Winter Wheat Nitrogen Estimation Based on Ground-Level and UAV-Mounted Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sensor Data Collection
2.2.1. Leaf- and Canopy-Level Data Collection
2.2.2. UAV-Level Data Collection
2.3. Plant Sampling Data Collection
2.4. Data Analysis Methods
3. Results
3.1. Variation in Winter Wheat N Indicators
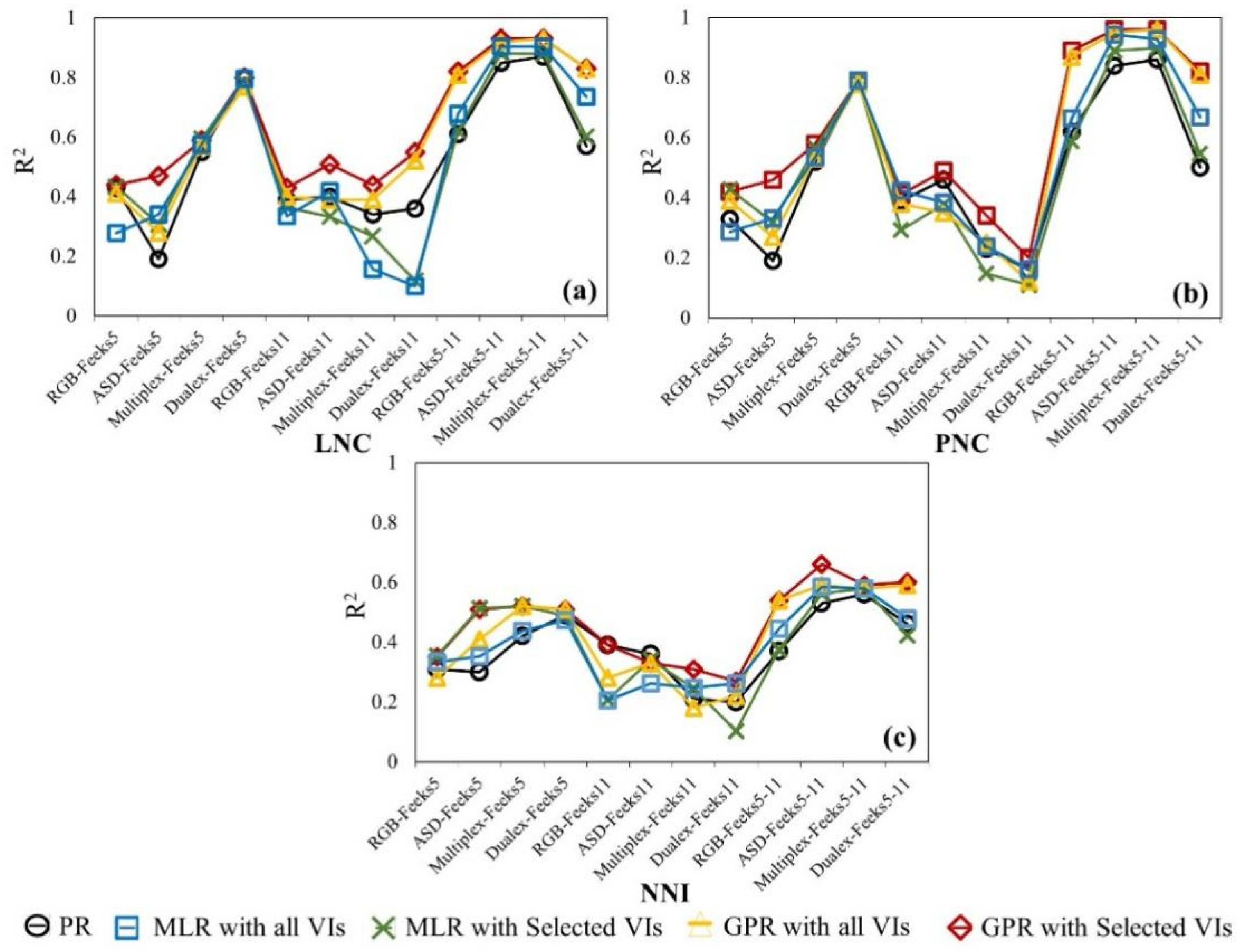
3.2. Relationships between Wheat N and VIs for Different Sensors
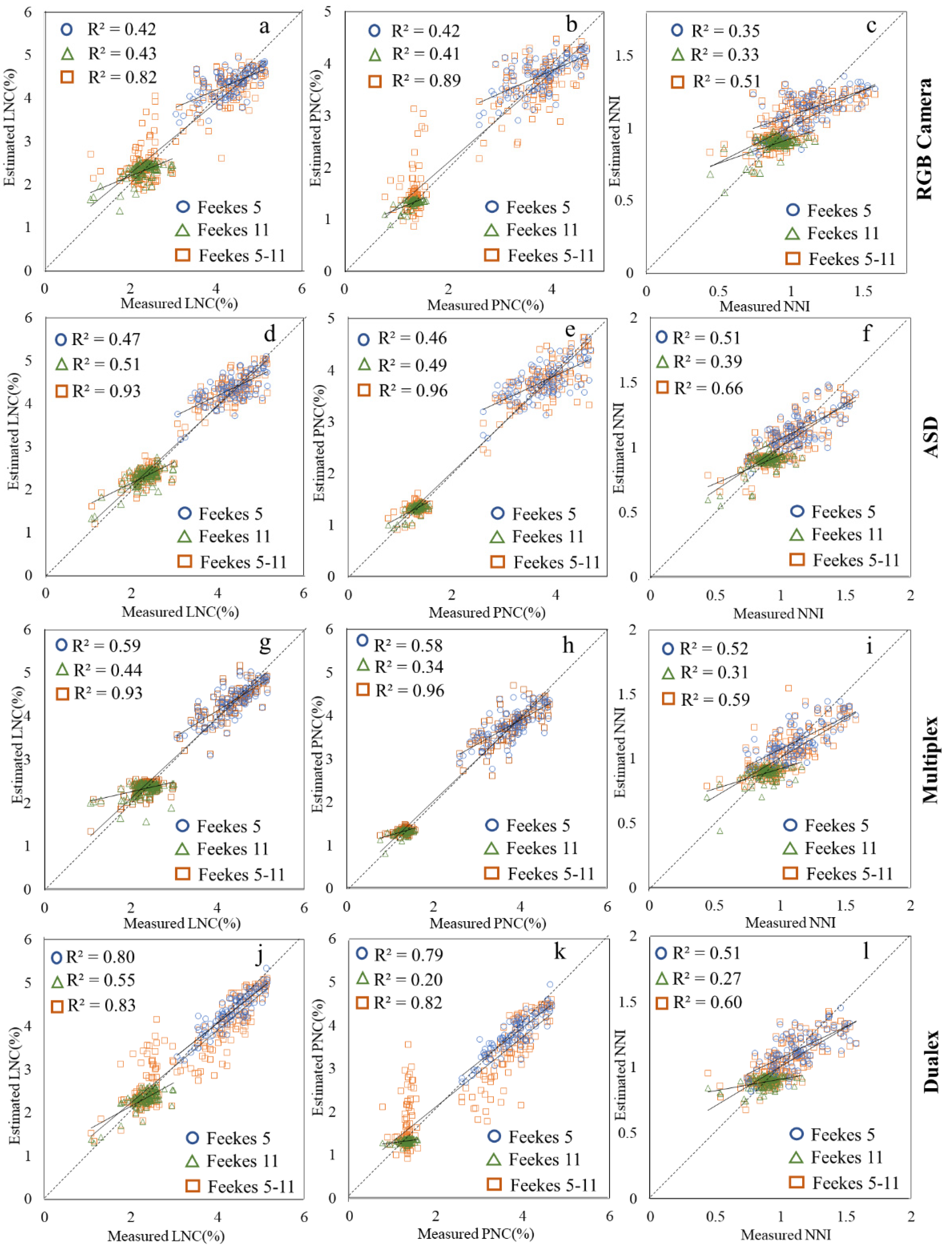
3.3. Wheat N Estimation through UAV-Level VIs
3.4. Wheat N Estimation through Canopy-Level VIs
3.5. Wheat N Estimation through Leaf-Level VIs
4. Discussion
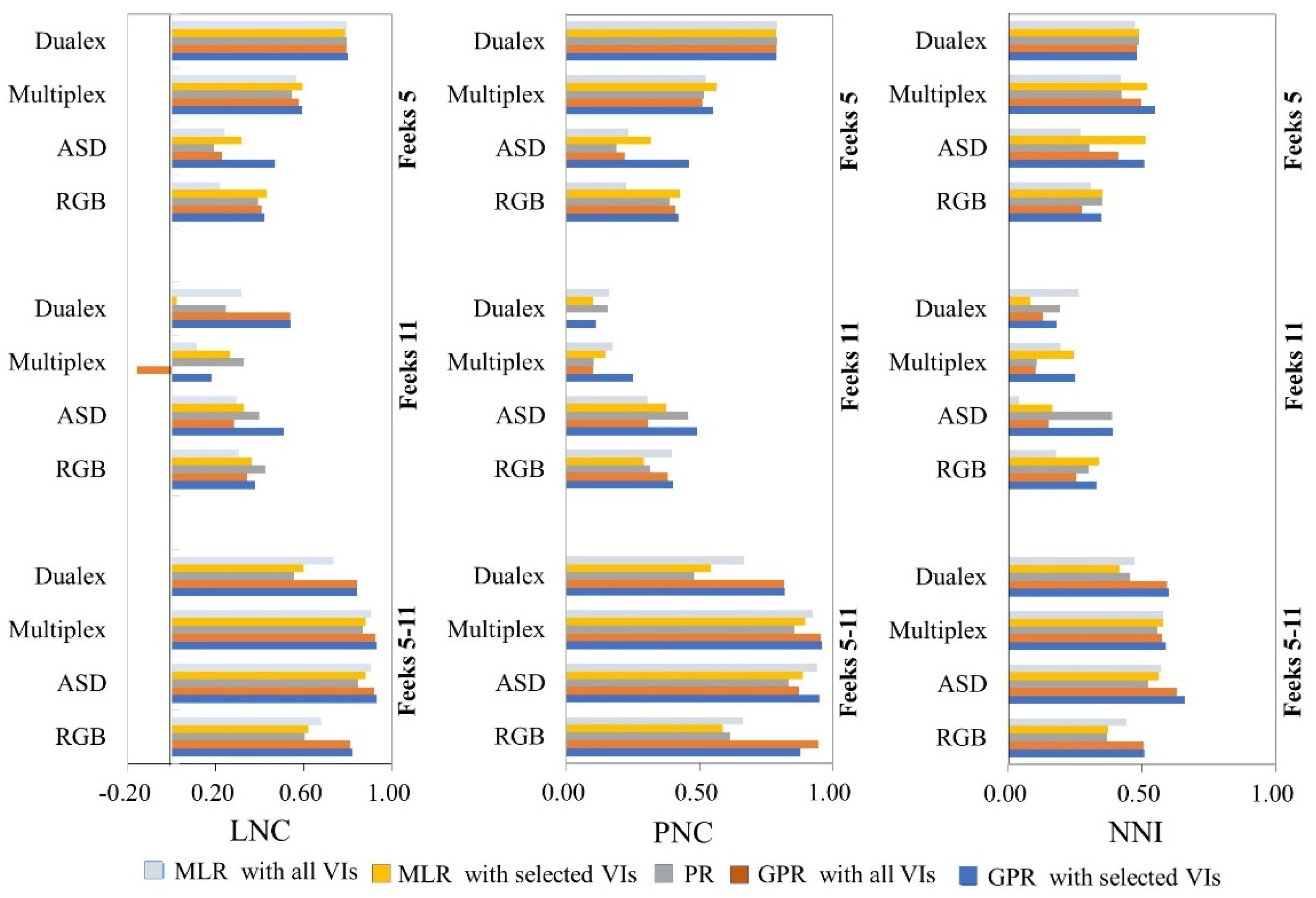
4.1. N Estimation Comparison for Different Sensors
4.2. Accuracy Evaluation of GPR, MLR, and PR Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mistele, B.; Schmidhalter, U. Estimating the nitrogen nutrition index using spectral canopy reflectance measurements. Eur. J. Agron. 2008, 29, 184–190. [Google Scholar] [CrossRef]
- Ziadi, N.; Brassard, M.; Bélanger, G.; Claessens, A.; Tremblay, N.; Cambouris, A.N.; Nolin, M.C.; Parent, L.E. Chlorophyll measurements and nitrogen nutrition index for the evaluation of corn nitrogen status. Agron. J. 2008, 100, 1264–1273. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Wang, H.; Huang, S.; Cheng, S.; Khosla, R.; Jiang, R. Nondestructive estimation of rice plant nitrogen status with Crop Circle multispectral active canopy sensor. Field Crops Res. 2013, 154, 133–144. [Google Scholar] [CrossRef]
- Diacono, M.; Rubino, P.; Montemurro, F. Precision nitrogen management of wheat: A review. Agron. Sustain. Dev. 2013, 33, 219–241. [Google Scholar] [CrossRef]
- Hansen, P.M.; Schjoerring, J.K. Reflectance measurement of canopy biomass and nitrogen status in wheat crops using normalized difference vegetation indices and partial least squares regression. Remote Sens. Environ. 2003, 86, S0034–S4257. [Google Scholar] [CrossRef]
- Ecarnot, M.; Compan, F.; Roumet, P. Assessing leaf nitrogen content and leafmass per unit area of wheat in the field throughout plant cycle with a portable spectrometer. Field Crops Res. 2013, 140, 44–50. [Google Scholar] [CrossRef]
- Debaeke, P.; Rouet, P.; Justes, E. Relationship between the normalized SPAD index and the nitrogen nutrition index: Application to durum wheat. J. Plant Nutr. 2006, 29, 75–92. [Google Scholar] [CrossRef]
- Prost, L.; Jeuffroy, M.H. Replacing the nitrogen nutrition index by the chlorophyll meter to assess wheat N status. Agron. Sustain. Dev. 2007, 27, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Cui, Z.; Chen, X.; Khosla, R.; Dao, T.H.; Miao, Y. Quantifying spatial variability of indigenous nitrogen supply for precision nitrogen management in small scale farming. Precis. Agric. 2012, 13, 45–61. [Google Scholar] [CrossRef]
- Tremblay, N.; Wang, Z.; Cerovic, Z.G. Sensing crop nitrogen status with fluorescence indicators—A review. Agron. Sust. Dev. 2012, 32, 451–464. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, J.L.; Zarco-Tejada, P.J.; López-Herrera, P.J.; Pérez-Martín, E.; Alonso-Ayuso, M.; Quemada, M. Airborne and ground level sensors for monitoring nitrogen status in a maize crop. Biosyst. Eng. 2017, 160, 124–133. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Ounis, A.; Cartelat, A.; Latouche, G.; Goulas, Y.; Meyer, S. The use of chlorophyll fluorescence excitation spectra for the non-destructive in situ assessment of UV absorbing compounds in leaves. Plant Cell Environ. 2002, 25, 1663–1676. [Google Scholar] [CrossRef]
- Cartelat, A.; Cerovic, Z.G.; Goulas, Y.; Meyer, S.; Lelarge, C.; Prioul, J.L. Optically assessed contents of leaf polyphenolics and chlorophyll as indicators of nitrogen deficiency in wheat (Triticum aestivum L.). Field Crops Res. 2005, 91, 35–49. [Google Scholar] [CrossRef]
- Lamb, D.W.; Steyn-Ross, M.; Schaare, P.; Hanna, M.M. Estimating leaf nitrogen concentration in ryegrass (Lolium spp.) pasture using the chlorophyll rededge: Theoretical modeling and experimental observations. Int. J. Remote Sens. 2002, 23, 3619–3648. [Google Scholar] [CrossRef]
- Reyniers, M.; Vrindts, E. Measuring wheat nitrogen status from space and ground-based platform. Int. J. Remote Sens. 2006, 27, 549–567. [Google Scholar] [CrossRef]
- Cho, M.A.; Skidmore, A.K. A new technique for extracting the red edge position from hyperspectral data: The linear extrapolation method. Remote Sens. Environ. 2006, 101, 181–193. [Google Scholar] [CrossRef]
- Ben, G.N.; Cerovic, Z.G.; Germain, C.; Toutain, S.; Latouche, G. Non-destructive optical monitoring of grape maturation by proximal sensing. Sensors 2010, 10, 10040–10068. [Google Scholar] [CrossRef]
- Mukhamediev, R.I.; Symagulov, A.; Kuchin, Y.; Zaitseva, E.; Bekbotayeva, A.; Yakunin, K.; Assanov, I.; Levashenko, V.; Popova, Y.; Akzhalova, A.; et al. Review of Some Applications of Unmanned Aerial Vehicles Technology in the Resource-Rich Country. Appl. Sci. 2021, 11, 10171. [Google Scholar] [CrossRef]
- Shakhatreh, H.; Sawalmeh, A.H.; Al-Fuqaha, A.; Dou, Z.; Almaita, E.; Khalil, I.; Othman, N.S.; Khreishah, A.; Guizani, M. Unmanned aerial vehicles (UAVs): A survey on civil applications and key research challenges. IEEE Access 2019, 7, 48572–48634. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, J.; Sun, X.; Zhang, X. Estimation of leaf chlorophyll content of rice using image color analysis. Can. J. Remote Sens. 2013, 39, 185–190. [Google Scholar] [CrossRef]
- Jia, B.; He, H.B.; Ma, F.Y.; Diao, M.; Jiang, G.Y.; Zheng, Z.; Cui, J.; Fan, H. Use of a digital camera to monitor the growth and nitrogen status of cotton. Sci. World J. 2014, 2014, 602647. [Google Scholar] [CrossRef]
- Adamsen, F.G.; Pinter, J.P.; Barnes, M.E.; LaMorte, L.R.; Wall, W.G.; Leavitt, W.S. Measuring wheat senescence with a digital camera. Crop. Sci. 1999, 39, 719–724. [Google Scholar] [CrossRef]
- Lukina, E.V.; Stone, M.L.; Raun, W.R. Estimating vegetation coverage in wheat using digital images. J. Plant Nutr. 1999, 22, 341–350. [Google Scholar] [CrossRef]
- Elsayed, S.; Barmeier, G.; Schmidhalter, U. Passive Reflectance Sensing and Digital Image Analysis Allows for Assessing the Biomass and Nitrogen Status of Wheat in Early and Late Tillering Stages. Front. Plant. Sci. 2018, 9, 1478. [Google Scholar] [CrossRef]
- Song, S.; Chong, H.; Chun, S.; Cheng, Y.; Dai, H. Research advancement on crop nitrogen nutrition diagnosis. Chin. J. Soil Sci. 2006, 37, 369–372, (In Chinese with English Abstract). [Google Scholar]
- Guo, B.B.; Qi, S.L.; Heng, Y.R.; Duan, J.; Zhao, H.Y.; Zhang, Y.P.; Wu, W.F.; Xie, Y.X.; Zhu, Y.J. Remotely assessing leaf N uptake in winter wheat based on canopy hyperspectral red-edge absorption. Eur. J. Agron. 2017, 82, 113–124. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Baret, F. PROSPECT: A Model of Leaf Optical Properties Spectra. Remote Sens. Environ. 1990, 34, 75–91. [Google Scholar] [CrossRef]
- Ollinger, S.V. Sources of variability in canopy reflectance and the convergent properties of plants. New Phytol. 2011, 189, 375–394. [Google Scholar] [CrossRef] [PubMed]
- Verrelst, J.; Romijn, E.; Kooistra, L. Mapping vegetation density in aheterogeneous river floodplain ecosystem using pointable CHRIS/PROBA data. Remote Sens. 2012, 4, 2866–2889. [Google Scholar] [CrossRef] [Green Version]
- Verrelst, J.; Camps-Valls, G.; Muñoz-Marí, J.; Rivera, J.P.; Veroustraete, F.; Clevers, J.G.P.W.; Moreno, J. Optical remote sensing and the retrieval of terrestrial vegetation bio-geophysical properties—A review. ISPRS J. Photogramm. Remote Sens. 2015, 108, 273–290. [Google Scholar] [CrossRef]
- Clevers, J.G.P.W. Beyond NDVI: Extraction of biophysical variables from remote sensing imagery. In Land Use and Land Cover Mapping in Europe: Practices and Trends; Manakos, I., Braun, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 363–381. [Google Scholar]
- Glenn, E.P.; Huete, A.R.; Nagler, P.L.; Nelson, S.G. Relationship between remotely-sensed vegetation indices, canopy attributes and plant physiological processes: What vegetation indices can and cannot tell us about the landscape. Sensors 2008, 8, 2136–2160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, A.; Langley, P. Selection of relevant features and examples in machine learning. Artif. Intell. 1997, 97, 245–271. [Google Scholar] [CrossRef] [Green Version]
- Verrelst, J.; Rivera, J.P.; Gitelson, A.; Delegido, J.; Moreno, J.; Camps, V.G. Spectral band selection for vegetation properties retrieval using Gaussian processes regression. Int. J. Appl. Earth Observ. Geoinf. 2016, 52, 554–567. [Google Scholar] [CrossRef]
- Miphokasap, P.; Honda, K.; Vaiphasa, C.; Souris, M.; Nagai, M. Estimating canopy nitrogen concentration in sugarcane using field imaging spectroscopy. Remote Sens. 2012, 4, 1651–1670. [Google Scholar] [CrossRef] [Green Version]
- Verrelst, J.; Alonso, L.; Rivera Caicedo, J.; Moreno, J.; Camps-Valls, G. Gaussian process retrieval of chlorophyll content from imaging spectroscopy data. IEEE J. Sel. Top. Appl. Earth Observ. Remote Sens. 2013, 6, 867–874. [Google Scholar] [CrossRef]
- Verrelst, J.; Alonso, L.; Camps-Valls, G.; Delegido, J.; Moreno, J. Retrieval of vegetation biophysical parameters using gaussian process techniques. IEEE Trans. Geosci. Remote Sens. 2012, 50, 1832–1843. [Google Scholar] [CrossRef]
- Verrelst, J.; Malenovský, Z.; Van der Tol, C.; Camps-Valls, G.; Gastellu-Etchegorry, J.P.; Lewis, P.; North, P.; Moreno, J. Quantifying Vegetation Biophysical Variables from Imaging Spectroscopy Data: A Review on Retrieval Methods. Surv. Geophys. 2018, 40, 589–629. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, C.E.; Williams, C.K.I. Gaussian Processes for Machine Learning; The MIT Press: Cambridge, MA, USA, 2006; pp. 7–30. Available online: http://www.gaussianprocess.org/gpml/chapters/RW.pdf (accessed on 28 July 2021).
- Li, F.; Miao, Y.; Hennig, S.D.; Gnyp, M.L.; Chen, X.; Jia, L. Evaluating hyperspectral vegetation indices for estimating nitrogen concentration of winter wheat at different growth stages. Precis. Agric. 2010, 11, 335–357. [Google Scholar] [CrossRef]
- Ranjan, R.; Chopra, U.K.; Sahoo, R.N.; Singh, A.K.; Pradhan, S. Assessment of plant nitrogen stress in wheat (Triticum aestivum L.) through hyperspectral indices. Int. J. Remote Sens. 2012, 33, 6342–6360. [Google Scholar] [CrossRef]
- Tian, Y.; Gu, K.; Chu, X.; Yao, X.; Cao, W.; Zhu, Y. Comparison of different hyperspectral vegetation indices for canopy leaf nitrogen concentration estimation in rice. Plant. Soil. 2013, 376, 193–209. [Google Scholar] [CrossRef]
- Song, X.; Feng, W.; He, L.; Xu, D.; Zhang, H.; Li, X.; Wang, Z.; Coburn, C.A.; Wang, C.; Guo, T. Examining view angle effects on leaf N estimation in wheat using field reflectance spectroscopy. ISPRS J. Photo. Remote Sens. 2016, 122, 57–67. [Google Scholar] [CrossRef]
- Tian, Y.; Yao, X.; Yang, J.; Cao, W.; Hannaway, D.B.; Zhu, Y. Assessing newly developed and published vegetation indices for estimating rice leaf nitrogen concentration with ground-and space-based hyperspectral reflectance. Field Crops Res. 2011, 120, 299–310. [Google Scholar] [CrossRef]
- Feng, W.; Yao, X.; Zhu, Y.; Tian, Y.C.; Cao, W.X. Monitoring leaf nitrogen status with hyperspectral reflectance in wheat. Eur. J. Agron. 2008, 28, 394–404. [Google Scholar] [CrossRef]
- Daniela, S.; Mirco, B.; Pietroalessandro, B.; Stefano, B. Plant nitrogen concentration in paddy rice from field canopy hyperspectral radiometry. Field Crops Res. 2009, 111, 119–129. [Google Scholar] [CrossRef]
- Wang, W.; Yao, X.; Tian, Y.; Liu, X.; Ni, J. Estimating leaf nitrogen concentration with three-band vegetation indices in rice and wheat. Field Crops Res. 2012, 129, 90–98. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W.; Harlan, J.C. Monitoring the Vernal Advancement and Retrogradation of Natural Vegetation; NASA/GSFC, Type III, Final Report; NTRS: Greenbelt, MD, USA, 1973; pp. 7–15. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19740004927.pdf (accessed on 26 July 2021).
- Reujean, J.L.; Breon, F.M. Estimating PAR absorbed by vegetation from bidirectional reflectance measurements. Remote Sens Enviro. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.N. Spectral reflectance changes associated with autumn senescence of Aesculus hippocastanum L. and Acer platanoides L. leaves: Spectral features and relation to chlorophyll estimation. J. Plant Phys. 1994, 143, 286. [Google Scholar] [CrossRef]
- Penuelas, J.; Filella, I.; Serrano, L.; Save, R. The reflectance at the 950–970 nm region as an indicator of plant water status. Int. J. Remote Sens. 1992, 14, 1887–1905. [Google Scholar] [CrossRef]
- Gao, B.C. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Hardisky, M.A.; Klemas, V.; Smart, R.M. The influence of Soil Salinity, Growth Form, and Leaf Moisture on the Spectral Radiance of Spartina alterniflora Canopies. Photo. Eng. Remote Sens. 1983, 48, 77–83. [Google Scholar]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; de colstoun, E.B.; Mcmurtrey, J.E. Estimating corn leaf chlorophyll content from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. Feb. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Qi, J.; Chehbouni, A.; Huete, A.R.; Kerr, Y.H. A Modified Soil Adjusted Vegetation Index (MSAVI). Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Metternicht, G. Vegetation indices derived from high resolution airborne videography for precision crop management. Int. J. Rem. Sens. 2003, 24, 2855–2877. [Google Scholar] [CrossRef]
- SpecTerra, S. Presentation and Analysis of Data; SpecTerra Services Pty Ltd.: Leederville, Australia, 1999; Available online: http://www.specterra.com.au/dmsv_data_frame.html (accessed on 10 July 2021).
- Demmig, A.B.; Adams, W.W. The role of xanthophylls cycle carotenoids in the protection of photosynthesis. Trends Plant. Sci. 1996, 1, 21–26. [Google Scholar] [CrossRef]
- Dawson, T.P.; Curran, P.J.; Plummer, S.E. LIBERTY-Modeling the effects of leaf biochemical concentration on reflectance spectra. Remote Sens. Environ. 1998, 65, 50–60. [Google Scholar] [CrossRef]
- Miller, J.; Hare, E.; Wu, J. Quantitative characterization of the vegetation red edge reflectance I. An inverted-Gaussian reflectance model. Int. J. Remote Sens. 1990, 11, 1755–1773. [Google Scholar] [CrossRef]
- Yue, J.B.; Feng, H.K.; Jin, X.L.; Yuan, H.H.; Li, Z.H.; Zhou, C.Q.; Yang, G.J.; Tian, Q.J. A Comparison of Crop Parameters Estimation Using Images from UAV-Mounted Snapshot Hyperspectral Sensor and High-Definition Digital Camera. Remote Sens. 2018, 10, 1138. [Google Scholar] [CrossRef] [Green Version]
- Woebbecke, D.; Meyer, G.; Von Bargen, K.; Mortensen, D. Color indices for weed identification under various soil, residue, and lighting conditions. Trans. ASAE 1995, 38, 259–269. [Google Scholar] [CrossRef]
- Meyer, G.E.; Neto, J.C. Verification of color vegetation indices for automated crop imaging applications. Comput. Electron. Agric. 2008, 63, 282–293. [Google Scholar] [CrossRef]
- Peñuelas, J.; Gamon, J.A.; Fredeen, A.L.; Merino, J.; Field, C.B. Reflectance indices associated with physiological changes in nitrogen- and water-limited sunflower leaves. Remote Sens. Environ. 1994, 48, 135–146. [Google Scholar] [CrossRef]
- Bradstreet, R.B. The Kjeldahl Method for Organic Nitrogen; Academic Press Incorporated: New York, NY, USA, 1965. [Google Scholar]
- Zhao, H.T.; Song, X.Y.; Yang, G.J.; Li, Z.H.; Zhang, D.Y.; Feng, H.K. Monitoring of Nitrogen and Grain Protein Content in Winter Wheat Based on Sentinel-2A Data. Remote Sens. 2019, 11, 1724. [Google Scholar] [CrossRef] [Green Version]
- Lemaire, G.; Jeuffroy, M.H.; Gastal, F. Diagnosis tool for plant and crop N status in vegetative stage: Theory and practices for crop N management. Eur. J. Agron. 2008, 28, 614–624. [Google Scholar] [CrossRef]
- Yue, S.; Meng, Q.; Zhao, R.; Li, F.; Chen, X.; Zhang, F.; Cui, Z. Critical nitrogen dilution curve for optimizing nitrogen management of winter wheat production in the North China Plain. Agron. J. 2012, 104, 523–529. [Google Scholar] [CrossRef]
- Willmott, C.J.; Matsuura, K. Advantages of the mean absolute error (MAE) over the root mean square error (RMSE) in assessing average model performance. Clim. Res. 2005, 30, 79–82. [Google Scholar] [CrossRef]
- Schaeffer, D.L. A model evaluation methodology applicable to environmental assessment models. Ecol. Model. 1980, 8, 275–295. [Google Scholar] [CrossRef] [Green Version]
- Nash, J.E.; Sutcliffe, J.V. River flow forecasting through conceptual models part I-A discussion of principles. J. Hydrol. 1970, 10, 282–290. [Google Scholar] [CrossRef]
- Saberioon, M.; Amin, M.S.M.; Gholizadeh, A.; Ezri, M.H. A review of optical methods for assessing nitrogen contents during rice growth. Appl. Eng. Agric. 2014, 30, 657–669. [Google Scholar] [CrossRef]
- Karim, S.T.; Cheng, T.; Liu, X.; Tian, Y.; Zhu, Y.; Cao, W.; Cao, Q. Potential of UAV-based active sensing for monitoring rice leaf nitrogen status. Front. Plant Sci. 2018, 9, 1834. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Liu, J.; Zhao, C.; Li, Z.; Huang, Y.; Yu, H. Unmanned aerial vehicle remote sensing for field-based crop phenotyping: Current status and perspectives. Front. Plant Sci. 2017, 8, 1111. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.A.; Ustin, S.L.; Ogunjemiyo, S.; Greenberg, J.; Dobrowski, S.Z.; Chen, J.Q.; Hinckley, T.M. Spectral and structural measures of northwest forest vegetation at leaf to landscape scales. Ecosystems 2004, 7, 545–562. [Google Scholar] [CrossRef]
- Jayme, G.A.B. Detection of nutrition deficiencies in plants using proximal images and machine learning: A review. Comput. Electron. Agric. 2019, 162, 482–492. [Google Scholar] [CrossRef]
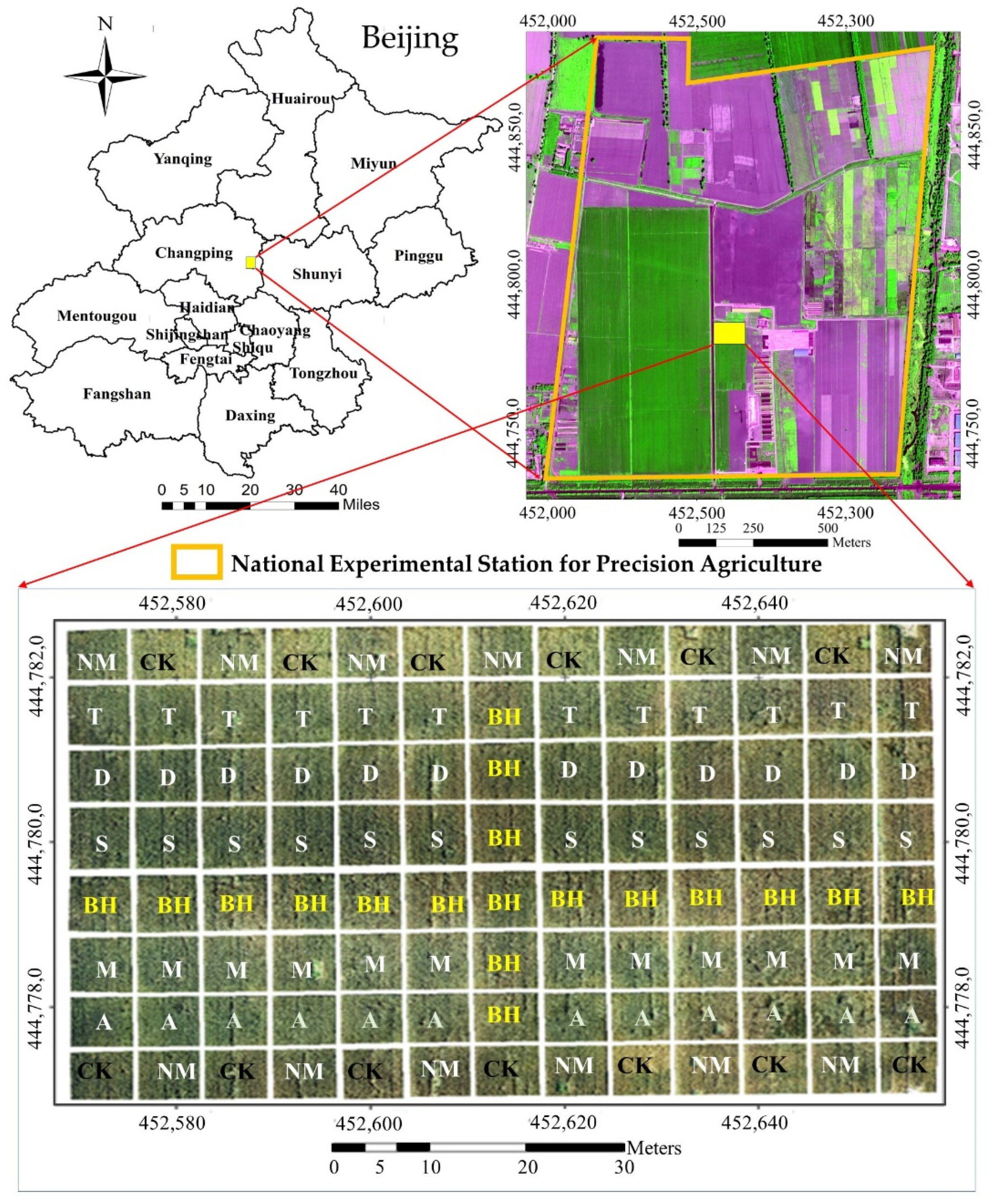

| Treatment | Plot | Base Fertilizer | Topdressing Fertilizer | Fertilizer Treatment Rate Statistic | |||||
|---|---|---|---|---|---|---|---|---|---|
| Number | Time | N kg/ha | Time | N kg/ha | Mean kg/ha | Min kg/ha | Max kg/ha | CV % | |
| BH | 18 | Seed | 72 | Feekes 2, 4 | 51,102 | 225 | 225 | 225 | 0 |
| NM | 13 | Seed | 72 | Feekes 6 | 78 | 150 | 150 | 150 | 0 |
| CK | 13 | Seed | 0 | Feekes 6 | 0 | 0 | 0 | 0 | 0 |
| A | 12 | Seed | 72 | Feekes 6 | 78 | 150 | 147 | 154.1 | 1.78 |
| M | 12 | Seed | 72 | Feekes 6 | 78 | 150 | 138.6 | 162.2 | 5.15 |
| D | 12 | Seed | 72 | Feekes 6 | 78 | 150 | 141.6 | 160 | 3.04 |
| S | 12 | Seed | 72 | Feekes 6 | 78 | 150 | 144.7 | 154.8 | 1.92 |
| T | 12 | Seed | 72 | Feekes 6 | 78 | 150 | 131 | 183.1 | 10.25 |
| Sensor Information | Polyphenol and Chlorophyll Meter | Polyphenol and Chlorophyll Meter | Field Spectrometer | UAV-Based Digital Camera |
|---|---|---|---|---|
| Sensor Type | Dualex | Multiplex | ASD | RGB Camera |
| Sensor name | Force-A Dualex Scientific | Force-A MULTIPLEX 3 | ASD FieldSpec 4 | Sony DSC–QX100 |
| Target sample | Plant leaves | Plant canopy | Plant canopy | Plant canopy |
| Field of view | - | - | 25° | 64° |
| Image size | - | - | - | 3000 × 4000 |
| Working height | - | 10 cm | 1.3 m | 50 m |
| Measurement area | 5 mm in diameter | 10 cm in diameter | 50 cm in diameter | Full field |
| Spectral information | Excitation channels: UV (357 nm) and red (650 nm). Detection channels: red and far-red. | Excitation channels: UV (375 nm), blue (450 nm), green (510 nm), and red (630 nm). Detection channels: yellow, red, and far-red. | 350–2500 nm | R,G,B |
| Original spectral resolution | - | - | 3 nm @ 700 nm; 10 nm @ 1400 nm | - |
| Data spectral resolution | 1 nm | |||
| Image spatial resolution | - | - | - | 2 cm |
| Sensor | ID | Vegetation Index | Formula | Reference |
|---|---|---|---|---|
| Multiplex | 1 | SFR_G | FRF_G/RF_G | [17] |
| 2 | SFR_R | FRF_R/RF_R | [17] | |
| 3 | BRR_FRF | BGF_UV/FRF_UV | [17] | |
| 4 | FER_RUV | FRF_R/FRF_UV | [17] | |
| 5 | FER_RG | FRF_R/FRF_G | [17] | |
| 6 | FLAV | Log(FER_RUV) | [17] | |
| 7 | ANTH | Log(FER_RG) | [17] | |
| 8 | NBI_G | FRF_UV/RF_G | [17] | |
| 9 | NBI_R | FRF_UV/RF_R | [17] | |
| ASD | 1 | SR(700,670) | (R700)/(R670) | [40] |
| 2 | SR(418,450) | (R418)/(R450) | [40] | |
| 3 | VOGa | (R740)/(R720) | [41] | |
| 4 | SR(553,537) | (R553)/(R537) | [42] | |
| 5 | NDCI | (R762 − R527)/(R762 + R527) | [41] | |
| 6 | NDRE | (R790 − R720)/(R790 + R720) | [43] | |
| 7 | TBI1 | (R434)/(R496 + R401) | [44] | |
| 8 | mND705 | (R750 − R705)/(R750 + R705 − 2R445) | [45] | |
| 9 | NDIopt | (R503 − R483)/(R503 + R483) | [46] | |
| 10 | TBI2 | (R924 − R703)/(R924 − R703) | [47] | |
| 11 | NDVI(670,780 | (R780 − R670)/(R780 + R670) | [48] | |
| 12 | RDVI | (R800 − R670)/(R800 + R670)1/2 | [49] | |
| 13 | SR(750,700) | (R750)/(R700) | [50] | |
| 14 | WI | (R900)/(R950) | [51] | |
| 15 | NDWI | (R860 − R1240)/(R860 + R1240) | [52] | |
| 16 | NDII | (R819 − R1600)/(R819 + R1600) | [53] | |
| 17 | MCARI | [(R700 − R670) − 0.2(R700 − R550)](R700/R670) | [54] | |
| 18 | TCARI | 3[(R700 − R670) − 0.2(R700 − R550)(R700/R670)] | [54] | |
| 19 | OSAVI | 1.16(R800 − R670)/(R800 + R670 + 0.16) | [55] | |
| 20 | MSAVI | 0.5[2R800 + 1 − ((2R800 + 1)2 − 8(R800 − R670))1/2] | [56] | |
| 21 | MCARI 1 | 1.2[2.5(R800 − R670) − 1.3(R800 − R550)] | [57] | |
| 22 | MCARI 2 | 1.5[2.5(R800 − R670) − 1.3(R800 − R550)]/[(2R800 + 1)2 − (6R800 − 5(R670)1/2) − 0.5]1/2 | [57] | |
| 23 | PPR | (R550 − R450)/(R550 + R450) | [58] | |
| 24 | PVR | (R550 − R650)/(R550 + R650) | [59] | |
| 25 | PRI | (R531 − R570)/(R531 + R570) | [60] | |
| 26 | REP | 700 + 40[(R670 + R780)/2) − R700)/(R740 − R700)] | [61] | |
| 27 | REV | Reflectance value at REP | [62] | |
| 28 | REFD | First deviation of red edge | [62] |
| ID | Vegetation Index | Full Name | Formula | Reference |
|---|---|---|---|---|
| 1 | R | DN values for red band | DNR/255 | [64] |
| 2 | G | DN values for green band | DNG/255 | [64] |
| 3 | B | DN values for blue band | DNB/255 | [64] |
| 4 | r | Chromatic coordinates for red | R/(R + G + B) | [64] |
| 5 | g | Chromatic coordinates for green | G/(R + G + B) | [64] |
| 6 | b | Chromatic coordinates for blue | B/(R + G + B) | [64] |
| 7 | ExR | Excess red | 1.4 × r − b | [65] |
| 8 | ExG | Excess green | 2g − (r + b) | [65] |
| 9 | NDI | The normalized difference vegetation index | (b − g)/(b + g) | [65] |
| 10 | CVI1 | Color vegetation index 1 | (r − g) | [65] |
| 11 | CVI2 | Color vegetation index 2 | (g − b) | [65] |
| 12 | CVI3 | Color vegetation index 3 | (g − b)/(r − g) | [65] |
| 13 | GRVI | Green–red vegetation index | (g − r)/(g + r) | [65] |
| 14 | NPCI | Normalized pigment chlorophyll ratio index | (b − r)/(b + r) | [66] |
| Statistics | Formula | Character | Reference |
|---|---|---|---|
| Root mean square error (RMSE) | from 0 to +∞, optimum 0 | [71] | |
| Mean absolute error (MAE) | from 0 to +∞, optimum 0 | [72] | |
| Nash–Sutcliffe efficiency (NSE) | from −∞ to 1, optimum 1 | [73] |
| Growth Stage | Parameter | Min | Max | Mean | Range | Std | CV (%) |
|---|---|---|---|---|---|---|---|
| Feekes 5 | LNC (%) | 3.07 | 5.16 | 4.33 | 2.09 | 0.49 | 11.30 |
| PNC (%) | 2.60 | 4.67 | 3.77 | 2.07 | 0.51 | 13.42 | |
| NNI | 0.74 | 1.58 | 1.14 | 0.84 | 0.19 | 17.17 | |
| Feekes 11 | LNC (%) | 1.08 | 2.99 | 2.32 | 1.92 | 0.31 | 13.42 |
| PNC (%) | 0.77 | 1.58 | 1.33 | 0.81 | 0.12 | 8.99 | |
| NNI | 0.44 | 1.17 | 0.89 | 0.73 | 0.11 | 11.97 | |
| Feekes 5–11 | LNC (%) | 1.08 | 5.16 | 3.33 | 4.08 | 1.08 | 32.34 |
| PNC (%) | 0.77 | 4.67 | 2.55 | 3.90 | 1.26 | 49.46 | |
| NNI | 0.44 | 1.58 | 1.00 | 1.14 | 0.20 | 18.59 |
| Sensor | Feekes Stage | LNC (%) | PNC (%) | NNI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | VI | R2 | RMSE | Model | VI | R2 | RMSE | Model | VI | R2 | RMSE | ||
| RGB | 5 | Poly | NPCI | 0.43 ** | 0.23 | Poly | ExR | 0.33 ** | 0.10 | Poly | ExR | 0.31 ** | 0.09 |
| 11 | Poly | NDI | 0.39 ** | 0.38 | Poly | NDI | 0.39 ** | 0.26 | Exp | NDI | 0.36 ** | 0.16 | |
| 5–11 | Poly | r | 0.61 ** | 0.68 | Pow | ExR | 0.62 ** | 0.40 | Log | ExR | 0.37 ** | 0.16 | |
| ASD | 5 | Poly | NDIopt | 0.19 | 0.44 | Poly | NDIopt | 0.19 | 0.30 | Poly | SR(553,537) | 0.30 ** | 0.16 |
| 11 | Poly | mND705 | 0.40 ** | 0.24 | Poly | NDRE | 0.46 ** | 0.09 | Poly | NDRE | 0.39 ** | 0.08 | |
| 5–11 | Poly | NDIopt | 0.85 ** | 0.43 | Poly | NDIopt | 0.84 ** | 0.26 | Log | NDIopt | 0.53 ** | 0.14 | |
| Multiplex | 5 | Poly | FLAV | 0.55 ** | 0.33 | Poly | FLAV | 0.52 ** | 0.24 | Poly | NBI_R | 0.42 ** | 0.15 |
| 11 | Poly | SFR_R | 0.34 ** | 0.25 | Poly | SFR_R | 0.23 | 0.11 | Poly | SFR_R | 0.21* | 0.10 | |
| 5–11 | Poly | BRR_FRF | 0.87 ** | 0.39 | Poly | BRR_FRF | 0.86 ** | 0.24 | Poly | NBI_G | 0.56 ** | 0.13 | |
| Dualex | 5 | Log | NBI | 0.80 ** | 0.22 | Pow | NBI | 0.79 ** | 0.23 | Lin | NBI | 0.49 ** | 0.14 |
| 11 | Poly | CHI | 0.36 ** | 0.27 | Log | CHI | 0.16 | 0.11 | Log | CHI | 0.20 * | 0.10 | |
| 5–11 | Poly | NBI | 0.57 ** | 0.72 | Poly | NBI | 0.50 ** | 0.92 | Exp | NBI | 0.46 ** | 0.15 | |
| N | Feekes | VI | GPR-SBBR | MLR | VIs | GPR | MLR | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Stage | R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | ||
| LNC | 5 | B, b | 0.44 | 0.37 | 0.43 | 0.37 | All 14 VIs | 0.41 | 0.37 | 0.28 | 0.43 |
| 11 | NPCI | 0.43 | 0.24 | 0.36 | 0.25 | All 14 VIs | 0.40 | 0.25 | 0.33 | 0.26 | |
| 5–11 | B, g, CVI2 | 0.82 | 0.46 | 0.62 | 0.67 | All 14 VIs | 0.81 | 0.47 | 0.68 | 0.62 | |
| PNC | 5 | B, b | 0.42 | 0.38 | 0.41 | 0.38 | All 14 VIs | 0.39 | 0.39 | 0.29 | 0.44 |
| 11 | G, NDI | 0.41 | 0.09 | 0.29 | 0.10 | All 14 VIs | 0.38 | 0.09 | 0.40 | 0.09 | |
| 5–11 | B, g, CVI2 | 0.89 | 0.43 | 0.59 | 0.82 | All 14 VIs | 0.87 | 0.45 | 0.67 | 0.74 | |
| NNI | 5 | B, b | 0.35 | 0.16 | 0.35 | 0.16 | All 14 VIs | 0.28 | 0.16 | 0.33 | 0.16 |
| 11 | R, NDI, CVI2 | 0.33 | 0.09 | 0.34 | 0.09 | All 14 VIs | 0.33 | 0.09 | 0.26 | 0.10 | |
| 5–11 | B, G, g, NDI, CVI2, CVI3 | 0.54 | 0.14 | 0.38 | 0.16 | All 14 VIs | 0.54 | 0.13 | 0.45 | 0.15 | |
| N | Feekes | VI | GPR-SBBR | MLR | VIs | GPR | MLR | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Stage | R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | ||
| LNC | 5 | TBI1, NDIopt, TBI2 | 0.47 | 0.36 | 0.31 | 0.41 | All 28 VIs | 0.28 | 0.42 | 0.34 | 0.42 |
| 11 | SR(700,670), SR(418,405), SR(740,720) | 0.51 | 0.22 | 0.33 | 0.25 | All 28 VIs | 0.39 | 0.26 | 0.42 | 0.26 | |
| 5–11 | SR(418,405), TBI1, PPR, PRI, REFD | 0.93 | 0.29 | 0.88 | 0.37 | All 28 VIs | 0.92 | 0.30 | 0.91 | 0.34 | |
| PNC | 5 | TBI1, NDIopt, TBI2 | 0.46 | 0.37 | 0.32 | 0.42 | All 28 VIs | 0.27 | 0.44 | 0.33 | 0.44 |
| 11 | SR(418,405), NDIopt, RDVI, REFD | 0.49 | 0.09 | 0.38 | 0.09 | All 28 VIs | 0.35 | 0.10 | 0.38 | 0.10 | |
| 5–11 | TBI1, PPR, REV, REFD | 0.96 | 0.27 | 0.89 | 0.42 | All 28 VIs | 0.95 | 0.28 | 0.94 | 0.31 | |
| NNI | 5 | MSAVI, PPR | 0.51 | 0.14 | 0.51 | 0.14 | All 28 VIs | 0.41 | 0.15 | 0.35 | 0.17 |
| 11 | NDWI, MCARI, MSAVI, PVR | 0.39 | 0.08 | 0.21 | 0.10 | All 28 VIs | 0.28 | 0.10 | 0.21 | 0.10 | |
| 5–11 | SR(418,405), NDIopt, MSAVI, MCARI1, PPR | 0.66 | 0.12 | 0.56 | 0.13 | All 28 VIs | 0.59 | 0.12 | 0.59 | 0.13 | |
| N | Feekes | VI | GPR-SBBR | MLR | VIs | GPR | MLR | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Stage | R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | ||
| LNC | 5 | SFR_G, FLAV, NBI_R | 0.59 | 0.31 | 0.59 | 0.31 | All 9 VIs | 0.57 | 0.31 | 0.58 | 0.32 |
| 11 | SFR_R | 0.44 | 0.28 | 0.27 | 0.27 | All 9 VIs | 0.39 | 0.30 | 0.16 | 0.29 | |
| 5–11 | SFR_R, BRR_FRF, NBI_R | 0.93 | 0.29 | 0.88 | 0.38 | All 9 VIs | 0.93 | 0.30 | 0.90 | 0.34 | |
| PNC | 5 | SFR_R, FLAV, NBI_R | 0.58 | 0.34 | 0.57 | 0.34 | All 9 VIs | 0.53 | 0.34 | 0.53 | 0.35 |
| 11 | SFR_R | 0.34 | 0.10 | 0.15 | 0.11 | All 9 VIs | 0.25 | 0.11 | 0.24 | 0.11 | |
| 5–11 | SFR_R, BRR_FRF, NBI_G | 0.96 | 0.25 | 0.90 | 0.41 | All 9 VIs | 0.96 | 0.26 | 0.93 | 0.35 | |
| NNI | 5 | FLAV, FER_RG | 0.52 | 0.34 | 0.52 | 0.14 | All 9 VIs | 0.52 | 0.14 | 0.44 | 0.15 |
| 11 | SFR_G, SFR_R | 0.31 | 0.09 | 0.24 | 0.09 | All 9 VIs | 0.18 | 0.10 | 0.25 | 0.10 | |
| 5–11 | SFR_G, SFR_R, NBI_G | 0.59 | 0.13 | 0.58 | 0.13 | All 9 VIs | 0.58 | 0.13 | 0.58 | 0.13 | |
| N | Feekes | VI | GPR-SBBR | MLR | VIs | GPR | MLR | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Stage | R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | ||
| LNC | 5 | NBI | 0.80 | 0.22 | 0.79 | 0.23 | All 3 VIs | 0.77 | 0.22 | 0.80 | 0.22 |
| 11 | Chl | 0.55 | 0.21 | 0.12 | 0.31 | All 3 VIs | 0.52 | 0.21 | 0.10 | 0.36 | |
| 5–11 | NBI, Chl | 0.83 | 0.43 | 0.60 | 0.69 | All 3 VIs | 0.83 | 0.43 | 0.74 | 0.56 | |
| PNC | 5 | NBI | 0.79 | 0.23 | 0.79 | 0.23 | All 3 VIs | 0.78 | 0.22 | 0.79 | 0.23 |
| 11 | Chl | 0.20 | 0.11 | 0.11 | 0.11 | All 3 VIs | 0.12 | 0.12 | 0.16 | 0.11 | |
| 5–11 | NBI, Chl | 0.82 | 0.55 | 0.55 | 0.86 | All 3 VIs | 0.81 | 0.54 | 0.67 | 0.74 | |
| NNI | 5 | NBI | 0.51 | 0.14 | 0.49 | 0.14 | All 3 VIs | 0.51 | 0.14 | 0.47 | 0.14 |
| 11 | Chl | 0.27 | 0.10 | 0.10 | 0.10 | All 3 VIs | 0.22 | 0.10 | 0.26 | 0.09 | |
| 5–11 | NBI, Chl | 0.60 | 0.13 | 0.42 | 0.15 | All 3 VIs | 0.59 | 0.13 | 0.48 | 0.15 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Yang, G.; Xu, X.; Zhang, D.; Yang, C.; Feng, H. Winter Wheat Nitrogen Estimation Based on Ground-Level and UAV-Mounted Sensors. Sensors 2022, 22, 549. https://doi.org/10.3390/s22020549
Song X, Yang G, Xu X, Zhang D, Yang C, Feng H. Winter Wheat Nitrogen Estimation Based on Ground-Level and UAV-Mounted Sensors. Sensors. 2022; 22(2):549. https://doi.org/10.3390/s22020549
Chicago/Turabian StyleSong, Xiaoyu, Guijun Yang, Xingang Xu, Dongyan Zhang, Chenghai Yang, and Haikuan Feng. 2022. "Winter Wheat Nitrogen Estimation Based on Ground-Level and UAV-Mounted Sensors" Sensors 22, no. 2: 549. https://doi.org/10.3390/s22020549
APA StyleSong, X., Yang, G., Xu, X., Zhang, D., Yang, C., & Feng, H. (2022). Winter Wheat Nitrogen Estimation Based on Ground-Level and UAV-Mounted Sensors. Sensors, 22(2), 549. https://doi.org/10.3390/s22020549







