Design, Calibration, and Application of a Robust, Cost-Effective, and High-Resolution Lensless Holographic Microscope
Abstract
:1. Introduction
2. Materials and Methods
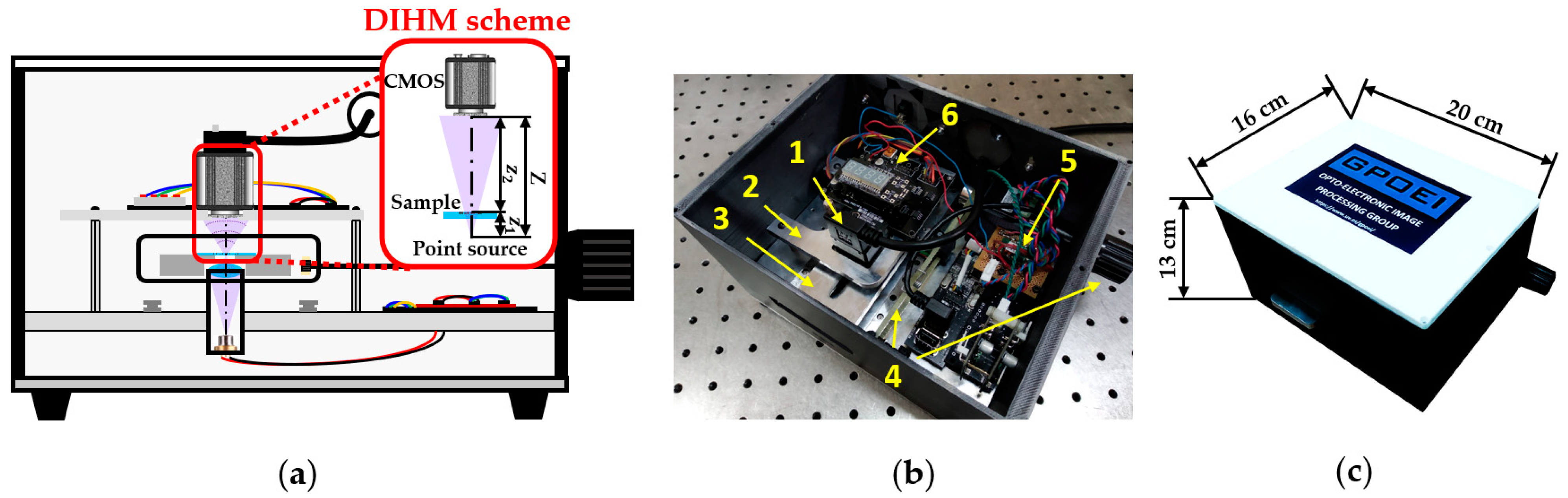
2.1. Description of the Lensless Holographic Microscope
2.2. Mathematical Foundations
2.2.1. Recording and Reconstruction Processes
2.2.2. Optical Parameters
2.3. Sample Preparation
3. Results
3.1. Calibration of the Lensless Holographic Microscope
3.1.1. Lateral Magnification
3.1.2. Field of View
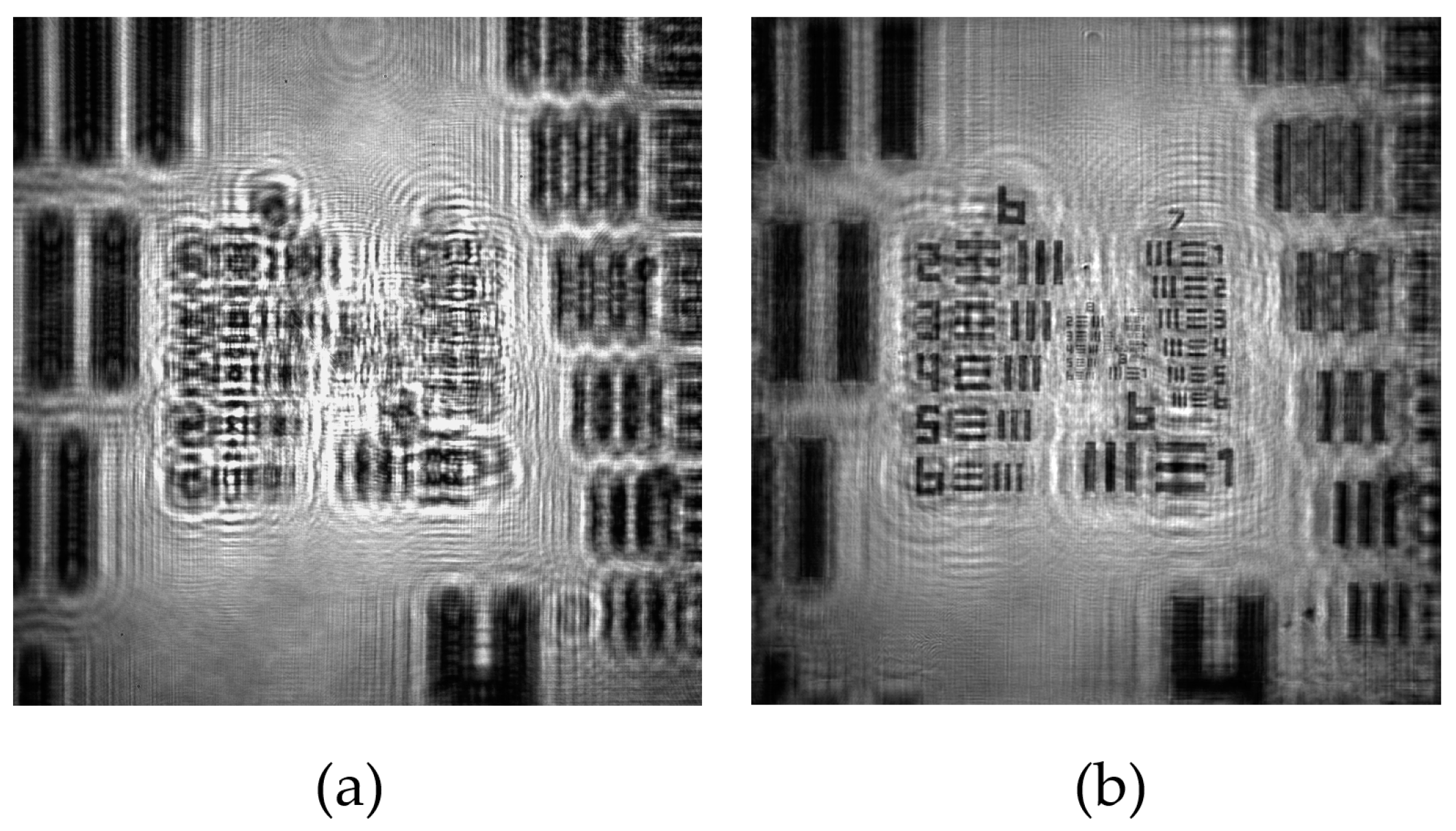
3.1.3. Lateral Resolution
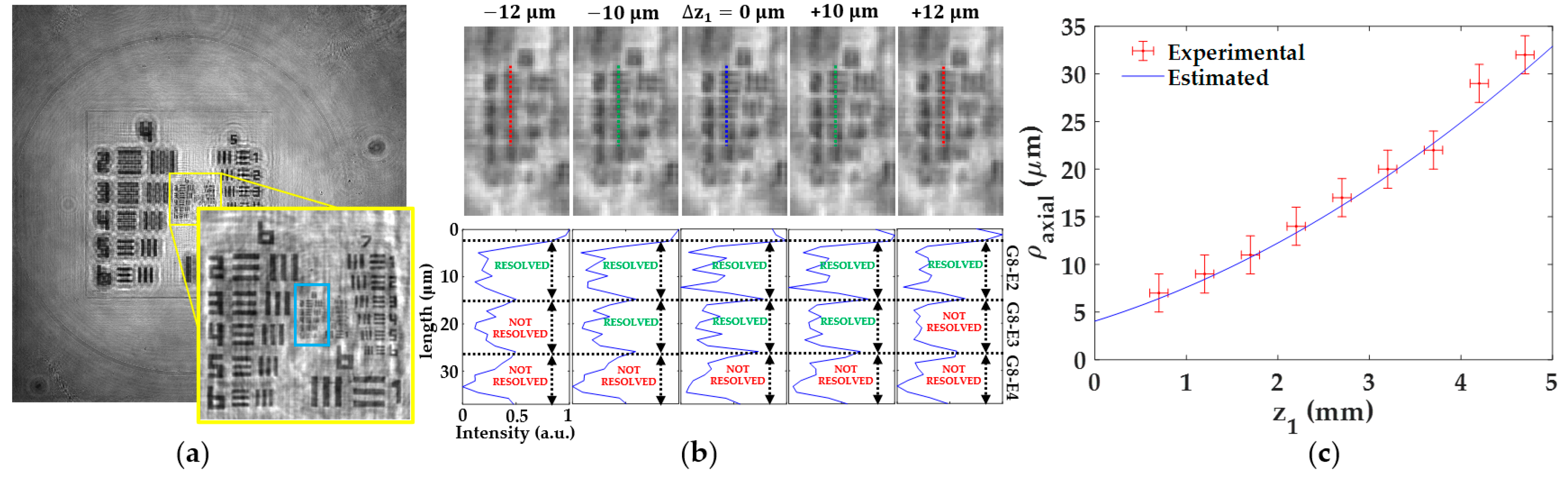
3.1.4. Axial Resolution
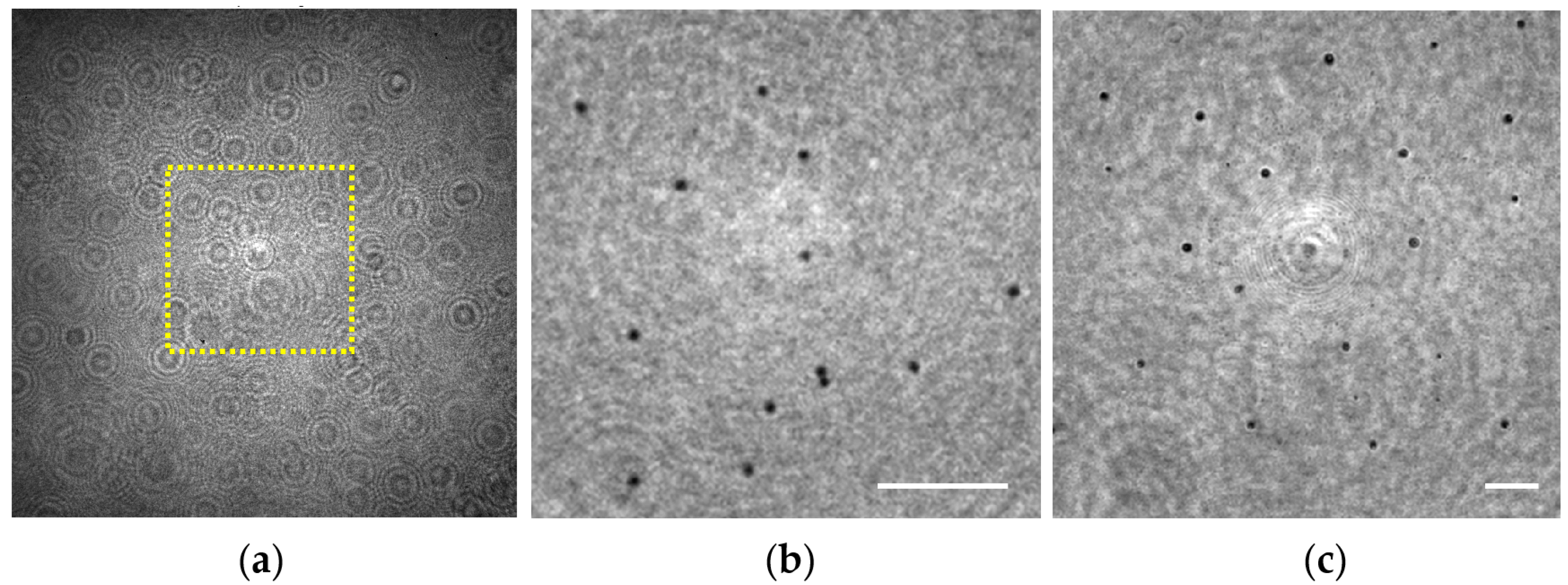
3.1.5. Extended Depth of Field
3.1.6. Phase Imaging
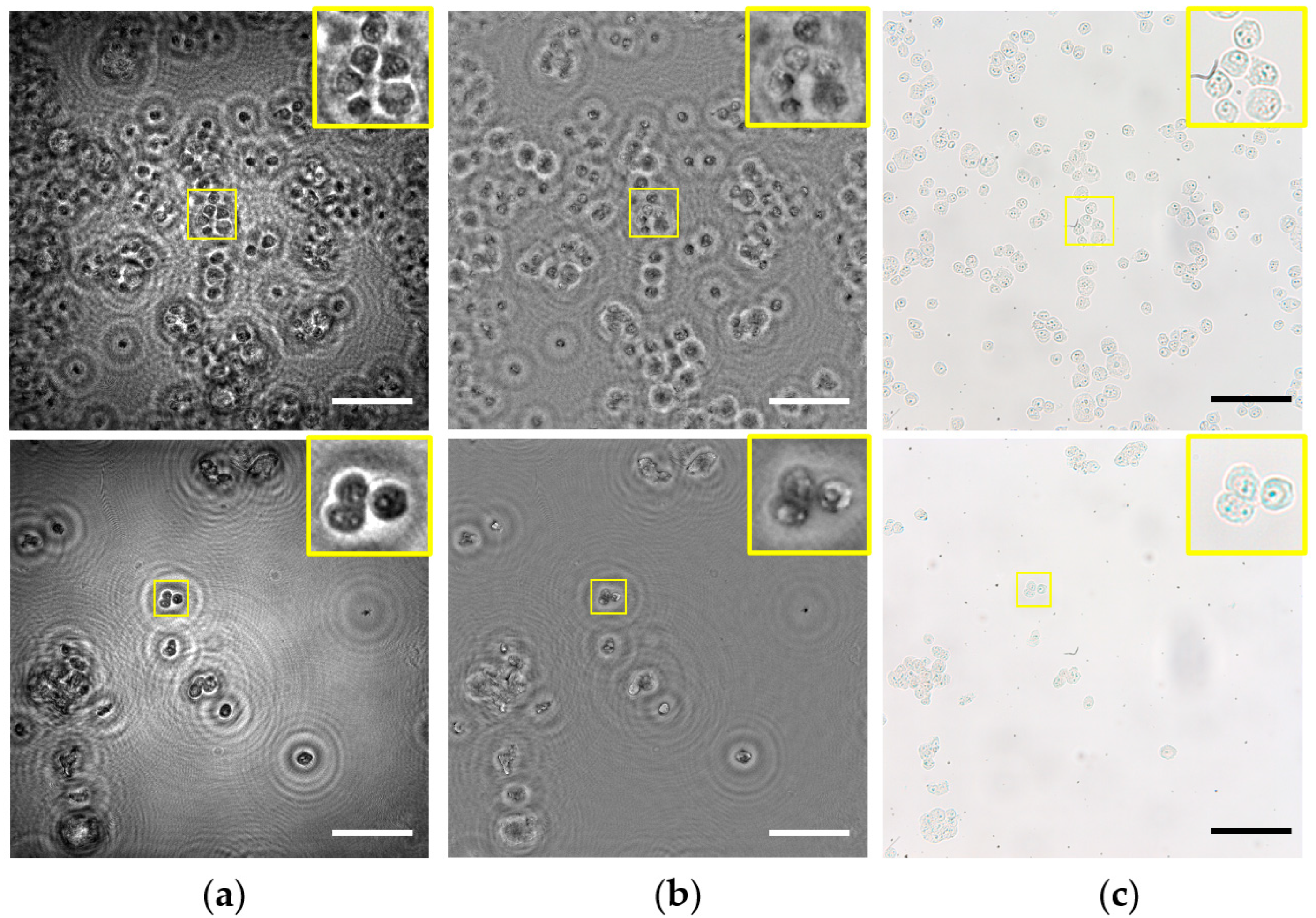
3.2. Application to Static Biosamples Inspection
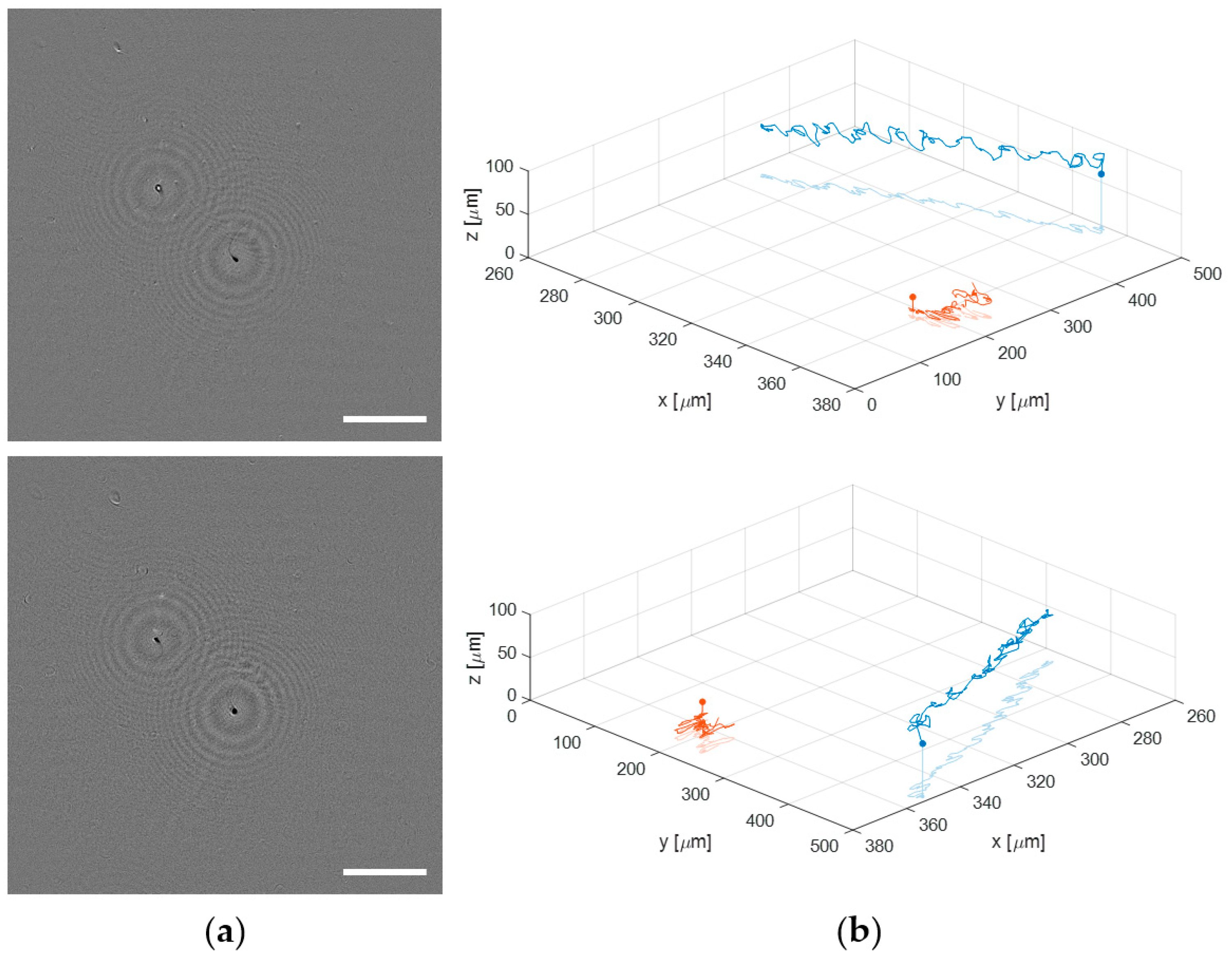
3.3. Application to Dynamic Biosamples
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, H.; Isikman, S.O.; Mudanyali, O.; Greenbaum, A.; Ozcan, A. Optical imaging techniques for point-of-care diagnostics. Lab Chip 2013, 13, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, A.; Luo, W.; Su, T.-W.; Göröcs, Z.; Xue, L.; Isikman, S.O.; Coskun, A.; Mudanyali, O.; Ozcan, A. Imaging without lenses: Achievements and remaining challenges of wide-field on-chip microscopy. Nat. Methods 2012, 9, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Seo, D.; Oh, S.; Yang, J.W.; Seo, S. A review of recent progress in lens-free imaging and sensing. Biosens. Bioelectron. 2017, 88, 130–143. [Google Scholar] [CrossRef]
- Wu, Y.; Ozcan, A. Lensless digital holographic microscopy and its applications in biomedicine and environmental monitoring. Methods 2018, 136, 4–16. [Google Scholar] [CrossRef]
- Repetto, L.; Piano, E.; Pontiggia, C. Lensless digital holographic microscope with light-emitting diode illumination. Opt. Lett. 2004, 29, 1132–1134. [Google Scholar] [CrossRef]
- Jericho, S.K.; Garcia-Sucerquia, J.; Xu, W.; Jericho, M.H.; Kreuzer, H.J. Submersible digital in-line holographic microscope. Rev. Sci. Instrum. 2006, 77, 043706. [Google Scholar] [CrossRef]
- Mudanyali, O.; Tseng, D.; Oh, C.; Isikman, S.O.; Sencan, I.; Bishara, W.; Oztoprak, C.; Seo, S.; Khademhosseini, B.; Ozcan, A. Compact, light-weight and cost-effective microscope based on lensless incoherent holography for telemedicine applications. Lab Chip 2010, 10, 1417–1428. [Google Scholar] [CrossRef] [Green Version]
- Mudanyali, O.; Oztoprak, C.; Tseng, D.; Erlinger, A.; Ozcan, A. Detection of waterborne parasites using field-portable and cost-effective lensfree microscopy. Lab Chip 2010, 10, 2419–2423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isikman, S.O.; Bishara, W.; Sikora, U.; Yaglidere, O.; Yeah, J.; Ozcan, A. Field-portable lensfree tomographic microscope. Lab Chip 2011, 11, 2222–2230. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.B.; Bae, H.; Cha, J.M.; Moon, S.J.; Dokmeci, R.D.; Cropek, D.M.; Khademhosseini, A. A cell-based biosensor for real-time detection of cardiotoxicity using lensfree imaging. Lab Chip 2011, 11, 1801–1807. [Google Scholar] [CrossRef]
- Lim, Y.; Lee, S.-Y.; Lee, B. Transflective digital holographic microscopy and its use for probing plasmonic light beaming. Opt. Express 2011, 19, 5202. [Google Scholar] [CrossRef]
- Greenbaum, A.; Akbari, N.; Feizi, A.; Luo, W.; Ozcan, A. Field-Portable Pixel Super-Resolution Colour Microscope. PLoS ONE 2013, 8, e76475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pushkarsky, I.; Liu, Y.; Weaver, W.M.; Su, T.-W.; Mudanyali, O.; Ozcan, A.; Di Carlo, D. Automated single-cell motility analysis on a chip using lensfree microscopy. Sci. Rep. 2014, 4, 4717. [Google Scholar] [CrossRef] [Green Version]
- Serabyn, E.; Liewer, K.; Lindensmith, C.; Wallace, K.; Nadeau, J. Compact, lensless digital holographic microscope for remote microbiology. Opt. Express 2016, 24, 28540–28548. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Picazo-Bueno, J.Á.; Granero, L.; Garciá, J.; Micó, V. Compact, cost-effective and field-portable microscope prototype based on MISHELF microscopy. Sci. Rep. 2017, 7, 43291. [Google Scholar] [CrossRef] [Green Version]
- Eom, J.; Moon, S. Three-Dimensional High-Resolution Digital Inline Hologram Reconstruction with a Volumetric Deconvolution Method. Sensors 2018, 18, 2918. [Google Scholar] [CrossRef] [Green Version]
- Seo, D.; Oh, S.; Lee, M.; Hwang, Y.; Seo, S. A Field-Portable Cell Analyzer without a Microscope and Reagents. Sensors 2018, 18, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amann, S.; Witzleben, M.; Breuer, S. 3D-printable portable open-source platform for low-cost lens-less holographic cellular imaging. Sci. Rep. 2019, 9, 11260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunnhofer, G.; Bergmann, A.; Klug, A.; Kraft, M. Design and Validation of a Holographic Particle Counter. Sensors 2019, 19, 4899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholz, G.; Mariana, S.; Dharmawan, A.B.; Syamsu, I.; Hörmann, P.; Reuse, C.; Hartmann, J.; Hiller, K.; Prades, J.D.; Wasisto, H.S.; et al. Continuous Live-Cell Culture Imaging and Single-Cell Tracking by Computational Lensfree LED Microscopy. Sensors 2019, 19, 1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobon-Maya, H.; Zapata-Valencia, S.; Zora-Guzmán, E.; Buitrago-Duque, C.; Garcia-Sucerquia, J. Open-source, cost-effective, portable, 3D-printed digital lensless holographic microscope. Appl. Opt. 2021, 60, A214. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, Y.; Xu, X.; Wang, R.; Yao, J.; Han, W.; Wei, M.; Chen, J.; Xuan, W.; Sun, L. High-precision lensless microscope on a chip based on in-line holographic imaging. Sensors 2021, 21, 720. [Google Scholar] [CrossRef] [PubMed]
- Gabor, D. A new microscopic principle. Nature 1948, 161, 777–778. [Google Scholar] [CrossRef]
- Rogers, G.L. XIV—Experiments in diffraction microscopy. Proc. R. Soc. Edinburgh Sect. A Math. Phys. Sci. 1952, 63, 193–221. [Google Scholar] [CrossRef]
- Xu, W.; Jericho, M.H.; Meinertzhagen, I.A.; Kreuzer, H.J. Digital in-line holography for biological applications. Proc. Natl. Acad. Sci. USA 2001, 98, 11301–11305. [Google Scholar] [CrossRef] [Green Version]
- Ferraro, P.; Wax, A.; Zalevsky, Z. Coherent Light Microscopy: Imaging and Quantitative Phase Analysis; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Garcia-sucerquia, J.; Xu, W.; Jericho, S.K.; Klages, P.; Jericho, M.H.; Kreuzer, H.J. Digital in-line holographic microscopy. Appl. Opt. 2006, 45, 836–850. [Google Scholar] [CrossRef]
- Frentz, Z.; Kuehn, S.; Hekstra, D.; Leibler, S. Microbial population dynamics by digital in-line holographic microscopy. Rev. Sci. Instrum. 2010, 81, 84301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenbaum, A.; Ozcan, A. Maskless imaging of dense samples using pixel super-resolution based multi-height lensfree on-chip microscopy. Opt. Express 2012, 20, 3143. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Yero, O.; Calabuig, A.; Tajahuerce, E.; Lancis, J.; Andrés, P.; Garcia-Sucerquia, J. Femtosecond digital lensless holographic microscopy to image biological samples. Opt. Lett. 2013, 38, 3205–3207. [Google Scholar] [CrossRef] [Green Version]
- Perucho, B.; Micó, V. Wavefront holoscopy: Application of digital in-line holography for the inspection of engraved marks in progressive addition lenses. J. Biomed. Opt. 2014, 19, 016017. [Google Scholar] [CrossRef]
- Sanz, M.; Trusiak, M.; García, J.; Micó, V. Variable zoom digital in-line holographic microscopy. Opt Lasers Eng. 2020, 127, 105939. [Google Scholar] [CrossRef]
- Rong, L.; Latychevskaia, T.; Chen, C.; Wang, D.; Yu, Z.; Zhou, X.; Li, Z.; Huang, H.; Wang, Y.; Zhou, Z. Terahertz in-line digital holography of human hepatocellular carcinoma tissue. Sci. Rep. 2015, 5, 8445. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Sucerquia, J.; Alvarez-Palacio, D.C.; Jericho, M.H.; Kreuzer, H.J. Comment on “Reconstruction algorithm for high-numerical-aperture holograms with diffraction-limited resolution”. Opt. Lett. 2006, 31, 2847. [Google Scholar] [CrossRef] [PubMed]
- Kanka, M.; Riesenberg, R.; Kreuzer, H.J. Reconstruction of high-resolution holographic microscopic images. Opt. Lett. 2009, 34, 1164. [Google Scholar] [CrossRef] [PubMed]
- Micó, V.; Zalevsky, Z. Superresolved digital in-line holographic microscopy for high-resolution lensless biological imaging. J. Biomed. Opt. 2010, 15, 046027. [Google Scholar] [CrossRef] [PubMed]
- Kanka, M.; Riesenberg, R.; Petruck, P.; Graulig, C. High resolution (NA = 0.8) in lensless in-line holographic microscopy with glass sample carriers. Opt. Lett. 2011, 36, 3653. [Google Scholar] [CrossRef] [PubMed]
- Bishara, W.; Su, T.-W.; Coskun, A.F.; Ozcan, A. Lensfree on-chip microscopy over a wide field-of-view using pixel super-resolution. Opt. Express 2010, 18, 11181. [Google Scholar] [CrossRef]
- Bishara, W.; Sikora, U.; Mudanyali, O.; Su, T.-W.; Yaglidere, O.; Luckhart, S.; Ozcan, A. Holographic pixel super-resolution in portable lensless on-chip microscopy using a fiber-optic array. Lab Chip 2011, 11, 1276–1279. [Google Scholar] [CrossRef]
- Lee, M.; Yaglidere, O.; Ozcan, A. Field-portable reflection and transmission microscopy based on lensless holography. Biomed. Opt. Express 2011, 2, 2730. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.K. Digital Holographic Microscopy. Principles, Techniques, and Applications; Springer: New York, NY, USA, 2011. [Google Scholar]
- Agbana, T.E.; Gong, H.; Amoah, A.S.; Bezzubik, V.; Verhaegen, M.; Vdovin, G. Aliasing, coherence, and resolution in a lensless holographic microscope. Opt. Lett. 2017, 42, 2271–2274. [Google Scholar] [CrossRef] [Green Version]
- Latychevskaia, T. Lateral and axial resolution criteria in incoherent and coherent optics and holography, near-and far-field regimes. Appl. Opt. 2019, 58, 3597–3603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodman, J.W. Introduction to Fourier Optics; Roberts and Company Publishers: Greenwood Village, CO, USA, 2005. [Google Scholar]
- Trusiak, M.; Picazo-Bueno, J.A.; Zdankowski, P.; Micó, V. DarkFocus: Numerical autofocusing in digital in-line holographic microscopy using variance of computational dark-field gradient. Opt. Lasers Eng. 2020, 134, 106195. [Google Scholar] [CrossRef]
- Su, T.W.; Xue, L.; Ozcan, A. High-throughput lensfree 3D tracking of human sperms reveals rare statistics of helical trajectories. Proc. Natl. Acad. Sci. USA 2012, 109, 16018–16022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Caprio, G.; El Mallahi, A.; Ferraro, P.; Dale, R.; Coppola, G.; Dale, B.; Coppola, G.; Dubois, F. 4D tracking of clinical seminal samples for quantitative characterization of motility parameters. Biomed. Opt. Express 2014, 5, 690–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerchberg, R.W.; Saxton, W.O. A practical algorithm for the determination of phase from image and diffraction plane pictures. Optik 1972, 35, 237–246. [Google Scholar]
- Latychevskaia, T.; Fink, H.W. Solution to the twin image problem in holography. Phys. Rev. Lett. 2007, 98, 233901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latychevskaia, T.; Fink, H.W. Reconstruction of purely absorbing, absorbing and phase-shifting, and strong phase-shifting objects from their single-shot in-line holograms. Appl. Opt. 2015, 54, 3925–3932. [Google Scholar] [CrossRef] [Green Version]
- Sanz, M.; Picazo-Bueno, J.A.; García, J.; Micó, V. Improved quantitative phase imaging in lensless microscopy by single-shot multi-wavelength illumination using a fast convergence algorithm. Opt. Express 2015, 23, 21365. [Google Scholar] [CrossRef]
- Latychevskaia, T. Iterative phase retrieval for digital holography: Tutorial. J. Opt. Soc. Am. A 2019, 36, D40. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picazo-Bueno, J.A.; Trindade, K.; Sanz, M.; Micó, V. Design, Calibration, and Application of a Robust, Cost-Effective, and High-Resolution Lensless Holographic Microscope. Sensors 2022, 22, 553. https://doi.org/10.3390/s22020553
Picazo-Bueno JA, Trindade K, Sanz M, Micó V. Design, Calibration, and Application of a Robust, Cost-Effective, and High-Resolution Lensless Holographic Microscope. Sensors. 2022; 22(2):553. https://doi.org/10.3390/s22020553
Chicago/Turabian StylePicazo-Bueno, Jose Angel, Karina Trindade, Martin Sanz, and Vicente Micó. 2022. "Design, Calibration, and Application of a Robust, Cost-Effective, and High-Resolution Lensless Holographic Microscope" Sensors 22, no. 2: 553. https://doi.org/10.3390/s22020553
APA StylePicazo-Bueno, J. A., Trindade, K., Sanz, M., & Micó, V. (2022). Design, Calibration, and Application of a Robust, Cost-Effective, and High-Resolution Lensless Holographic Microscope. Sensors, 22(2), 553. https://doi.org/10.3390/s22020553






