevens, J.; Drake, C. Medical costs of fatal and nonfatal falls in older adults. J. Am. Geriatr. Soc. 2018, 66, 693–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillespie, L.D.; Robertson, M.C.; Gillespie, W.J.; Sherrington, C.; Gates, S.; Clemson, L.M.; Lamb, S.E. Interventions for preventing falls in older people living in the community. Cochrane Database Syst. Rev. 2012, 2012, CD007146. [Google Scholar]
- Moncada, L.V.V.; Mire, L.G. Preventing Falls in Older Persons. Am. Fam. Physician 2017, 96, 240–247. [Google Scholar] [PubMed]
- Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society. Summary of the updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J. Am. Geriatr. Soc. 2011, 59, 148–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youssef, E.F.; Shanb, A.A. Supervised Versus Home Exercise Training Programs on Functional Balance in Older Subjects. Malays. J. Med. Sci. 2016, 23, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.L.; Chen, L.K.; Chern, C.M.; Hsu, L.C.; Chen, C.C.; Hwang, S.J. Rehabilitation outcome in home-based versus supervised exercise programs for chronically dizzy patients. Arch. Gerontol. Geriatr. 2010, 51, 264–267. [Google Scholar] [CrossRef]
- Laufer, Y.; Dar, G.; Kodesh, E. Does a Wii-based exercise program enhance balance control of independently functioning older adults? A systematic review. Clin. Interv. Aging 2014, 9, 1803–1813. [Google Scholar] [CrossRef] [Green Version]
- de Vries, A.W.; Faber, G.; Jonkers, I.; Van Dieen, J.H.; Verschueren, S.M.P. Virtual reality balance training for elderly: Similar skiing games elicit different challenges in balance training. Gait Posture 2018, 59, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.B.; Horslen, B.C.; Davis, J.R.; Allum, J.H.J.; Carpenter, M.G. Benefits of multi-session balance and gait training with multi-modal biofeedback in healthy older adults. Gait Posture 2016, 47, 10–17. [Google Scholar] [CrossRef]
- Schwenk, M.; Grewal, G.S.; Holloway, D.; Muchna, A.; Garland, L.; Najafi, B. Interactive Sensor-Based Balance Training in Older Cancer Patients with Chemotherapy-Induced Peripheral Neuropathy: A Randomized Controlled Trial. Gerontology 2016, 62, 553–563. [Google Scholar] [CrossRef]
- Sienko, K.H.; Balkwill, M.D.; Oddsson, L.I.E.; Wall, C. Effects of multi-directional vibrotactile feedback on vestibular-deficient postural performance during continuous multi-directional support surface perturbations. J. Vestib. Res. 2008, 18, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Basta, D.; Rossi-Izquierdo, M.; Soto-Varela, A.; Greters, M.E.; Bittar, R.S.; Steinhagen-Thiessen, E.; Eckardt, R.; Harada, T.; Goto, F.; Ogawa, K.; et al. Efficacy of a vibrotactile neurofeedback training in stance and gait conditions for the treatment of balance deficits: A double-blind, placebo-controlled multicenter study. Otol. Neurotol. 2011, 32, 1492–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brugnera, C.; Bittar, R.S.M.; Greters, M.E.; Basta, D. Effects of vibrotactile vestibular substitution on vestibular rehabilitation—preliminary study. Braz. J. Otorhinolaryngol. 2015, 81, 616–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sienko, K.H.; Seidler, R.D.; Carender, W.J.; Goodworth, A.D.; Whitney, S.L.; Peterka, R.J. Potential Mechanisms of Sensory Augmentation Systems on Human Balance Control. Front. Neurol. 2018, 9, 944. [Google Scholar] [CrossRef]
- Kingma, H.; Felipe, L.; Cecile, M.; Peter, G.; Nils, G.; Angelica, G.; Fornos, P.; Demkin, V.; Berg, R. Van De Vibrotactile feedback improves balance and mobility in patients with severe bilateral vestibular loss. J. Neurol. 2019, 266, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Shull, P.B.; Jirattigalachote, W.; Hunt, M.A.; Cutkosky, M.R.; Delp, S.L. Quantified self and human movement: A review on the clinical impact of wearable sensing and feedback for gait analysis and intervention. Gait Posture 2014, 40, 11–19. [Google Scholar] [CrossRef]
- Yen, C.; Lin, K.; Hu, M.; Wu, R.; Lu, T.; Lin, C. Effects of Virtual Reality—Augmented Balance Training on Sensory Organization and Attentional Demand for Postural Control in People With Parkinson Disease: A Randomized Controlled Trial. Phys. Ther. 2011, 91, 862–874. [Google Scholar] [CrossRef] [Green Version]
- Rossi-Izquierdo, M.; Ernst, A.; Soto-Varela, A.; Santos-Pérez, S.; Faraldo-García, A.; Sesar-Ignacio, A.; Basta, D. Vibrotactile neurofeedback balance training in patients with Parkinson’s disease: Reducing the number of falls. Gait Posture 2013, 37, 195–200. [Google Scholar] [CrossRef]
- American College of Sports Medicin. ACSM’s Health-Related Physical Fitness Assessment Manual; Lippincott & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Bao, T.; Klatt, B.N.; Carender, W.J.; Kinnaird, C.; Alsubaie, S. Effects of long-term vestibular rehabilitation therapy with vibrotactile sensory augmentation for people with unilateral vestibular disorders—A randomized preliminary study. J. Vestib. Res. 2019, 29, 323–334. [Google Scholar] [CrossRef] [Green Version]
- Bao, T.; Carender, W.J.; Kinnaird, C.; Barone, V.J.; Peethambaran, G.; Whitney, S.L.; Kabeto, M.; Seidler, R.D.; Sienko, K.H. Effects of long-term balance training with vibrotactile sensory augmentation among community-dwelling healthy older adults. J. Neuroeng. Rehabil. 2018, 15, 5. [Google Scholar] [CrossRef] [Green Version]
- Sienko, K.H.; Whitney, S.L.; Carender, W.J.; Wall III, C. The role of sensory augmentation for people with vestibular deficits: Real-time balance aid and/or rehabilitation device? J. Vestib. Res. 2017, 27, 63–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumann, F.; Keyser, J.; Goeke, C.; Ko, S.U.; Krause, C.; Wache, S.; Lytochkin, A.; Ebert, M.; Brunsch, V.; Wahn, B.; et al. Learning New Sensorimotor Contingencies: Effects of Long-Term Use of Sensory Augmentation on the Brain and Conscious Perception. PLoS ONE 2016, 11, e0166647. [Google Scholar]
- Clendaniel, R.A. Outcome measures for assessment of treatment of the dizzy and balance disorder patient. Otolaryngol. Clin. N. Am. 2000, 33, 519–533. [Google Scholar] [CrossRef]
- Rehabilitation Measures Database. Available online: https://www.sralab.org/statistical-terms-use (accessed on 9 October 2017).
- Bao, T.; Kinnaird, C.; Carender, W.J.; Sienko, K.H. Effects of sensory augmentation activation thresholds on balance performance in people with vestibular disorders. In Proceedings of the ISPGR—The International Society of Posture and Gait Research World Congress, Edinburgh, UK, 30 June–4 July 2019. [Google Scholar]
- Lee, B.-C.; Kim, J.; Chen, S.; Sienko, K.H. Cell phone based balance trainer. J. Neuroeng. Rehabil. 2012, 9, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klatt, B.N.; Carender, W.J.; Lin, C.C.; Alsubaie, S.F.; Kinnaird, C.R.; Sienko, K.H.; Whitney, S.L. A Conceptual Framework for the Progression of Balance Exercises in Persons with Balance and Vestibular Disorders. Phys. Med. Rehabil. Int. 2015, 2, 1044. [Google Scholar]
- Noohi, F.; Kinnaird, C.; DeDios, Y.; Kofman, I.S.; Wood, S.; Bloomberg, J.; Mulavara, A.; Seidler, R. Functional Brain Activation in Response to a Clinical Vestibular Test Correlates with Balance. Front. Syst. Neurosci. 2017, 11, 11. [Google Scholar] [CrossRef] [Green Version]
- Hupfeld, K.E.; McGregor, H.R.; Koppelmans, V.; Beltran, N.E.; Kofman, I.S.; De Dios, Y.E.; Riascos, R.F.; Reuter-Lorenz, P.A.; Wood, S.J.; Bloomberg, J.J.; et al. Brain and Behavioral Evidence for Reweighting of Vestibular Inputs with Long-Duration Spaceflight. Cereb. Cortex 2022, 32, 755–769. [Google Scholar] [CrossRef]
- Friston, K.J.; Holmes, A.P.; Worsley, K.J.; Poline, J.-P.; Frith, C.D.; Frackowiak, R.S.J. Statistical parametric maps in functional imaging: A general linear approach. Hum. Brain Mapp. 1994, 2, 189–210. [Google Scholar] [CrossRef]
- Glover, G.H.; Li, T.; Ress, D. Image-Based Method for Retrospective Correction of Physiological Motion Effects in fMRI: RETROICOR. Magn. Reson. Med. 2000, 44, 162–167. [Google Scholar] [CrossRef]
- Friston, K.J.; Ashburner, J.; Frith, C.D.; Poline, J.; Heather, J.D.; Frackowiak, R.S.J. Spatial Registration and Normalization of Images. Hum. Brain Mapp. 1995, 3, 165–189. [Google Scholar] [CrossRef]
- Diedrichsen, J.; Balsters, J.H.; Flavell, J.; Cussans, E.; Ramnani, N. A probabilistic MR atlas of the human cerebellum. Neuroimage 2009, 46, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Diedrichsen, J.; Maderwald, S.; Küper, M.; Thürling, M.; Rabe, K.; Gizewski, E.R. NeuroImage Imaging the deep cerebellar nuclei: A probabilistic atlas and normalization procedure. Neuroimage 2011, 54, 1786–1794. [Google Scholar] [CrossRef]
- Diedrichsen, J.; Zotow, E. Surface-Based Display of Volume-Averaged Cerebellar Imaging Data. PLoS ONE 2015, 10, e0133402. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002, 15, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, V.; Keeser, D.; Hergenroeder, T.; Erat, O.; Brandt, T.; Dieterich, M. Structural and functional connectivity mapping of the vestibular circuitry from human brainstem to cortex. Brain Struct. Funct. 2016, 221, 1291–1308. [Google Scholar] [CrossRef] [PubMed]
- Wrisley, D.M.; Stephens, M.J.; Mosley, S.; Wojnowski, A.; Duffy, J.; Burkard, R. Learning Effects of Repetitive Administrations of the Sensory Organization Test in Healthy Young Adults. Arch. Phys. Med. Rehabil. 2007, 88, 1049–1054. [Google Scholar] [CrossRef]
- Goldberg, A.; Chavis, M.; Watkins, J.; Wilson, T. The five-times-sit-to-stand test: Validity, reliability and detectable change in older females. Aging Clin. Exp. Res. 2012, 24, 339–344. [Google Scholar] [CrossRef]
- Miller, W.L.; Maffei, V.; Bosco, G.; Iosa, M.; Zago, M.; Macaluso, E.; Lacquaniti, F. Vestibular nuclei and cerebellum put visual gravitational motion in context. J. Neurophysiol. 2008, 99, 1969–1982. [Google Scholar] [CrossRef] [Green Version]
- Peterka, R.J. Sensorimotor Integration in Human Postural Control. J. Neurophysiol. 2002, 88, 1097–1118. [Google Scholar] [CrossRef] [Green Version]
- Horak, F.B. Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age Ageing 2006, 35, 7–11. [Google Scholar] [CrossRef] [Green Version]
- King, L.; Horak, F.B. The role of the vestibular system in postural control. In Vestibular Rehabilitation, 4th ed.; FA Davis: Philadelphia, PA, USA, 2014; pp. 29–48. [Google Scholar]
- Wildenberg, J.C.; Tyler, M.E.; Danilov, Y.P.; Kaczmarek, K.A.; Meyerand, M.E. High-resolution fMRI detects neuromodulation of individual brainstem nuclei by electrical tongue stimulation in balance-impaired individuals. Neuroimage 2011, 56, 2129–2137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wildenberg, J.C.; Tyler, M.E.; Danilov, Y.P.; Kaczmarek, K.A.; Meyerand, M.E. Sustained cortical and subcortical neuromodulation induced by electrical tongue stimulation. Brain Imaging Behav. 2010, 4, 199–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wildenberg, J.C.; Tyler, M.E.; Danilov, Y.P.; Kaczmarek, K.A.; Meyerand, M.E. Electrical tongue stimulation normalizes activity within the motion-sensitive brain network in balance-impaired subjects as revealed by group independent component analysis. Brain Connect. 2011, 1, 255–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cullen, K.E.; Brooks, J.X. Neural correlates of sensory prediction errors in monkeys: Evidence for internal models of voluntary self-motion in the cerebellum. Cerebellum 2015, 14, 31–34. [Google Scholar] [CrossRef] [Green Version]
- Dieterich, M.; Bauermann, T.; Best, C.; Stoeter, P.; Schlindwein, P. Evidence for cortical visual substitution of chronic bilateral vestibular failure (an fMRI study). Brain 2007, 130, 2108–2116. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Hu, X.; Zhang, Y.; Pan, Q.; Zhan, Q.; Tan, G.; Wang, K. Effect of Vestibular Rehabilitation on Spontaneous Brain Activity in Patients With Vestibular Migraine: A Resting-State Functional Magnetic Resonance Imaging Study. Front. Hum. Neurosci. 2020, 14, 227. [Google Scholar] [CrossRef]
- Wiesmeier, I.K.; Dalin, D.; Wehrle, A.; Granacher, U.; Muehlbauer, T.; Dietterle, J.; Weiller, C.; Gollhofer, A.; Maurer, C. Balance training enhances vestibular function and reduces overactive proprioceptive feedback in elderly. Front. Aging Neurosci. 2017, 9, 273. [Google Scholar] [CrossRef]
- Lesinski, M.; Hortobagyi, T.; Muehlbauer, T.; Gollhofer, A.; Granacher, U. Effects of Balance Training on Balance Performance in Healthy Older Adults: A Systematic Review and Meta-analysis. Sports Med. 2015, 45, 1721–1738. [Google Scholar] [CrossRef] [Green Version]
- Reinthal, A. Getting the Dosage Right in Balance Exercise Prescription: The Intensity Problem. J. Nov. Physiother. 2017, 7, e147. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).