A Wearable System Based on Multiple Magnetic and Inertial Measurement Units for Spine Mobility Assessment: A Reliability Study for the Evaluation of Ankylosing Spondylitis
Abstract
:1. Introduction
2. Materials and Methods
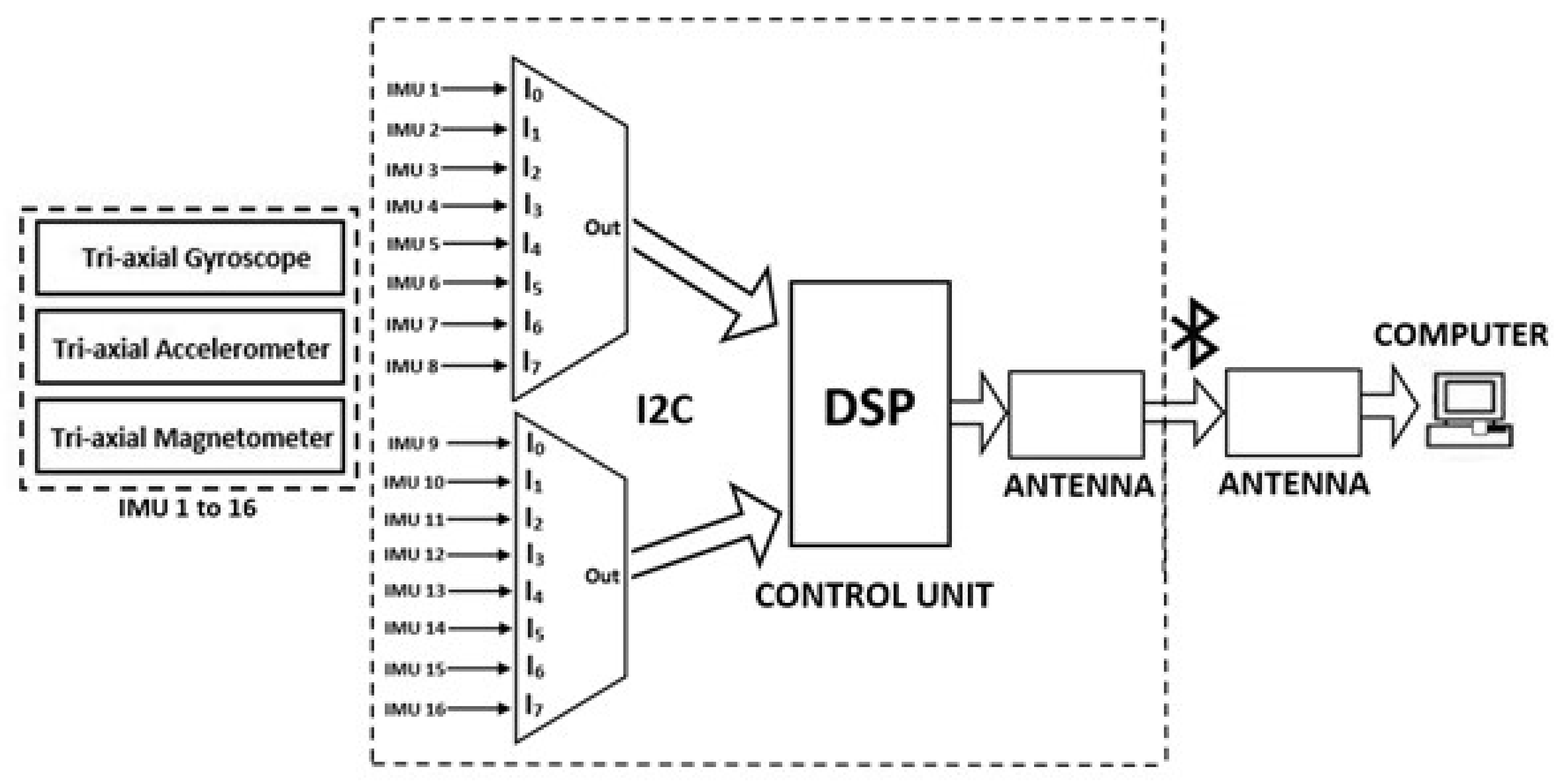
2.1. Multi-MIMU System
2.2. Movement Estimation Algorithm
2.2.1. Sensor Modeling
2.2.2. Kalman Filter Design
2.3. Experimental Study
2.3.1. The Objective of the Study
2.3.2. Recruitment
2.3.3. Experimental Procedure
- Movement 1: anterior hip flexion.
- Movement 2: trunk lateral flexions to the left/right sides.
- Movement 3: trunk axial rotation.
- Movement 4: cervical axial rotation.
- Movement 5: cervical flexion/extension.
- Movement 6: cervical lateral flexion.
2.3.4. Measurements
2.3.5. Statistical Analysis
3. Results
Spinal Mobility Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Woolf, A.D.; Pfleger, B. Burden of major musculoskeletal conditions. Bull. World Health Organ. 2003, 81, 646–656. [Google Scholar]
- Rudwaleit, M.; Landewe, R.; Van Der Heijde, D.; Listing, J.; Brandt, J.; Braun, J.; Burgos-Vargas, R.; Estévez, E.C.; Davis, J.; Dijkmans, B.; et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (Part I): Classification of paper patients by expert opinion including uncertainty appraisal. Ann. Rheum. Dis. 2009, 68, 770–776. [Google Scholar] [CrossRef]
- Rudwaleit, M.; Van Der Heijde, D.; Landewé, R.; Listing, J.; Akkoç, N.; Brandt, J.; Braun, J.; Chou, C.T.; Estévez, E.C.; Dougados, M.; et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): Validation and final selection. Ann. Rheum. Dis. 2009, 68, 777–783. [Google Scholar] [CrossRef] [Green Version]
- Peláez-Ballestas, I.; Navarro-Zarza, J.E.; Julian, B.; Lopez, A.; Flores-Camacho, R.; Casasola-Vargas, J.C.; Sanin, L.H.; Rivas, L.; Vázquez-Mellado, J.; Burgos-Vargas, R. A community-based study on the prevalence of spondyloarthritis and inflammatory back pain in mexicans. JCR J. Clin. Rheumatol. 2013, 19, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Burgos-Vargas, R.; Peláez-Ballestas, I. Epidemiology of Spondyloarthritis in México. Am. J. Med. Sci. 2011, 341, 298–300. [Google Scholar] [CrossRef]
- Cardiel, M.H.; Rojas-Serrano, J. Community based study to estimate prevalence, burden of illness and help seeking behavior in rheumatic diseases in Mexico City. A COPCORD study. Clin. Exp. Rheumatol. 2002, 20, 617–624. [Google Scholar]
- Sieper, J.; Rudwaleit, M.; Baraliakos, X.; Brandt, J.; Braun, J.; Burgos-Vargas, R.; Dougados, M.; Hermann, K.-G.; Landewé, R.; Maksymowych, W.; et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: A guide to assess spondyloarthritis. Ann. Rheum. Dis. 2009, 68 (Suppl. S2), ii1–ii44. [Google Scholar] [CrossRef]
- Jenkinson, T.R.; Mallorie, P.A.; Whitelock, H.C.; Kennedy, L.G.; Garrett, S.L.; Calin, Y.A. Defining spinal mobility in ankylosing spondylitis (AS). The Bath AS Metrology Index. J. Rheumatol. 1994, 21, 1694–1698. [Google Scholar]
- Martindale, J.H.; Sutton, C.J.; Goodacre, L. An exploration of the inter- and intra-rater reliability of the Bath Ankylosing Spondylitis Metrology Index. Clin. Rheumatol. 2012, 31, 1627–1631. [Google Scholar] [CrossRef]
- Calvo-Gutiérrez, J.; Garrido-Castro, J.L.; González-Navas, C.; Villegas, M.D.C.C.; Ortega-Castro, R.; López-Medina, C.; Font-Ugalde, P.; Escudero-Contreras, A.; Collantes-Estévez, E. Inter-rater reliability of clinical mobility measures in ankylosing spondylitis. BMC Musculoskelet. Disord. 2016, 17, 382. [Google Scholar] [CrossRef] [Green Version]
- Madsen, O.R.; Hansen, L.B.; Rytter, A.; Suetta, C.; Egsmose, C. The Bath metrology index as assessed by a trained and an untrained rater in patients with spondylarthropathy: A study of intra- and inter-rater agreements. Clin. Rheumatol. 2009, 28, 35–40. [Google Scholar] [CrossRef]
- Aghazadeh, F.; Arjmand, N.; Nasrabadi, A. Coupled artificial neural networks to estimate 3D whole-body posture, lumbosacral moments, and spinal loads during load-handling activities. J. Biomech. 2020, 102, 109332. [Google Scholar] [CrossRef]
- Asadi, F.; Arjmand, N. Marker-less versus marker-based driven musculoskeletal models of the spine during static load-handling activities. J. Biomech. 2020, 112, 110043. [Google Scholar] [CrossRef]
- Garrido-Castro, J.L.; Escudero, A.; Medina-Carnicer, R.; Galisteo, A.M.; Gonzalez-Navas, C.; Carmona, L.; Collantes-Estevez, E. Validation of a new objective index to measure spinal mobility: The University of Cordoba Ankylosing Spondylitis Metrology Index (UCOASMI). Rheumatol. Int. 2013, 34, 401–406. [Google Scholar] [CrossRef]
- Baghdadi, A.; Cavuoto, L.A.; Crassidis, J.L. Hip and Trunk Kinematics Estimation in Gait Through Kalman Filter Using IMU Data at the Ankle. IEEE Sens. J. 2018, 18, 4253–4260. [Google Scholar] [CrossRef]
- Noamani, A.; Nazarahari, M.; Lewicke, J.; Vette, A.H.; Rouhani, H. Validity of using wearable inertial sensors for assessing the dynamics of standing balance. Med. Eng. Phys. 2020, 77, 53–59. [Google Scholar] [CrossRef]
- Filippeschi, A.; Schmitz, N.; Miezal, M.; Bleser, G.; Ruffaldi, E.; Stricker, D. Survey of Motion Tracking Methods Based on Inertial Sensors: A Focus on Upper Limb Human Motion. Sensors 2017, 17, 1257. [Google Scholar] [CrossRef] [Green Version]
- Kobsar, D.; Osis, S.T.; Boyd, J.E.; Hettinga, B.A.; Ferber, R. Wearable sensors to predict improvement following an exercise intervention in patients with knee osteoarthritis. J. Neuroeng. Rehabil. 2017, 14, 94. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Shin, Y.B.; Kim, S.-Y.; Kim, J. A novel sensor-based assessment of lower limb spasticity in children with cerebral palsy. J. Neuroeng. Rehabil. 2018, 15, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Giggins, O.M.; Sweeney, K.T.; Caulfield, B. Rehabilitation exercise assessment using inertial sensors: A cross-sectional analytical study. J. Neuroeng. Rehabil. 2014, 11, 158. [Google Scholar] [CrossRef] [Green Version]
- Milosevic, B.; Leardini, A.; Farella, E. Kinect and wearable inertial sensors for motor rehabilitation programs at home: State of the art and an experimental comparison. Biomed. Eng. Online 2020, 19, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Zihajehzadeh, S.; Loh, D.; Lee, T.J.; Hoskinson, R.; Park, E.J. A cascaded Kalman filter-based GPS/MEMS-IMU integration for sports applications. Measurement 2015, 73, 200–210. [Google Scholar] [CrossRef]
- Hajibozorgi, M.; Arjmand, N. Sagittal range of motion of the thoracic spine using inertial tracking device and effect of measurement errors on model predictions. J. Biomech. 2016, 49, 913–918. [Google Scholar] [CrossRef] [Green Version]
- Gholipour, A.; Arjmand, N. Artificial neural networks to predict 3D spinal posture in reaching and lifting activities; Applications in biomechanical models. J. Biomech. 2016, 49, 2946–2952. [Google Scholar] [CrossRef]
- Papi, E.; Koh, W.S.; McGregor, A.H. Wearable technology for spine movement assessment: A systematic review. J. Biomech. 2017, 64, 186–197. [Google Scholar] [CrossRef]
- Simpson, L.; Maharaj, M.M.; Mobbs, R.J. The role of wearables in spinal posture analysis: A systematic review. BMC Musculoskelet. Disord. 2019, 20, 1–14. [Google Scholar] [CrossRef]
- Raya, R.; Garcia-Carmona, R.; Sanchez, C.; Urendes, E.; Ramirez, O.; Martin, A.; Otero, A. An Inexpensive and Easy to Use Cervical Range of Motion Measurement Solution Using Inertial Sensors. Sensors 2018, 18, 2582. [Google Scholar] [CrossRef] [Green Version]
- Fathi, A.; Curran, K. Detection of spine curvature using wireless sensors. SI Smart Mater. Appl. New Mater. 2017, 29, 553–560. [Google Scholar] [CrossRef]
- Mjøsund, H.L.; Boyle, E.; Kjaer, P.; Mieritz, R.M.; Skallgård, T.; Kent, P. Clinically acceptable agreement between the ViMove wireless motion sensor system and the Vicon motion capture system when measuring lumbar region inclination motion in the sagittal and coronal planes. BMC Musculoskelet. Disord. 2017, 18, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Molnar, M.; Kok, M.; Engel, T.; Kaplick, H.; Mayer, F.; Seel, T. A Method for Lower Back Motion Assessment Using Wearable 6D Inertial Sensors. In Proceedings of the 2018 21st International Conference on Information Fusion (FUSION), Cambridge, UK, 10–13 July 2018. [Google Scholar] [CrossRef]
- Aranda-Valera, I.; Cuesta-Vargas, A.; Garrido-Castro, J.; Gardiner, P.; López-Medina, C.; Machado, P.; Condell, J.; Connolly, J.; Williams, J.; Muñoz-Esquivel, K.; et al. Measuring Spinal Mobility Using an Inertial Measurement Unit System: A Validation Study in Axial Spondyloarthritis. Diagnostics 2020, 10, 426. [Google Scholar] [CrossRef]
- O’Grady, M.; O’Dwyer, T.; Connolly, J.; Condell, J.; Esquivel, K.; O’Shea, F.; Gardiner, P.; Wilson, F. Measuring Spinal Mobility Using an Inertial Measurement Unit System: A Reliability Study in Axial Spondyloarthritis. Diagnostics 2021, 11, 490. [Google Scholar] [CrossRef]
- Franco, L.; Sengupta, R.; Wade, L.; Cazzola, D. A novel IMU-based clinical assessment protocol for Axial Spondyloarthritis: A protocol validation study. PeerJ. 2021, 9, e10623. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, P.V.; Small, D.; Muñoz-Esquivel, K.; Condell, J.; Cuesta-Vargas, A.; Williams, J.; Machado, P.M.; Garrido-Castro, J.L. Validity and reliability of a sensor-based electronic spinal mobility index for axial spondyloarthritis. Rheumatol. Oxf. Engl. 2020, 59, 3415–3423. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hernández, A.; Padilla-Castañeda, M.A.; Pérez Lomeli, J.S.; Casasola-Vargas, J.; Burgos-Vargas, Y.R. Preliminary tests of an Inertial Measurement Units based System for Spine mobility assessment in patients with Ankylosing Spondylitis. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Guadalajara, México, 30 October–5 November 2021. [Google Scholar]
- Martínez-Hernández, J.S.; Pérez Lomeli, J.; Casasola-Vargas, M.A.; Padilla-Castañeda, Y.R.; Burgos, -V. Evaluation of The Spine Mobility In Patients With Ankylosing Spondyloarthritis Through A Novel Multi-Sensor Inertial System: A Pilot Test. In Proceedings of the PANLAR 23rd Congress Abstracts, Virtual, 12–15 August 2021; Volume 27. [Google Scholar] [CrossRef]
- Martínez-Hernández, A. Sistema de Estimación de Orientación Basado en Sensores Inerciales Para Aplicaciones en Evaluación de Movimientos del Cuerpo Humano. Master’s Thesis, Universidad Nacional Autónoma De México, Ciudad de México, México, 2017. (In Mexican). [Google Scholar]
- Roetenberg, D.; Luinge, H.; Slycke, Y.P. Xsens MVN: Full 6DOF human motion tracking using miniature inertial sensors. Xsens Motion Technol. BV Tech. Rep. 2009, 3, 1–9. [Google Scholar]
- Lee, J.K.; Park, E.J.; Robinovitch, S. Estimation of Attitude and External Acceleration Using Inertial Sensor Measurement During Various Dynamic Conditions. IEEE Trans. Instrum. Meas. 2012, 61, 2262–2273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ligorio, G.; Sabatini, A.M. A Novel Kalman Filter for Human Motion Tracking With an Inertial-Based Dynamic Inclinometer. IEEE Trans. Biomed. Eng. 2015, 62, 2033–2043. [Google Scholar] [CrossRef] [PubMed]
- Luinge, H.J.; Veltink, P.H. Measuring orientation of human body segments using miniature gyroscopes and accelerometers. Med. Biol. Eng. Comput. 2005, 43, 273–282. [Google Scholar] [CrossRef]
- Ozyagcilar, T. Calibrating an eCompass in the Presence of Hard- and Soft-Iron Interference. Freescale Semiconductor NXP. 2015. Available online: https://www.nxp.com/search?keyword=AN4246&start=0 (accessed on 18 November 2021).
- Liu, D.; Pei, L.; Qian, J.; Wang, L.; Liu, C.; Liu, P.; Yu, W. Simplified Ellipsoid Fitting-Based Magnetometer Calibration for Pedestrian Dead Reckoning. In Proceedings of the 2016 China Satellite Navigation Conference (CSNC), Xi’an, China, 13–15 May 2016; Volume 2, pp. 473–486. [Google Scholar]
- Welch, G.; Bishop, G. An Introduction to the Kalman Filter. Available online: https://www.cs.unc.edu/~welch/media/pdf/kalman_intro.pdf (accessed on 10 October 2021).
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Jonas, R.; Wilke, H.-J. Chapter 2—The Cervical Spine. In Biomechanics of the Spine; Galbusera, F., Wilke, Y.H.-J., Eds.; Academic Press: London, UK, 2018; pp. 11–34. [Google Scholar] [CrossRef]
- Liebsch, C.; Wilke, H.-J. Basic Biomechanics of the Thoracic Spine and Rib Cage. In Biomechanics of the Spine; Galbusera, F., Wilke, Y.H.-J., Eds.; Academic Press: London, UK, 2018; pp. 35–50. [Google Scholar] [CrossRef]
- Wilke, H.-J.; Volkheimer, D. Basic Biomechanics of the Lumbar Spine. In Biomechanics of the Spine; Galbusera, F., Wilke, Y.H.-J., Eds.; Academic Press: London, UK, 2018; pp. 51–67. [Google Scholar] [CrossRef]
- Roetenberg, D.; Luinge, H.; Baten, C.; Veltink, P. Compensation of magnetic disturbances improves inertial and magnetic sensing of human body segment orientation. IEEE Trans. Neural Syst. Rehabil. Eng. 2005, 13, 395–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zihajehzadeh, S.; Loh, D.; Lee, M.; Hoskinson, R.; Park, E.J. A cascaded two-step Kalman filter for estimation of human body segment orientation using MEMS-IMU. In Proceedings of the 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 6270–6273. [Google Scholar] [CrossRef]
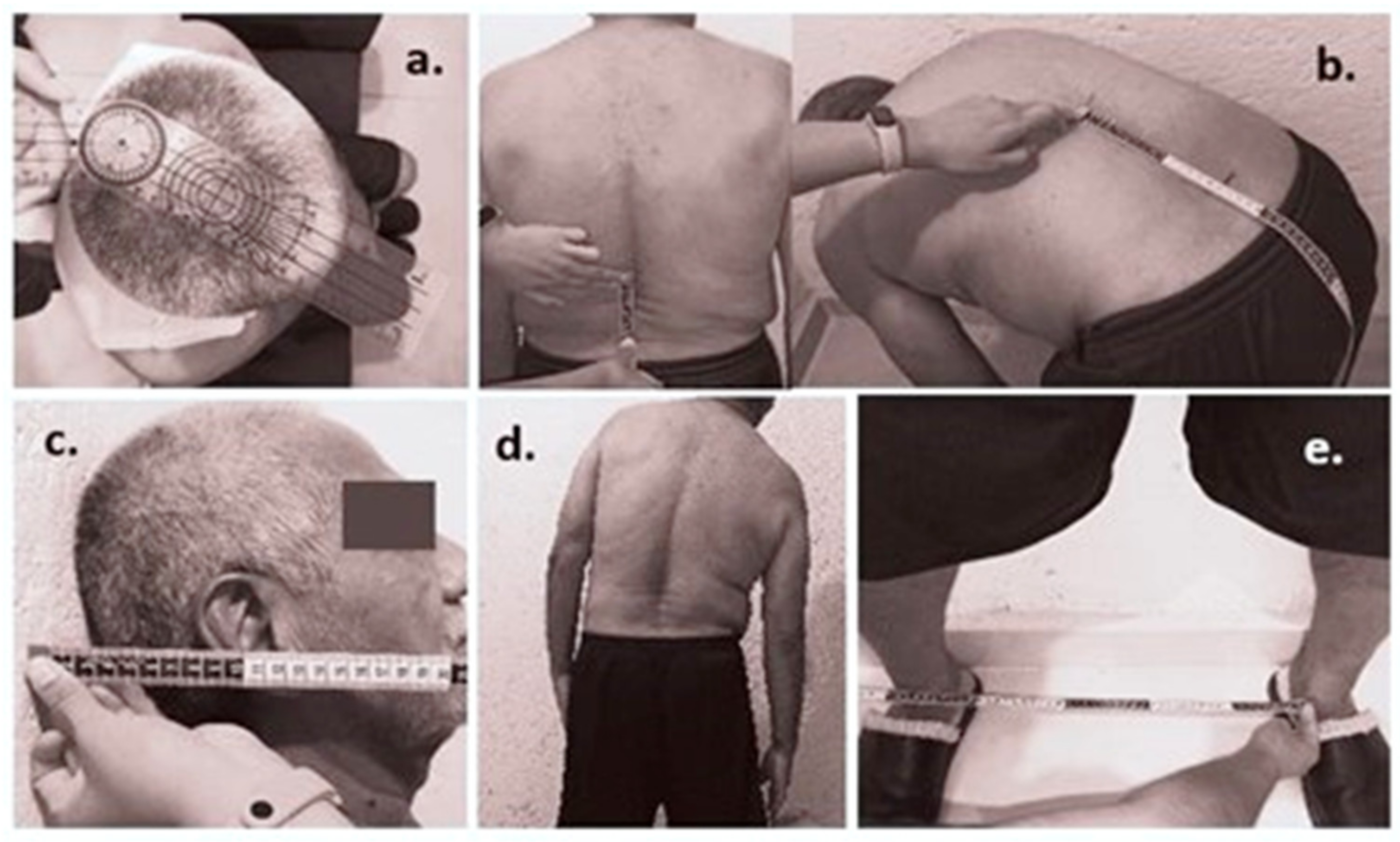


| Subjects Anthropometric Data | ||
|---|---|---|
| Participants | ||
| Women | 7 | |
| Men | 8 | |
| mean ± SD | p value (t-test) | |
| Age | 31.26 ± 5.79 | <0.0001 |
| Weight (kg) | 67.15 ± 10.18 | <0.0001 |
| Height (m) | 1.67 ± 0.07 | <0.0001 |
| BMI (kg/m2) | 24.04 ± 2.43 | <0.0001 |
| Back height (cm) | 49.39 ± 3.02 | <0.0001 |
| Separation between MIMU (cm) | 3.52 ± 0.21 | <0.0001 |
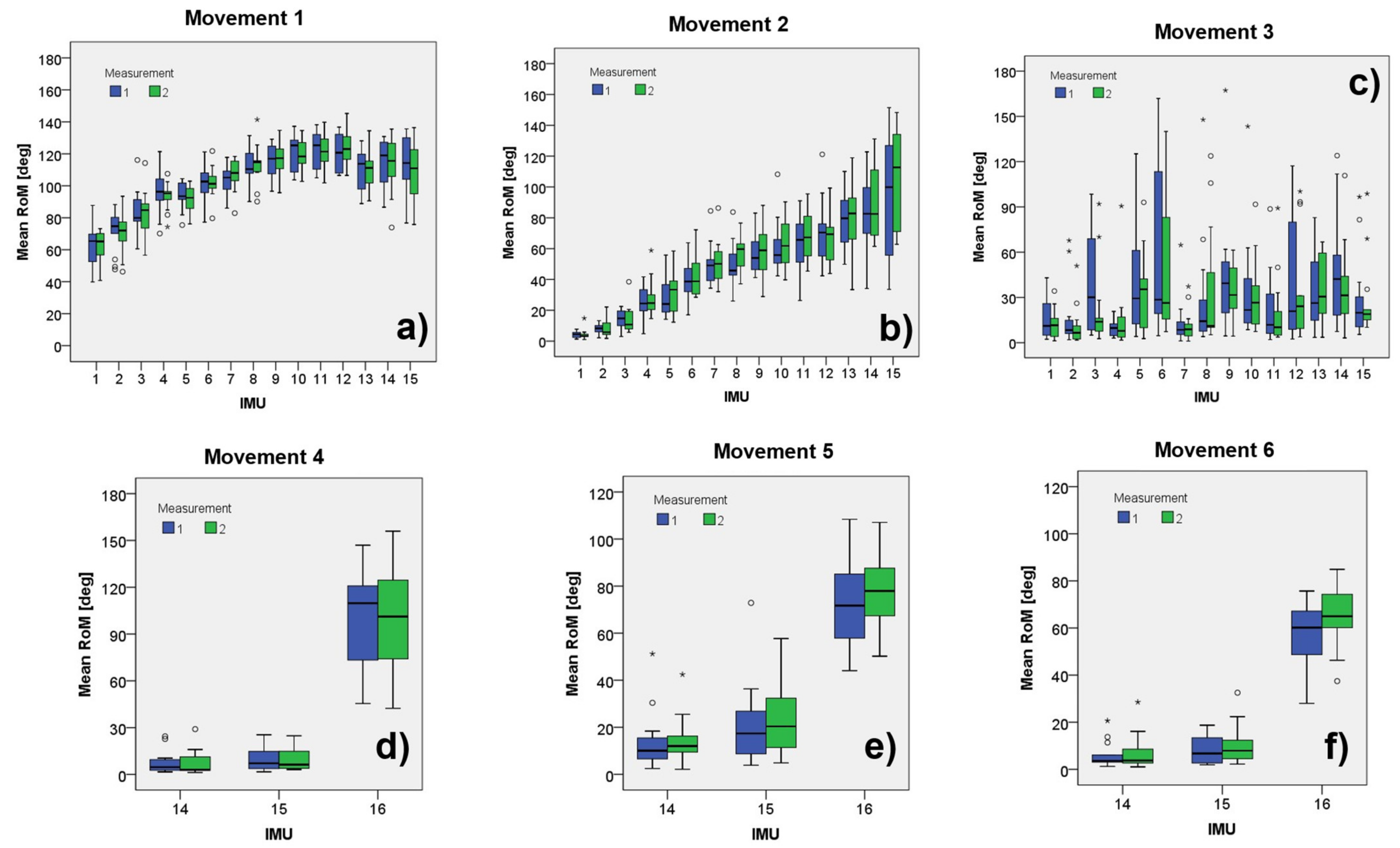
| Trunk Movements | Head Movements | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Movement 1 | Movement 2 | Movement 3 | Movement 4 | Movement 5 | Movement 6 | ||||||||||||||
| IMU | Mean | SD | ICC | Mean | SD | ICC | Mean | SD | ICC | IMU | Mean | SD | ICC | Mean | SD | ICC | Mean | SD | ICC |
| 1 | 61.8 | 12.0 | 0.60 | 4.4 | 2.9 | 0.26 | 13.5 | 10.8 | 0.59 | 14 | 7.3 | 7.4 | 0.84 | 14.0 | 11.2 | 0.92 | 7.3 | 7.4 | 0.86 |
| 2 | 69.0 | 12.8 | 0.52 | 9.2 | 6.8 | 0.22 | 12.8 | 16.7 | 0.44 | 15 | 9.1 | 7.3 | 0.87 | 24.7 | 17.1 | 0.82 | 10.9 | 8.7 | 0.71 |
| 3 | 84.7 | 15.1 | 0.91 | 14.1 | 8.4 | 0.45 | 29.8 | 29.9 | 0.54 | 16 | 96.1 | 35.3 | 0.67 | 73.8 | 18.8 | 0.72 | 60.8 | 12.5 | 0.66 |
| 4 | 95.2 | 11.8 | 0.63 | 26.0 | 11.6 | 0.71 | 11.9 | 13.8 | 0.01 | ||||||||||
| 5 | 92.7 | 8.7 | 0.83 | 28.8 | 13.7 | 0.57 | 38.5 | 32.6 | 0.38 | ||||||||||
| 6 | 102.0 | 10.6 | 0.85 | 40.7 | 14.1 | 0.67 | 55.6 | 50.5 | 0.84 | ||||||||||
| 7 | 105.8 | 9.7 | 0.74 | 49.6 | 15.4 | 0.74 | 11.7 | 13.0 | 0.03 | ||||||||||
| 8 | 113.6 | 12.7 | 0.82 | 53.2 | 18.1 | 0.37 | 36.0 | 41.8 | 0.84 | ||||||||||
| 9 | 116.5 | 11.7 | 0.80 | 59.4 | 23.6 | 0.77 | 38.7 | 28.4 | 0.45 | ||||||||||
| 10 | 120.8 | 11.7 | 0.77 | 62.9 | 20.0 | 0.67 | 31.3 | 29.5 | 0.43 | ||||||||||
| 11 | 123.8 | 12.3 | 0.82 | 66.2 | 19.6 | 0.81 | 19.1 | 23.7 | 0.87 | ||||||||||
| 12 | 123.8 | 11.9 | 0.73 | 68.0 | 21.0 | 0.65 | 34.3 | 36.0 | 0.77 | ||||||||||
| 13 | 110.1 | 13.6 | 0.77 | 81.6 | 27.9 | 0.68 | 37.6 | 25.4 | 0.90 | ||||||||||
| 14 | 114.0 | 15.7 | 0.84 | 86.3 | 29.0 | 0.70 | 38.8 | 32.2 | 0.46 | ||||||||||
| 15 | 112.8 | 17.7 | 0.79 | 102.3 | 40.7 | 0.79 | 26.5 | 26.2 | 0.90 | ||||||||||
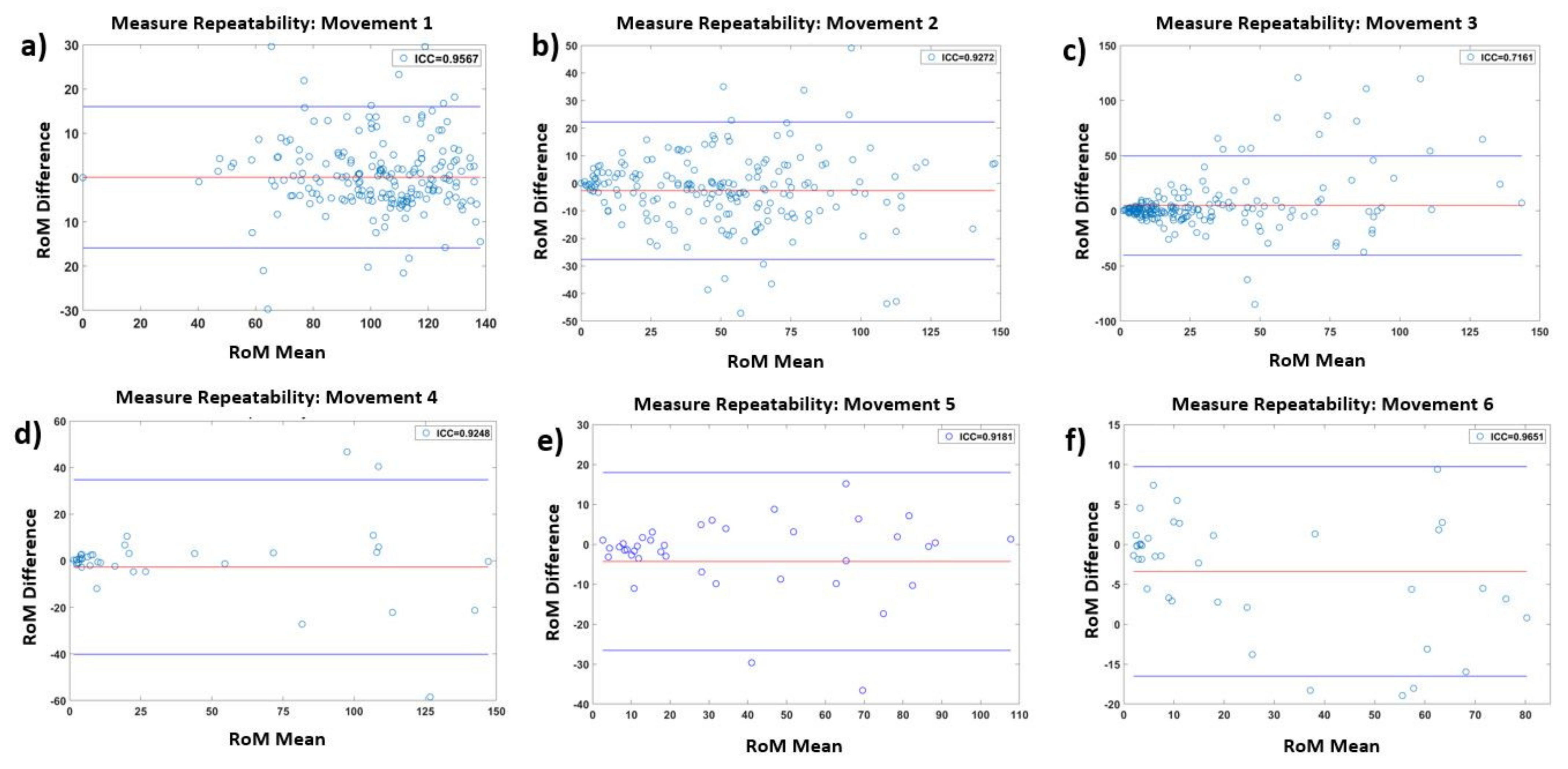
| Movement | Articulations | Excercise | IMUs | ICC |
|---|---|---|---|---|
| 1 | Lumbar–thoracic | Anterior hip flexion | MIMU1–MIMU15 | 0.96 |
| 2 | Lumbar–thoracic | Lateral flexion | MIMU1–MIMU15 | 0.93 |
| 3 | Lumbar–thoracic | Axial rotation | MIMU1–MIMU15 | 0.72 |
| 4 | Cervical | Axial rotation | MIMU14–MIMU16 | 0.93 |
| 5 | Cervical | Flexion/extension | MIMU14–MIMU16 | 0.92 |
| 6 | Cervical | Lateral flexion | MIMU14–MIMU16 | 0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Hernández, A.; Perez-Lomelí, J.S.; Burgos-Vargas, R.; Padilla-Castañeda, M.A. A Wearable System Based on Multiple Magnetic and Inertial Measurement Units for Spine Mobility Assessment: A Reliability Study for the Evaluation of Ankylosing Spondylitis. Sensors 2022, 22, 1332. https://doi.org/10.3390/s22041332
Martínez-Hernández A, Perez-Lomelí JS, Burgos-Vargas R, Padilla-Castañeda MA. A Wearable System Based on Multiple Magnetic and Inertial Measurement Units for Spine Mobility Assessment: A Reliability Study for the Evaluation of Ankylosing Spondylitis. Sensors. 2022; 22(4):1332. https://doi.org/10.3390/s22041332
Chicago/Turabian StyleMartínez-Hernández, Adriana, Juan S. Perez-Lomelí, Ruben Burgos-Vargas, and Miguel A. Padilla-Castañeda. 2022. "A Wearable System Based on Multiple Magnetic and Inertial Measurement Units for Spine Mobility Assessment: A Reliability Study for the Evaluation of Ankylosing Spondylitis" Sensors 22, no. 4: 1332. https://doi.org/10.3390/s22041332
APA StyleMartínez-Hernández, A., Perez-Lomelí, J. S., Burgos-Vargas, R., & Padilla-Castañeda, M. A. (2022). A Wearable System Based on Multiple Magnetic and Inertial Measurement Units for Spine Mobility Assessment: A Reliability Study for the Evaluation of Ankylosing Spondylitis. Sensors, 22(4), 1332. https://doi.org/10.3390/s22041332






