Nanoporous Cauliflower-like Pd-Loaded Functionalized Carbon Nanotubes as an Enzyme-Free Electrocatalyst for Glucose Sensing at Neutral pH: Mechanism Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apparatus and Electrochemical Techniques
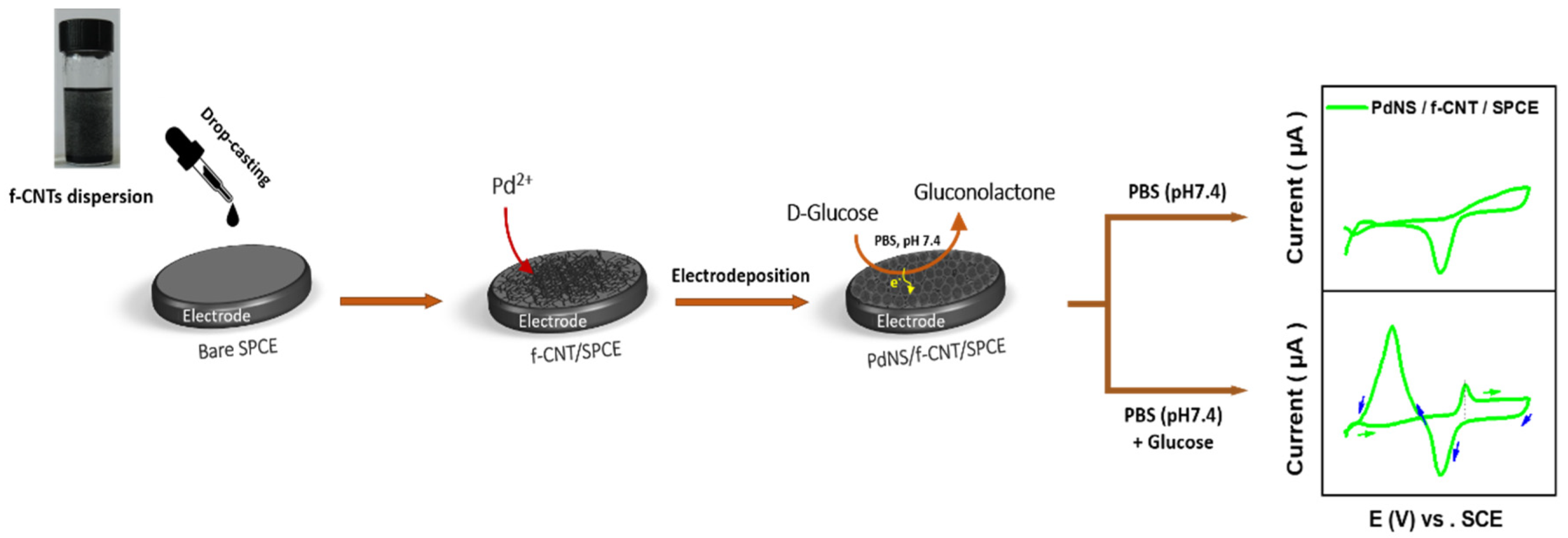
2.3. SPCE Production
2.4. Preparation of f-CNT/SPCE
2.5. Electrodeposition of PdNS Catalyst
2.6. Characterization of Electrocatalytic Activity for GOR
3. Results and Discussion
3.1. Parameters Affecting the Electrocatalytic Activity of Pd/f-CNT toward Glucose in Neutral pH
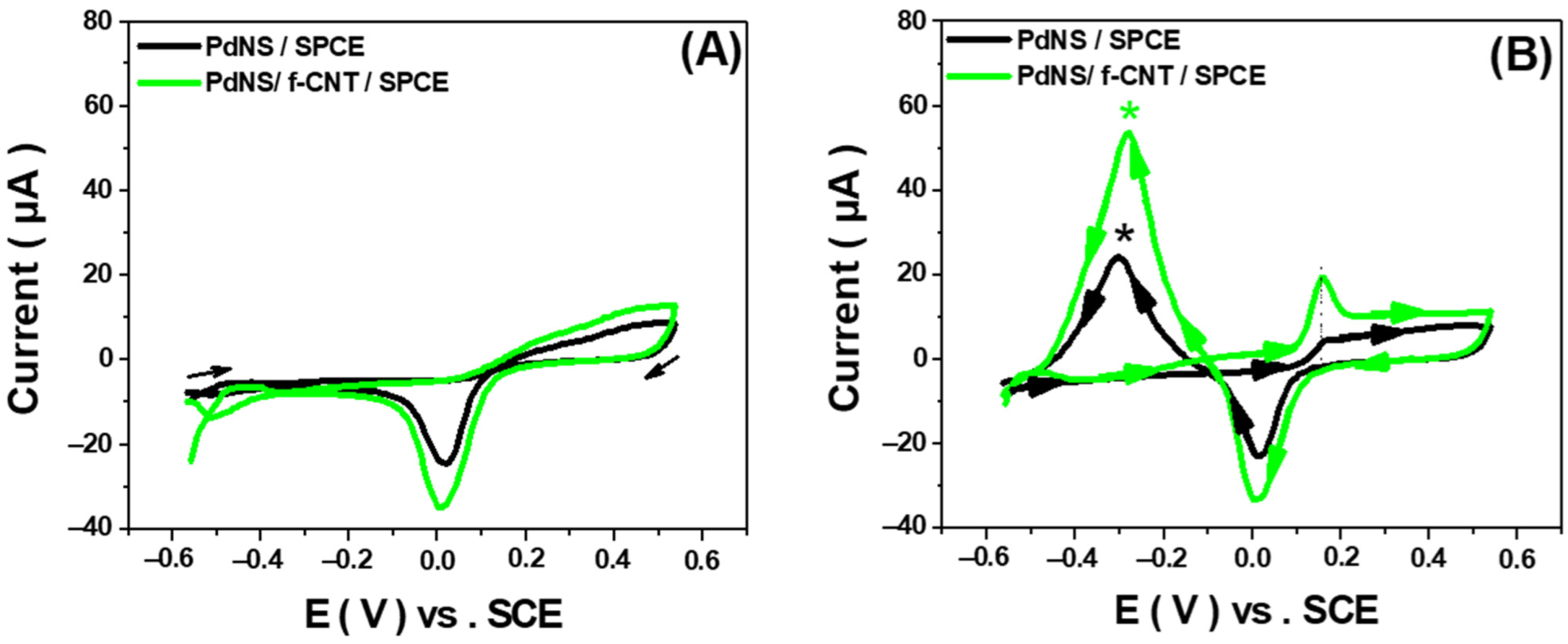
3.1.1. Effect of f-CNTs
3.1.2. Effect of Electrodeposition Potential
3.1.3. Effect of PdCl2 Precursor Concentration
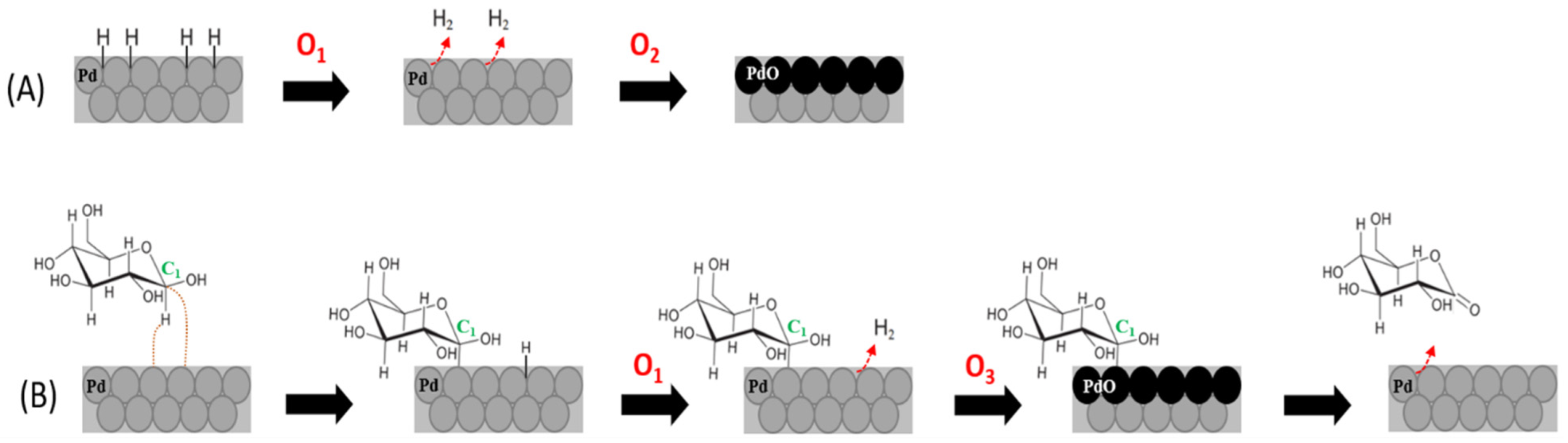
3.2. Mechanism Proposed for Glucose Oxidation on PdNS/f-CNT Biomimetic Nanocatalyst in Neutral pH
3.3. Performance of PdNS/f-CNT-Based Glucose Sensors
3.3.1. Performance Comparison of PdNS/f-CNT as Non-Enzymatic Glucose Sensors in Neutral pH
3.3.2. Selectivity of PdNS/f-CNT-Based Glucose Sensors
4. Conclusions
- Reducing the applied potential by inserting non-precious metals, polymers, etc.;
- The use of ion-exchange membranes over the sensor (e.g., electrostatic repulsion if charged interference species are present);
- Coupling the non-enzymatic sensor with a sample preparation procedure to select the target analyte.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ricci, F.; Amine, A.; Tuta, C.S.; Ciucu, A.A.; Lucarelli, F.; Palleschi, G.; Moscone, D. Prussian Blue and Enzyme Bulk-Modified Screen-Printed Electrodes for Hydrogen Peroxide and Glucose Determination with Improved Storage and Operational Stability. Anal. Chim. Acta 2003, 485, 111–120. [Google Scholar] [CrossRef]
- Zafar, H.; Channa, A.; Jeoti, V.; Stojanović, G.M. Comprehensive Review on Wearable Sweat-Glucose Sensors for Continuous Glucose Monitoring. Sensors 2022, 22, 638. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.H.; Vyas, C.; Grieve, B.; Bartolo, P. Recent Advances in Enzymatic and Non-Enzymatic Electrochemical Glucose Sensing. Sensors 2021, 21, 4672. [Google Scholar] [CrossRef] [PubMed]
- Chansaenpak, K.; Kamkaew, A.; Lisnund, S.; Prachai, P.; Ratwirunkit, P.; Jingpho, T.; Blay, V.; Pinyou, P. Development of a Sensitive Self-Powered Glucose Biosensor Based on an Enzymatic Biofuel Cell. Biosensors 2021, 11, 16. [Google Scholar] [CrossRef]
- Lueke, J.; Moussa, W.A. MEMS-Based Power Generation Techniques for Implantable Biosensing Applications. Sensors 2011, 11, 1433–1460. [Google Scholar] [CrossRef] [Green Version]
- Ramanavicius, S.; Ramanavicius, A. Charge Transfer and Biocompatibility Aspects in Conducting Polymer-Based Enzymatic Biosensors and Biofuel Cells. Nanomaterials 2021, 11, 371. [Google Scholar] [CrossRef]
- Chiao, M. A Microfabricated PDMS Microbial Fuel Cell. J. Microelectromech. Syst. 2008, 17, 1329–1341. [Google Scholar]
- Opallo, M.; Dolinska, J. Glucose Electrooxidation. In Encyclopedia of Interfacial Chemistry; Wandelt, K., Ed.; Elsevier: Oxford, UK, 2018; pp. 633–642. ISBN 9780128098943. [Google Scholar]
- Brouzgou, A.; Tsiakaras, P. Electrocatalysts for Glucose Electrooxidation Reaction: A Review. Top. Catal. 2015, 58, 1311–1327. [Google Scholar] [CrossRef]
- Adeel, M.; Rahman, M.M.; Caligiuri, I.; Canzonieri, V.; Rizzolio, F.; Daniele, S. Recent Advances of Electrochemical and Optical Enzyme-Free Glucose Sensors Operating at Physiological Conditions. Biosens. Bioelectron. 2020, 165, 112331. [Google Scholar] [CrossRef]
- Li, Y.; Deng, D.; Wang, H.; Huan, K.; Yan, X.; Luo, L. Controlled Synthesis of Cu-Sn Alloy Nanosheet Arrays on Carbon Fiber Paper for Self-Supported Nonenzymatic Glucose Sensing. Anal. Chim. Acta 2022, 1190, 339249. [Google Scholar] [CrossRef]
- Balkourani, G.; Damartzis, T.; Brouzgou, A.; Tsiakaras, P. Cost Effective Synthesis of Graphene Nanomaterials for Non-Enzymatic Electrochemical Sensors for Glucose: A Comprehensive Review. Sensors 2022, 22, 355. [Google Scholar] [CrossRef] [PubMed]
- Ghica, M.E.; Carvalho, R.C.; Amine, A.; Brett, C.M. Glucose Oxidase Enzyme Inhibition Sensors for Heavy Metals at Carbon Film Electrodes Modified with Cobalt or Copper Hexacyanoferrate. Sens. Actuators B Chem. 2013, 178, 270–278. [Google Scholar] [CrossRef]
- Wilson, R.; Turner, A.P.F. Glucose Oxidase: An Ideal Enzyme. Biosens. Bioelectron. 1992, 7, 165–185. [Google Scholar] [CrossRef]
- Huang, T.; Liu, Z.; Li, Y.; Li, Y.; Chao, L.; Chen, C.; Tan, Y.; Xie, Q.; Yao, S.; Wu, Y. Oxidative Polymerization of 5-Hydroxytryptamine to Physically and Chemically Immobilize Glucose Oxidase for Electrochemical Biosensing. Anal. Chim. Acta 2018, 1013, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Li, C.; Zhang, T.; Lang, Q.; Liu, A. Au@ Ag Heterogeneous Nanorods as Nanozyme Interfaces with Peroxidase-like Activity and Their Application for One-Pot Analysis of Glucose at Nearly Neutral PH. ACS Appl. Mater. Interfaces 2015, 7, 14463–14470. [Google Scholar] [CrossRef]
- Chen, M.; Cao, X.; Chang, K.; Xiang, H.; Wang, R. A Novel Electrochemical Non-Enzymatic Glucose Sensor Based on Au Nanoparticle-Modified Indium Tin Oxide Electrode and Boronate Affinity. Electrochim. Acta 2021, 368, 137603. [Google Scholar] [CrossRef]
- Shen, N.; Xu, H.; Zhao, W.; Zhao, Y.; Zhang, X. Highly Responsive and Ultrasensitive Non-Enzymatic Electrochemical Glucose Sensor Based on Au Foam. Sensors 2019, 19, 1203. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Pan, H.; Liu, H.; Du, M. Nonenzymatic Glucose Sensor Based on Flower-Shaped Au@ Pd Core–Shell Nanoparticles–Ionic Liquids Composite Film Modified Glassy Carbon Electrodes. Electrochim. Acta 2010, 56, 636–643. [Google Scholar] [CrossRef]
- Jiang, T.; Yan, L.; Meng, Y.; Xiao, M.; Wu, Z.; Tsiakaras, P.; Song, S. Glucose Electrooxidation in Alkaline Medium: Performance Enhancement of PdAu/C Synthesized by NH3 Modified Pulse Microwave Assisted Polyol Method. Appl. Catal. B Environ. 2015, 162, 275–281. [Google Scholar] [CrossRef]
- Sakr, M.A.; Elgammal, K.; Delin, A.; Serry, M. Performance-Enhanced Non-Enzymatic Glucose Sensor Based on Graphene-Heterostructure. Sensors 2020, 20, 145. [Google Scholar] [CrossRef] [Green Version]
- Samoson, K.; Thavarungkul, P.; Kanatharana, P.; Limbut, W. A Nonenzymatic Glucose Sensor Based on the Excellent Dispersion of a Graphene Oxide-Poly (Acrylic Acid)-Palladium Nanoparticle-Modified Screen-Printed Carbon Electrode. J. Electrochem. Soc. 2019, 166, B1079. [Google Scholar] [CrossRef]
- Baghayeri, M.; Amiri, A.; Farhadi, S. Development of Non-Enzymatic Glucose Sensor Based on Efficient Loading Ag Nanoparticles on Functionalized Carbon Nanotubes. Sens. Actuators B Chem. 2016, 225, 354–362. [Google Scholar] [CrossRef]
- Emir, G.; Dilgin, Y.; Ramanaviciene, A.; Ramanavicius, A. Amperometric Nonenzymatic Glucose Biosensor Based on Graphite Rod Electrode Modified by Ni-Nanoparticle/Polypyrrole Composite. Microchem. J. 2021, 161, 105751. [Google Scholar] [CrossRef]
- Huang, B.; Wang, Y.; Lu, Z.; Du, H.; Ye, J. One Pot Synthesis of Palladium-Cobalt Nanoparticles over Carbon Nanotubes as a Sensitive Non-Enzymatic Sensor for Glucose and Hydrogen Peroxide Detection. Sens. Actuators B Chem. 2017, 252, 1016–1025. [Google Scholar] [CrossRef]
- Shen, C.; Su, J.; Li, X.; Luo, J.; Yang, M. Electrochemical Sensing Platform Based on Pd–Au Bimetallic Cluster for Non-Enzymatic Detection of Glucose. Sens. Actuators B Chem. 2015, 209, 695–700. [Google Scholar] [CrossRef]
- Li, X.; Du, X. Molybdenum Disulfide Nanosheets Supported Au-Pd Bimetallic Nanoparticles for Non-Enzymatic Electrochemical Sensing of Hydrogen Peroxide and Glucose. Sens. Actuators B Chem. 2017, 239, 536–543. [Google Scholar] [CrossRef]
- Tang, L.; Huan, K.; Deng, D.; Han, L.; Zeng, Z.; Luo, L. Glucose Sensor Based on Pd Nanosheets Deposited on Cu/Cu2O Nanocomposites by Galvanic Replacement. Colloids Surf. B Biointerfaces 2020, 188, 110797. [Google Scholar] [CrossRef]
- Yan, L.; Brouzgou, A.; Meng, Y.; Xiao, M.; Tsiakaras, P.; Song, S. Efficient and Poison-Tolerant PdxAuy/C Binary Electrocatalysts for Glucose Electrooxidation in Alkaline Medium. Appl. Catal. B Environ. 2014, 150, 268–274. [Google Scholar] [CrossRef]
- Cheng, T.-M.; Huang, T.-K.; Lin, H.-K.; Tung, S.-P.; Chen, Y.-L.; Lee, C.-Y.; Chiu, H.-T. (110)-Exposed Gold Nanocoral Electrode as Low Onset Potential Selective Glucose Sensor. ACS Appl. Mater. Interfaces 2010, 2, 2773–2780. [Google Scholar] [CrossRef] [Green Version]
- Branagan, D.; Breslin, C.B. Electrochemical Detection of Glucose at Physiological PH Using Gold Nanoparticles Deposited on Carbon Nanotubes. Sens. Actuators B Chem. 2019, 282, 490–499. [Google Scholar] [CrossRef]
- Haghighi, B.; Karimi, B.; Tavahodi, M.; Behzadneia, H. Fabrication of a Nonenzymatic Glucose Sensor Using Pd-Nanoparticles Decorated Ionic Liquid Derived Fibrillated Mesoporous Carbon. Mater. Sci. Eng. C 2015, 52, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Şavk, A.; Cellat, K.; Arıkan, K.; Tezcan, F.; Gülbay, S.K.; Kızıldağ, S.; Işgın, E.Ş.; Şen, F. Highly Monodisperse Pd-Ni Nanoparticles Supported on RGO as a Rapid, Sensitive, Reusable and Selective Enzyme-Free Glucose Sensor. Sci. Rep. 2019, 9, 19228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, L.; Jin, J.; Yang, G.; Lu, T.; Zhang, H.; Cai, C. Nonenzymatic Electrochemical Detection of Glucose Based on Palladium-Single-Walled Carbon Nanotube Hybrid Nanostructures. Anal. Chem. 2009, 81, 7271–7280. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.-S.; Chen, C.-W.; Lee, C.-L. Pd Nanocube as Non-Enzymatic Glucose Sensor. Sens. Actuators B Chem. 2015, 208, 569–574. [Google Scholar] [CrossRef]
- Shu, H.; Cao, L.; Chang, G.; He, H.; Zhang, Y.; He, Y. Direct Electrodeposition of Gold Nanostructures onto Glassy Carbon Electrodes for Non-Enzymatic Detection of Glucose. Electrochim. Acta 2014, 132, 524–532. [Google Scholar] [CrossRef]
- Tian, N.; Zhou, Z.-Y.; Sun, S.-G. Electrochemical Preparation of Pd Nanorods with High-Index Facets. Chem. Commun. 2009, 12, 1502–1504. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Fan, Y.-J.; Wang, H.-H.; Tian, N.; Zhou, Z.-Y.; Sun, S.-G. Electrochemically Shape-Controlled Synthesis in Deep Eutectic Solvents of Pt Nanoflowers with Enhanced Activity for Ethanol Oxidation. Electrochim. Acta 2012, 76, 468–474. [Google Scholar] [CrossRef]
- Sun, I.-W.; Chang, J.-K. Electrodeposition of Nanomaterials. In Springer Handbook of Electrochemical Energy; Springer: Berlin/Heidelberg, Germany, 2017; pp. 835–895. [Google Scholar]
- Sun, Z.; Ma, X.; Hu, X. Electrocatalytic Dechlorination of 2, 3, 5-Trichlorophenol on Palladium/Carbon Nanotubes-Nafion Film/Titanium Mesh Electrode. Environ. Sci. Pollut. Res. 2017, 24, 14355–14364. [Google Scholar] [CrossRef]
- Low, C.T.J.; Wills, R.G.A.; Walsh, F.C. Electrodeposition of Composite Coatings Containing Nanoparticles in a Metal Deposit. Surf. Coat. Technol. 2006, 201, 371–383. [Google Scholar] [CrossRef]
- Chen, T.-W.; Rajaji, U.; Chen, S.-M.; Muthumariyappan, A.; Al Mogren, M.M.; Ramalingam, R.J.; Hochlaf, M. Facile Synthesis of Copper (II) Oxide Nanospheres Covered on Functionalized Multiwalled Carbon Nanotubes Modified Electrode as Rapid Electrochemical Sensing Platform for Super-Sensitive Detection of Antibiotic. Ultrason. Sonochem. 2019, 58, 104596. [Google Scholar] [CrossRef]
- Chu, T.-F.; Rajendran, R.; Kuznetsova, I.; Wang, G.-J. High-Power, Non-Enzymatic Glucose Biofuel Cell Based on a Nano/Micro Hybrid-Structured Au Anode. J. Power Sources 2020, 453, 227844. [Google Scholar] [CrossRef]
- Grdeń, M.; Lukaszewski, M.; Jerkiewicz, G.; Czerwiński, A. Electrochemical Behaviour of Palladium Electrode: Oxidation, Electrodissolution and Ionic Adsorption. Electrochim. Acta 2008, 53, 7583–7598. [Google Scholar] [CrossRef]
- Rand, D.A.J.; Woods, R. The Nature of Adsorbed Oxygen on Rhodium, Palladium and Gold Electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1971, 31, 29–38. [Google Scholar] [CrossRef]
- Lukaszewski, M.; Soszko, M.; Czerwiński, A. Electrochemical Methods of Real Surface Area Determination of Noble Metal Electrodes–an Overview. Int. J. Electrochem. Sci. 2016, 11, 4442–4469. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solution; National Association of Corrosion Engineers: Houston, TX, USA, 1974. [Google Scholar]
- Zhang, J.; Chen, M.; Li, H.; Li, Y.; Ye, J.; Cao, Z.; Fang, M.; Kuang, Q.; Zheng, J.; Xie, Z. Stable Palladium Hydride as a Superior Anode Electrocatalyst for Direct Formic Acid Fuel Cells. Nano Energy 2018, 44, 127–134. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Baba, K.; Flanagan, T.B. The Effect of Alloying of Palladium on the Hydrogen-Palladium Miscibility Gap. Z. Für Phys. Chem. 1988, 158, 223–235. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Yuwasa, K.; Hirayama, K. X-Ray Investigation of the Absorption of Hydrogen by Several Palladium and Nickel Solid Solution Alloys. J. Common Met. 1982, 88, 115–124. [Google Scholar] [CrossRef]
- Pletcher, D. Electrocatalysis: Present and Future. J. Appl. Electrochem. 1984, 14, 403–415. [Google Scholar] [CrossRef]
- Tomanin, P.P.; Cherepanov, P.V.; Besford, Q.A.; Christofferson, A.J.; Amodio, A.; McConville, C.F.; Yarovsky, I.; Caruso, F.; Cavalieri, F. Cobalt Phosphate Nanostructures for Non-Enzymatic Glucose Sensing at Physiological PH. ACS Appl. Mater. Interfaces 2018, 10, 42786–42795. [Google Scholar] [CrossRef]
- Masoomi-Godarzi, S.; Khodadadi, A.A.; Vesali-Naseh, M.; Mortazavi, Y. Highly Stable and Selective Non-Enzymatic Glucose Biosensor Using Carbon Nanotubes Decorated by Fe3O4 Nanoparticles. J. Electrochem. Soc. 2013, 161, B19. [Google Scholar] [CrossRef]
- Naghib, S.M.; Rahmanian, M.; Keivan, M.A.; Asiaei, S.; Vahidi, O. Novel Magnetic Nanocomposites Comprising Reduced Graphene Oxide/Fe3O4/Gelatin Utilized in Ultrasensitive Non-Enzymatic Biosensing. Int. J. Electrochem. Sci. 2016, 11, 10256–10269. [Google Scholar] [CrossRef]
- Zang, G.; Hao, W.; Li, X.; Huang, S.; Gan, J.; Luo, Z.; Zhang, Y. Copper Nanowires-MOFs-Graphene Oxide Hybrid Nanocomposite Targeting Glucose Electro-Oxidation in Neutral Medium. Electrochim. Acta 2018, 277, 176–184. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, F.; Yu, Q.; Fan, R.; Yang, M.; Rao, S.; Lan, Q.; Yang, Z.; Yang, Z. Three-Dimensional Porous Cu@Cu2O Aerogels for Direct Voltammetric Sensing of Glucose. Microchim. Acta 2019, 186, 192. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-S.; Wu, Z.-W.; Lee, C.-L. Concave Pd Core/Island Pt Shell Nanoparticles: Synthesis and Their Promising Activities toward Neutral Glucose Oxidation. Sens. Actuators B Chem. 2019, 281, 1–7. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, X.; Yin, Y.; Wu, P.; Cai, C. Nonenzymatic Electrochemical Detection of Glucose Based on Pd1Pt3–Graphene Nanomaterials. J. Electroanal. Chem. 2013, 690, 19–24. [Google Scholar] [CrossRef]
- Singh, B.; Dempsey, E.; Laffir, F. Carbon Nanochips and Nanotubes Decorated PtAuPd-Based Nanocomposites for Glucose Sensing: Role of Support Material and Efficient Pt Utilisation. Sens. Actuators B Chem. 2014, 205, 401–410. [Google Scholar] [CrossRef]



| Electrochemical Sensor | Regression Equation | R2 | Sensitivity (µA mM−1 cm−2) | LOD (µM) |
|---|---|---|---|---|
| PdNS (10 mM)/f-CNT/SPCE | Ipa (µA) = 0.004 [Glu] + 3.120 × 10−4 | 0.996 | 2000 | |
| PdNS (20 mM)/f-CNT/SPCE | Ipa (µA) = 0.023 [Glu] + 0.008 | 0.994 | 1000 | |
| PdNS (50 mM)/f-CNT/SPCE | Ipa (µA) = 0.106 [Glu] + 0.066 | 0.994 | 442 | |
| PdNS (100 mM)/f-CNT/SPCE | Ipa (µA) = 0.663 [Glu] + 0.564 | 0.997 | 95 |
| Electrode | Working Potential (V vs. Ag/AgCl) | Concentration Range (mM) | Sensitivity (µA mM−1 cm−2) | DL (µM) | Ref. |
|---|---|---|---|---|---|
| DGN-modified GCE 1 | +0.15 | 0.1–25 | 190.70 | 50 | [36] |
| Pd@f-SWNTs/GCE 2 | −0.5 | 0.5–17 | 160.00 | 0.2 | [34] |
| Fe3O4/f-MWCNTs/GCE 3 | −0.05 | 0.5–7.0 | 238.70 | 15 | [53] |
| rGO/Fe3O4/G/GCE 4 | +0.0011 | 0.1–10 | 2.30 | 0.024 | [54] |
| MWCNT-Au nano/SPE 5 | +0.15 | 0.1–25 | 2.770.55 | 4.1 | [31] |
| Cu NWs-MOFs-GO/GE 6 | +0.3 | 0.02–26.6 | 7.72 | 7 | [55] |
| 3D Cu@Cu2O AG/GCE 7 | +0.6 | 0.1–10 | 12.00 | 54 | [56] |
| Pd@Pt CINPs/GCE 8 | −0.1 | 1.0–8.5 | 15.14 | 0.82 | [57] |
| (Pd/Pt)-graphene/GCE 9 | +0.1 | 1.0–23.0 | -- | 5 | [58] |
| PtAuPd/f-CNC/GCE 10 | +0.43 | Up to 10 | 11.24 | 2.9 | [59] |
| PdNS/f-CNT/SPCE | +0.3 V vs. SCE | 1–41 | 9.26 | 95 | This work |
| Concentration | 1 mM | 10 mM | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analyte | Glu | Gal | Suc | Fru | DA | PCM | AA | Glu | Gal | Suc | Fru | DA | PCM | AA |
| Current (µA) | 0.15 | 0.35 | -- | -- | 17.21 | 9.86 | 25.80 | 2.28 | 2.80 | -- | -- | 219.38 | 71.41 | 217.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghanam, A.; Haddour, N.; Mohammadi, H.; Amine, A.; Sabac, A.; Buret, F. Nanoporous Cauliflower-like Pd-Loaded Functionalized Carbon Nanotubes as an Enzyme-Free Electrocatalyst for Glucose Sensing at Neutral pH: Mechanism Study. Sensors 2022, 22, 2706. https://doi.org/10.3390/s22072706
Ghanam A, Haddour N, Mohammadi H, Amine A, Sabac A, Buret F. Nanoporous Cauliflower-like Pd-Loaded Functionalized Carbon Nanotubes as an Enzyme-Free Electrocatalyst for Glucose Sensing at Neutral pH: Mechanism Study. Sensors. 2022; 22(7):2706. https://doi.org/10.3390/s22072706
Chicago/Turabian StyleGhanam, Abdelghani, Naoufel Haddour, Hasna Mohammadi, Aziz Amine, Andrei Sabac, and François Buret. 2022. "Nanoporous Cauliflower-like Pd-Loaded Functionalized Carbon Nanotubes as an Enzyme-Free Electrocatalyst for Glucose Sensing at Neutral pH: Mechanism Study" Sensors 22, no. 7: 2706. https://doi.org/10.3390/s22072706
APA StyleGhanam, A., Haddour, N., Mohammadi, H., Amine, A., Sabac, A., & Buret, F. (2022). Nanoporous Cauliflower-like Pd-Loaded Functionalized Carbon Nanotubes as an Enzyme-Free Electrocatalyst for Glucose Sensing at Neutral pH: Mechanism Study. Sensors, 22(7), 2706. https://doi.org/10.3390/s22072706








