Recent Trends in the Improvement of the Electrochemical Response of Screen-Printed Electrodes by Their Modification with Shaped Metal Nanoparticles
Abstract
1. Introduction
2. SPEs Modification by Physical Approaches
2.1. Drop-Casting Method
2.2. Spin Coating
2.3. Spray Coating
2.4. Sputter Coating
2.5. Electrospray
2.6. Chemical and Electrochemical Deposition
2.7. Ink Mixing and Printing Method
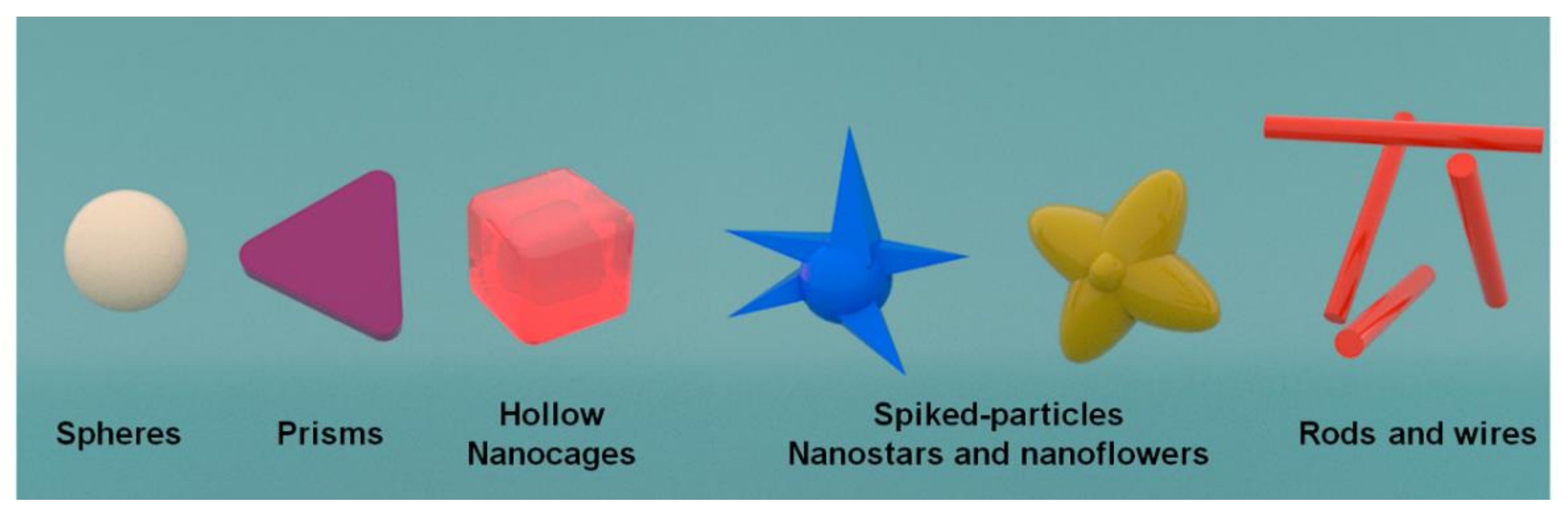
3. SPEs Modification with Morphologically Different NPs Systems
3.1. Spherical Nanoparticles
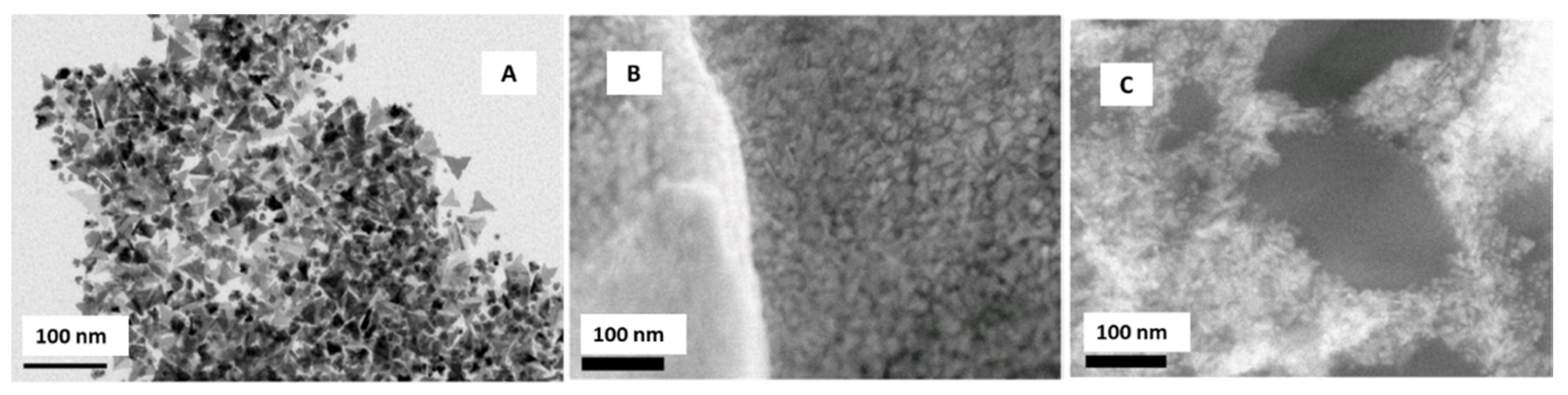
3.2. Triangle Shaped Nanoparticles
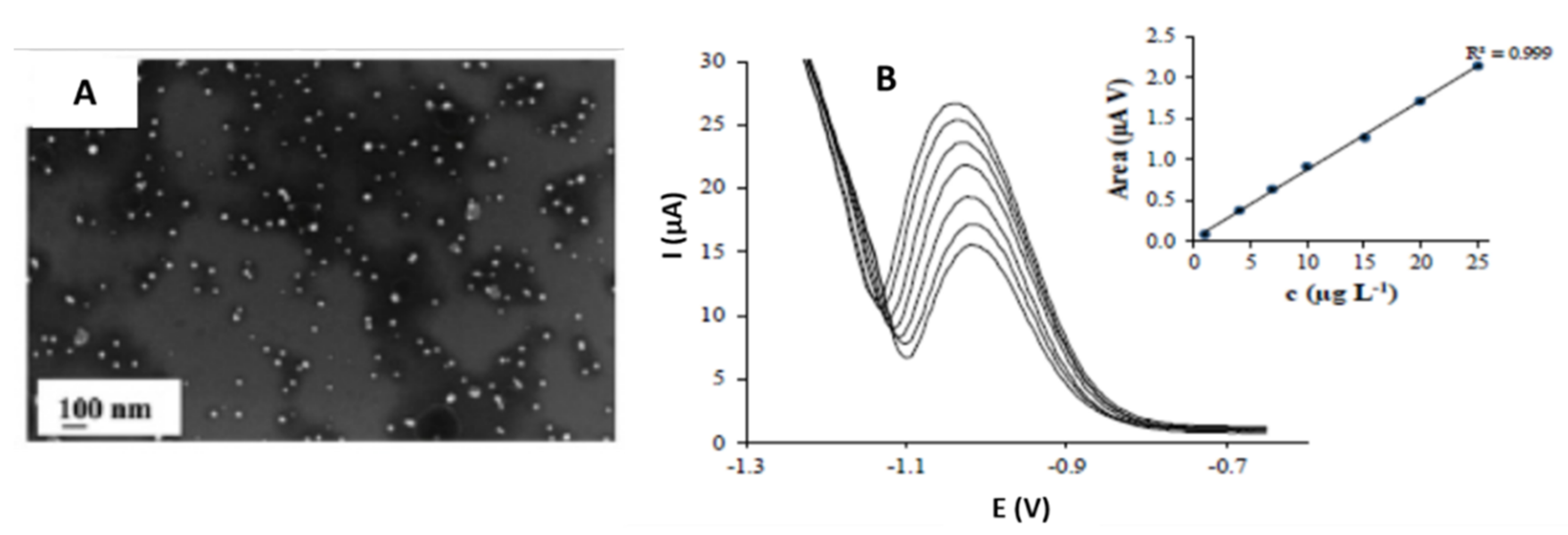
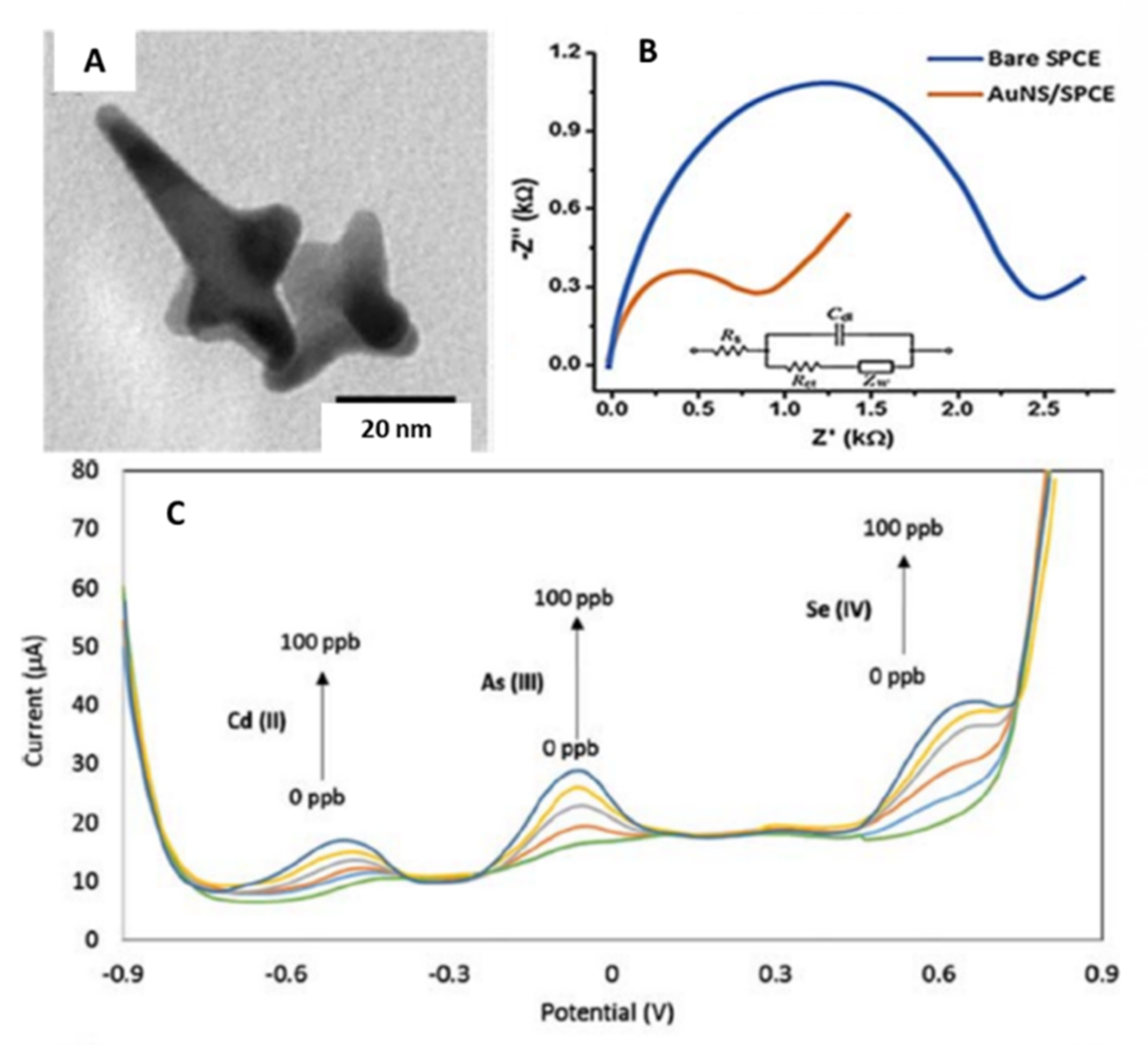
3.3. Star-Shaped Nanoparticles
3.4. Nanoflowers Shaped Nanoparticles
3.5. Nanowires
3.6. Nanocages
3.7. Nanocubes
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Economou, A. Screen-printed electrodes modified with “green” metals for electrochemical stripping analysis of toxic elements. Sensors 2018, 18, 1032. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.T.; Li, D.W.; Long, Y.T. Recent developments and applications of screen-printed electrodes in environmental assays—A review. Anal. Chim. Acta 2012, 734, 31–44. [Google Scholar] [CrossRef]
- Antuña-Jiménez, D.; González-García, M.B.; Hernández-Santos, D.; Fanjul-Bolado, P. Screen-printed electrodes modified with metal nanoparticles for small molecule sensing. Biosensors 2020, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ràfols, C.; Bastos-Arrieta, J.; Serrano, N.; Díaz-Cruz, J.M.; Ariño, C.; de Pablo, J.; Esteban, M. Ag nanoparticles drop-casting modification of screen-printed electrodes for the simultaneous voltammetric determination of Cu(II) and Pb(II). Sensors 2017, 17, 1458. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rivero, K.; Torralba-Cadena, L.; Espriu-Gascon, A.; Casas, I.; Bastos-Arrieta, J.; Florido, A. Strategies for Surface Modification with Ag-Shaped Nanoparticles: Electrocatalytic Enhancement of Screen-Printed Electrodes for the Detection of Heavy Metals. Sensors 2019, 19, 4249. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.; Schiøtz, J.; Liu, P.; Nørskov, J.K.; Stimming, U. Nano-scale effects in electrochemistry. Chem. Phys. Lett. 2004, 390, 440–444. [Google Scholar] [CrossRef]
- Campelo, J.M.; Luna, D.; Luque, R.; Marinas, J.M.; Romero, A.A. Sustainable preparation of supported metal nanoparticles and their applications in catalysis. ChemSusChem 2009, 2, 18–45. [Google Scholar] [CrossRef]
- Savage, N.; Diallo, M.S.; Duncan, J.; Street, A.; Sustich, R. Nanotechnology Applications for Clean Water; William Andrew Inc: Nueva York, NY, USA, 2009; ISBN 978-0-8155-1578-4. [Google Scholar]
- Murray, R.W. Nanoelectrochemistry: Metal nanoparticles, nanoelectrodes, and nanopores. Chem. Rev. 2008, 108, 2688–2720. [Google Scholar] [CrossRef]
- Li, C.M.; Hu, W. Electroanalysis in micro- and nano-scales. J. Electroanal. Chem. 2013, 688, 20–31. [Google Scholar] [CrossRef]
- Jirasirichote, A.; Punrat, E.; Suea-Ngam, A.; Chailapakul, O.; Chuanuwatanakul, S. Voltammetric detection of carbofuran determination using screen-printed carbon electrodes modified with gold nanoparticles and graphene oxide. Talanta 2017, 175, 331–337. [Google Scholar] [CrossRef]
- Shi, Z.; Lu, Y.; Chen, Z.; Cheng, C.; Xu, J.; Zhang, Q.; Yan, Z.; Luo, Z.; Liu, Q. Electrochemical non-enzymatic sensing of glycoside toxins by boronic acid functionalized nano-composites on screen-printed electrode. Sens. Actuators B Chem. 2021, 329, 129197. [Google Scholar] [CrossRef]
- Arduini, F.; Scognamiglio, V.; Covaia, C.; Amine, A.; Moscone, D.; Palleschi, G. A choline oxidase amperometric bioassay for the detection of mustard agents based on screen-printed electrodes modified with prussian blue nanoparticles. Sensors 2015, 15, 4353–4367. [Google Scholar] [CrossRef] [PubMed]
- Gevaerd, A.; Banks, C.E.; Bergamini, M.F.; Marcolino-Junior, L.H. Nanomodified Screen-Printed Electrode for direct determination of Aflatoxin B1 in malted barley samples. Sens. Actuators B Chem. 2020, 307, 1–7. [Google Scholar] [CrossRef]
- Shim, K.; Kim, J.; Shahabuddin, M.; Yamauchi, Y.; Hossain, M.S.A.; Kim, J.H. Efficient wide range electrochemical bisphenol-A sensor by self-supported dendritic platinum nanoparticles on screen-printed carbon electrode. Sens. Actuators B Chem. 2018, 255, 2800–2808. [Google Scholar] [CrossRef]
- Goia, D.V.; Matijević, E. Preparation of monodispersed metal particles. New J. Chem. 1998, 22, 1203–1215. [Google Scholar] [CrossRef]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Seed-Mediated Growth Approach for Shape-Controlled Synthesis of Spheroidal and Rod-like Gold Nanoparticles Using a Surfactant Template. Adv. Mater. 2001, 13, 1389–1393. [Google Scholar] [CrossRef]
- Lee, H.; Jang, Y.; Seo, J.; Nam, J.-M.; Char, K. Nanoparticle-Functionalized Polymer Platform for Controlling Metastatic Cancer Cell Adhesion, Shape, and Motility. ACS Nano 2011, 5, 5444–5456. [Google Scholar] [CrossRef]
- Torimoto, T.; Kamiya, Y.; Kameyama, T.; Nishi, H.; Uematsu, T.; Kuwabata, S.; Shibayama, T. Controlling Shape Anisotropy of ZnS–AgInS 2 Solid Solution Nanoparticles for Improving Photocatalytic Activity. ACS Appl. Mater. Interfaces 2016, 8, 27151–27161. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.G.M.; Rodrigues, T.S.; Slater, T.J.A.; Lewis, E.A.; Alves, R.S.; Fajardo, H.V.; Balzer, R.; da Silva, A.H.M.; de Freitas, I.C.; Oliveira, D.C.; et al. Controlling Size, Morphology, and Surface Composition of AgAu Nanodendrites in 15 s for Improved Environmental Catalysis under Low Metal Loadings. ACS Appl. Mater. Interfaces 2015, 7, 25624–25632. [Google Scholar] [CrossRef]
- Tiano, A.L.; Koenigsmann, C.; Santulli, A.C.; Wong, S.S. Solution-based synthetic strategies for one-dimensional metal-containing nanostructures. Chem. Commun. 2010, 46, 8093–8130. [Google Scholar] [CrossRef]
- Muñoz-Rojas, D.; Oró-Solé, J.; Ayyad, O.; Gómez-Romero, P. Facile One-Pot Synthesis of Self-Assembled Silver@Polypyrrole Core/Shell Nanosnakes. Small 2008, 4, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Domenech, B.; Bastos-Arrieta, J.; Alonso, A.; Macanas, J.; Munoz, M.; Muraviev, D. Bifunctional Polymer-Metal Nanocomposite Ion Exchange Materials. In Ion Exchange Technologies; Kilislioglu, A., Ed.; InTech: Rijeka, Croatia, 2012; pp. 35–72. ISBN 978-953-51-0836-8. [Google Scholar]
- Donnan, F.G. Theory of membrane equilibria and membrane potentials in the presence of non-dialysing electrolytes. A contribution to physical-chemical physiology. J. Memb. Sci. 1995, 100, 45–55. [Google Scholar] [CrossRef]
- Nagarajan, R.; Hatton, T. Nanoparticles: Synthesis, Stabilization, Passivation, and Functionalization; Nagarajan, R., Hatton, T.A., Eds.; American Chemical Society: Washington, DC, USA, 2008; Volume 996, ISBN 9780841269699. [Google Scholar]
- Yang, M.; Yang, Y.; Liu, Y.; Shen, G.; Yu, R. Platinum nanoparticles-doped sol-gel/carbon nanotubes composite electrochemical sensors and biosensors. Biosens. Bioelectron. 2006, 21, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic nanoparticles: Design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef]
- Luque, R.; Balu, A.M.; Campelo, J.M.; Gonzalez-Arellano, C.; Gracia, M.J.; Luna, D.; Marinas, J.M.; Romero, A.A. Tunable shapes in supported metal nanoparticles: From nanoflowers to nanocubes. Mater. Chem. Phys. 2009, 117, 408–413. [Google Scholar] [CrossRef]
- Bastos-Arrieta, J.; Muñoz, J.; Vigués, N.; Muraviev, D.N.; Céspedes, F.; Mas, J.; Baeza, M.; Muñoz, M. Intermatrix synthesis of Ag, AgAu and Au nanoparticles by the galvanic replacement strategy for bactericidal and electrocatalytically active nanocomposites. New J. Chem. 2016, 40, 10344–10352. [Google Scholar] [CrossRef]
- Sun, Y. Shape-Controlled Synthesis of Gold and Silver Nanoparticles. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef]
- Balan, L.; Melinte, V.; Buruiana, T.; Schneider, R.; Vidal, L. Controlling the morphology of gold nanoparticles synthesized photochemically in a polymer matrix through photonic parameters. Nanotechnology 2012, 23, 415705. [Google Scholar] [CrossRef]
- Langille, M.R.; Personick, M.L.; Zhang, J.; Mirkin, C.A. Defining rules for the shape evolution of gold nanoparticles. J. Am. Chem. Soc. 2012, 134, 14542–14554. [Google Scholar] [CrossRef]
- Lohse, S.E.; Burrows, N.D.; Scarabelli, L.; Liz-Marzán, L.M.; Murphy, C.J. Anisotropic noble metal nanocrystal growth: The role of halides. Chem. Mater. 2014, 26, 34–43. [Google Scholar] [CrossRef]
- Thota, S.; Crans, D.C. Metal Nanoparticles; Wiley-VCH GmbH & Co. KGaA: Weinheim, Germany, 2018; ISBN 9783527807093. [Google Scholar]
- Abdullaeva, Z. Synthesis of Nanoparticles and Nanomaterials; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-54074-0. [Google Scholar]
- Woehl, T.J.; Evans, J.E.; Arslan, I.; Ristenpart, W.D.; Browning, N.D. Direct in Situ Determination of the Mechanisms Controlling Nanoparticle Nucleation and Growth. ACS Nano 2012, 6, 8599–8610. [Google Scholar] [CrossRef]
- Personick, M.L.; Langille, M.R.; Zhang, J.; Mirkin, C.A. Shape control of gold nanoparticles by silver underpotential deposition. Nano Lett. 2011, 11, 3394–3398. [Google Scholar] [CrossRef]
- Bastos-Arrieta, J.; Montes, R.; Ocaña, C.; Espinoza, M.; Muñoz, M.; Baeza, M. In Situ Characterization of Size, Spatial Distribution, Chemical Composition, and Electroanalytical Response of Hybrid Nanocomposite Materials. In In-Situ Characterization Techniques for Nanomaterials; Springer: Berlin/Heidelberg, Gemany, 2018; pp. 251–288. ISBN 9783662563229. [Google Scholar]
- Singh, M.; Jaiswal, N.; Tiwari, I.; Foster, C.W.; Banks, C.E. A reduced graphene oxide-cyclodextrin-platinum nanocomposite modified screen printed electrode for the detection of cysteine. J. Electroanal. Chem. 2018, 829, 230–240. [Google Scholar] [CrossRef]
- Cunha-Silva, H.; Arcos-Martinez, M.J. A disposable rhodium nanoparticle-modified screen-printed sensor for direct determination of bromide anions. Sens. Actuators B Chem. 2019, 282, 603–608. [Google Scholar] [CrossRef]
- Baradoke, A.; Pastoriza-Santos, I.; González-Romero, E. Screen-printed GPH electrode modified with Ru nanoplates and PoPD polymer film for NADH sensing: Design and characterization. Electrochim. Acta 2019, 300, 316–323. [Google Scholar] [CrossRef]
- Torres-Rivero, K.; Pérez-Ràfols, C.; Bastos-Arrieta, J.; Florido, A.; Martí, V.; Serrano, N. Direct As(V) determination using screen-printed electrodes modified with silver nanoparticles. Nanomaterials 2020, 10, 1280. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Sullivan, C.; Brack, E.M.; Drew, C.P.; Kurup, P. Simultaneous voltammetric detection of cadmium(II), arsenic(III), and selenium(IV) using gold nanostar–modified screen-printed carbon electrodes and modified Britton-Robinson buffer. Anal. Bioanal. Chem. 2020, 412, 4113–4125. [Google Scholar] [CrossRef]
- Dutta, S.; Strack, G.; Kurup, P. Gold nanostar-based voltammetric sensor for chromium(VI). Microchim. Acta 2019, 186, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Su, J.; Zeng, D.; Liu, G.; Liu, L.; Xu, Y.; Wang, C.; Liu, X.; Wang, L.; Mi, X. Gold nano-flowers (Au NFs) modified screen-printed carbon electrode electrochemical biosensor for label-free and quantitative detection of glycated hemoglobin. Talanta 2019, 201, 119–125. [Google Scholar] [CrossRef]
- Rezaei, R.; Foroughi, M.M.; Beitollahi, H.; Tajik, S.; Jahani, S. Synthesis of lanthanium-doped ZnO nanoflowers: Supported on graphite screen printed electrode for selective and sensitive detection of hydrochlorothiazide. Int. J. Electrochem. Sci. 2019, 14, 2038–2048. [Google Scholar] [CrossRef]
- Kabir, M.F.; Rahman, M.T.; Gurung, A.; Qiao, Q. Electrochemical Phosphate Sensors Using Silver Nanowires Treated Screen Printed Electrodes. IEEE Sens. J. 2018, 18, 3480–3485. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, G.; Chu, G.; An, X.; Guo, Y.; Sun, X. The development of a novel biosensor based on gold nanocages/graphene oxide–chitosan modified acetylcholinesterase for organophosphorus pesticide detection. New J. Chem. 2019, 43, 13816–13826. [Google Scholar] [CrossRef]
- Mahmoudi-Moghaddam, H.; Tajik, S.; Beitollahi, H. Highly sensitive electrochemical sensor based on La3+ doped Co3O4 nanocubes for determination of sudan I content in food samples. Food Chem. 2019, 286, 191–196. [Google Scholar] [CrossRef]
- Khorshed, A.A.; Khairy, M.; Elsafty, S.A.; Banks, C.E. Disposable screen-printed electrodes modified with uniform iron oxide nanocubes for the simple electrochemical determination of meclizine, an antihistamine drug. Anal. Methods 2019, 11, 282–287. [Google Scholar] [CrossRef]
- Dhara, K.; Thiagarajan, R.; Nair, B.G.; Thekkedath, G.S.B. Highly sensitive and wide-range nonenzymatic disposable glucose sensor based on a screen printed carbon electrode modified with reduced graphene oxide and Pd-CuO nanoparticles. Microchim. Acta 2015, 182, 2183–2192. [Google Scholar] [CrossRef]
- Cadevall, M.; Ros, J.; Merkoçi, A. Bismuth nanoparticles integration into heavy metal electrochemical stripping sensor. Electrophoresis 2015, 36, 1872–1879. [Google Scholar] [CrossRef]
- Cinti, S.; Politi, S.; Moscone, D.; Palleschi, G.; Arduini, F. Stripping Analysis of As(III) by means of screen-printed electrodes modified with gold nanoparticles and carbon black nanocomposite. Electroanalysis 2014, 26, 931–939. [Google Scholar] [CrossRef]
- Khue, V.Q.; Huy, T.Q.; Phan, V.N.; Tuan-Le, A.; Thanh Le, D.T.; Tonezzer, M.; Hong Hanh, N.T. Electrochemical stability of screen-printed electrodes modified with Au nanoparticles for detection of methicillin-resistant Staphylococcus aureus. Mater. Chem. Phys. 2020, 255, 123562. [Google Scholar] [CrossRef]
- Kaliyaraj Selva Kumar, A.; Zhang, Y.; Li, D.; Compton, R.G. A mini-review: How reliable is the drop casting technique? Electrochem. Commun. 2020, 121, 106867. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Sami Yilbas, B.; Al-Sharafi, A.; Ali, H. Surfaces for Self-Cleaning. In Self-Cleaning of Surfaces and Water Droplet Mobility; Sami Yilbas, B., Al-Sharafi, A., Ali, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 45–98. ISBN 9780128147764. [Google Scholar]
- Mishra, A.; Batt, N.; Bajpai, A.K. Nanostructured superhydrophobic coatings for solar panel applications. In Nanomaterials-Based Coatings; Nguyen Tri, P., Rtimi, S., Ouellet Plamondon, C.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 397–424. ISBN 978-0-12-815884-5. [Google Scholar]
- Zhang, J.X.J.; Hoshino, K. Fundamentals of nano/microfabrication and scale effect. In Molecular Sensors and Nanodevices; Zhang, J.X.J., Hoshino, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 43–111. ISBN 978-0-12-814862-4. [Google Scholar]
- Chomoucka, J.; Prasek, J.; Businova, P.; Trnkova, L.; Drbohlavova, J.; Pekarek, J.; Hrdy, R.; Hubalek, J. Novel Electrochemical Biosensor for Simultaneous Detection of Adenine and Guanine Based on Cu2O Nanoparticles. Procedia Eng. 2012, 47, 702–705. [Google Scholar] [CrossRef]
- Mayousse, C.; Celle, C.; Moreau, E.; Mainguet, J.-F.; Carella, A.; Simonato, J.-P. Improvements in purification of silver nanowires by decantation and fabrication of flexible transparent electrodes. Application to capacitive touch sensors. Nanotechnology 2013, 24, 215501. [Google Scholar] [CrossRef] [PubMed]
- Crystals, W. Delivery of Nanoparticles on Surfaces. In Fundamentals and Applications of Nano Silicon in Plasmonics and Fullerines; Elsevier: Amsterdam, The Netherlands, 2018; pp. 341–362. ISBN 9780323480574. [Google Scholar]
- Girotto, C.; Rand, B.P.; Steudel, S.; Genoe, J.; Heremans, P. Nanoparticle-based, spray-coated silver top contacts for efficient polymer solar cells. Org. Electron. 2009, 10, 735–740. [Google Scholar] [CrossRef]
- Akhtar, M.A.; Batool, R.; Hayat, A.; Han, D.; Riaz, S.; Khan, S.U.; Nasir, M.; Nawaz, M.H.; Niu, L. Functionalized Graphene Oxide Bridging between Enzyme and Au-Sputtered Screen-Printed Interface for Glucose Detection. ACS Appl. Nano Mater. 2019, 2, 1589–1596. [Google Scholar] [CrossRef]
- Gasparotto, G.; Costa, J.P.C.; Costa, P.I.; Zaghete, M.A.; Mazon, T. Electrochemical immunosensor based on ZnO nanorods-Au nanoparticles nanohybrids for ovarian cancer antigen CA-125 detection. Mater. Sci. Eng. C 2017, 76, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.H. Sputter Processing. In Handbook of Thin Film Deposition; Elsevier: Amsterdam, The Netherlands, 2018; pp. 195–230. ISBN 9781437778731. [Google Scholar]
- Zhang, S.; Kawakami, K. One-step preparation of chitosan solid nanoparticles by electrospray deposition. Int. J. Pharm. 2010, 397, 211–217. [Google Scholar] [CrossRef]
- Mettakoonpitak, J.; Mehaffy, J.; Volckens, J.; Henry, C.S. AgNP/Bi/Nafion-modified Disposable Electrodes for Sensitive Zn(II), Cd(II), and Pb(II) Detection in Aerosol Samples. Electroanalysis 2017, 29, 880–889. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, C.; Gai, Z.; Yu, S.; Ren, X. Chemical vapor deposition and its application in surface modification of nanoparticles. Chem. Pap. 2020, 74, 767–778. [Google Scholar] [CrossRef]
- Cinti, S.; Arduini, F.; Vellucci, G.; Cacciotti, I.; Nanni, F.; Moscone, D. Carbon black assisted tailoring of Prussian Blue nanoparticles to tune sensitivity and detection limit towards H2O2 by using screen-printed electrode. Electrochem. Commun. 2014, 47, 63–66. [Google Scholar] [CrossRef]
- Mohanty, U.S. Electrodeposition: A versatile and inexpensive tool for the synthesis of nanoparticles, nanorods, nanowires, and nanoclusters of metals. J. Appl. Electrochem. 2011, 41, 257–270. [Google Scholar] [CrossRef]
- Dominguez Renedo, O.; Ruiz Espelt, L.; García Astorgano, N.; Arcos Martinez, M.J. Electrochemical determination of chromium(VI) using metallic nanoparticle-modified carbon screen-printed electrodes. Talanta 2008, 76, 854–858. [Google Scholar] [CrossRef] [PubMed]
- Sanllorente-Méndez, S.; Domínguez-Renedo, O.; Arcos-Martínez, M.J. Determination of arsenic(III) using platinum nanoparticle-modified screen-printed carbon-based electrodes. Electroanalysis 2009, 21, 635–639. [Google Scholar] [CrossRef]
- Pérez-Fernández, B.; Martín-Yerga, D.; Costa-García, A. Galvanostatic electrodeposition of copper nanoparticles on screen-printed carbon electrodes and their application for reducing sugars determination. Talanta 2017, 175, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, B.; Fakhari, A.R. Electrocatalytic oxidation and determination of insulin at nickel oxide nanoparticles-multiwalled carbon nanotube modified screen printed electrode. Biosens. Bioelectron. 2013, 46, 130–135. [Google Scholar] [CrossRef]
- González-Sánchez, M.I.; Gómez-Monedero, B.; Agrisuelas, J.; Iniesta, J.; Valero, E. Electrochemical performance of activated screen printed carbon electrodes for hydrogen peroxide and phenol derivatives sensing. J. Electroanal. Chem. 2019, 839, 75–82. [Google Scholar] [CrossRef]
- Kubendhiran, S.; Sakthinathan, S.; Chen, S.M.; Lee, C.M.; Lou, B.S.; Sireesha, P.; Su, C.C. Electrochemically Activated Screen Printed Carbon Electrode Decorated with Nickel Nano Particles for the Detection of Glucose in Human Serum and Human Urine Sample. Int. J. Electrochem. Sci. 2016, 11, 7934–7946. [Google Scholar] [CrossRef]
- Wang, J.; Pedrero, M.; Sakslund, H.; Hammerich, O.; Pingarron, J. Electrochemical activation of screen-printed carbon strips. Analyst 1996, 121, 345. [Google Scholar] [CrossRef]
- Niu, P.; Fernández-Sánchez, C.; Gich, M.; Navarro-Hernández, C.; Fanjul-Bolado, P.; Roig, A. Screen-printed electrodes made of a bismuth nanoparticle porous carbon nanocomposite applied to the determination of heavy metal ions. Microchim. Acta 2016, 183, 617–623. [Google Scholar] [CrossRef]
- Jadav, J.K.; Umrania, V.V.; Rathod, K.J.; Golakiya, B.A. Development of silver/carbon screen-printed electrode for rapid determination of vitamin C from fruit juices. LWT 2018, 88, 152–158. [Google Scholar] [CrossRef]
- Ghosale, A.; Shrivas, K.; Deb, M.K.; Ganesan, V.; Karbhal, I.; Bajpai, P.K.; Shankar, R. A low-cost screen printed glass electrode with silver nano-ink for electrochemical detection of H2O2. Anal. Methods 2018, 10, 3248–3255. [Google Scholar] [CrossRef]
- Ali, T.A.; Mohamed, G.G. Potentiometric determination of La(III) in polluted water samples using modified screen-printed electrode by self-assembled mercapto compound on silver nanoparticles. Sens. Actuators B Chem. 2015, 216, 542–550. [Google Scholar] [CrossRef]
- Deng, M.; Zhang, X.; Zhang, Z.; Xin, Z.; Song, Y. A Gold Nanoparticle Ink Suitable for the Fabrication of Electrochemical Electrode by Inkjet Printing. J. Nanosci. Nanotechnol. 2014, 14, 5114–5119. [Google Scholar] [CrossRef] [PubMed]
- Aherne, D.; Ledwith, D.M.; Gara, M.; Kelly, J.M. Optical properties and growth aspects of silver nanoprisms produced by a highly reproducible and rapid synthesis at room temperature. Adv. Funct. Mater. 2008, 18, 2005–2016. [Google Scholar] [CrossRef]
- Aherne, D.; Gara, M.; Kelly, J.M.; Gun’Ko, Y.K. From Ag Nanoprisms to Triangular AuAg Nanoboxes. Adv. Funct. Mater. 2010, 20, 1329–1338. [Google Scholar] [CrossRef]
- Habib, A.; Tabata, M.; Wu, Y.G. Formation of Gold Nanoparticles by Good’s Buffers. Bull. Chem. Soc. Jpn. 2005, 78, 262–269. [Google Scholar] [CrossRef]
- Dutta, S.; Strack, G.; Kurup, P. Gold nanostar electrodes for heavy metal detection. Sens. Actuators B Chem. 2019, 281, 383–391. [Google Scholar] [CrossRef]
- Song, S.Y.; Han, Y.D.; Park, Y.M.; Jeong, C.Y.; Yang, Y.J.; Kim, M.S.; Ku, Y.; Yoon, H.C. Bioelectrocatalytic detection of glycated hemoglobin (HbA1c) based on the competitive binding of target and signaling glycoproteins to a boronate-modified surface. Biosens. Bioelectron. 2012, 35, 355–362. [Google Scholar] [CrossRef]
- Cohen, R.M.; Haggerty, S.; Herman, W.H. HbA1c for the Diagnosis of Diabetes and Prediabetes: Is It Time for a Mid-Course Correction? J. Clin. Endocrinol. Metab. 2010, 95, 5203–5206. [Google Scholar] [CrossRef]
- González-Vargas, C.; Serrano, N.; Ariño, C.; Salazar, R.; Esteban, M.; Díaz-Cruz, J.M. Voltammetric Determination of Anti-Hypertensive Drug Hydrochlorothiazide Using Screen-Printed Electrodes Modified with L-Glutamic Acid. Chemosensors 2017, 5, 25. [Google Scholar] [CrossRef]
- Heli, H.; Pishahang, J.; Amiri, H.B.; Sattarahmady, N. Synthesis of nickel nanowrinkles and its application for the electrocatalytic oxidation and sensitive detection of hydrochlorothiazide. Microchem. J. 2017, 130, 205–212. [Google Scholar] [CrossRef]
- Jansi Rani, B.; Babu, E.S.; Praveenkumar, M.; Ravichandran, S.; Ravi, G.; Yuvakkumar, R. Morphology-Dependent Photoelectrochemical and Photocatalytic Performance of γ-Bi2O3 Nanostructures. J. Nanosci. Nanotechnol. 2019, 20, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Korte, K.E.; Skrabalak, S.E.; Xia, Y. Rapid synthesis of silver nanowires through a CuCl− or CuCl2−mediated polyol process. J. Mater. Chem. 2008, 18, 437–441. [Google Scholar] [CrossRef]
- Skrabalak, S.E.; Chen, J.; Sun, Y.; Lu, X.; Au, L.; Cobley, C.M.; Xia, Y. Gold Nanocages: Synthesis, Properties, and Applications. Acc. Chem. Res. 2008, 41, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, E. Biotransformation of Individual Pesticides. In Pesticide Biotransformation and Disposition; Elsevier: Amsterdam, The Netherlands, 2012; pp. 195–208. ISBN 9780123854810. [Google Scholar]
- Kato, K.; Dang, F.; Mimura, K.; Kinemuchi, Y.; Imai, H.; Wada, S.; Osada, M.; Haneda, H.; Kuwabara, M. Nano-sized cube-shaped single crystalline oxides and their potentials; composition, assembly and functions. Adv. Powder Technol. 2014, 25, 1401–1414. [Google Scholar] [CrossRef]
- Nano Composix Silver Nanocubes. Available online: https://nanocomposix.com/pages/silver-nanocubes (accessed on 9 February 2021).
- Patra, S.; Roy, E.; Madhuri, R.; Sharma, P.K. A technique comes to life for security of life: The food contaminant sensors. In Nanobiosensors: Nanotechnology in the Agri-Food Industry; Grumezescu, A., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 713–772. ISBN 9780128043721. [Google Scholar]
- Agència Catalana de Seguretat Alimentària Colorants Sudan. Available online: http://acsa.gencat.cat/ca/detall/article/Colorantes-Sudan (accessed on 9 February 2021).
- Center Memorial Sloan Kettering Cancer Meclizine. Available online: https://www.mskcc.org/cancer-care/patient-education/meclizine-01 (accessed on 17 March 2021).


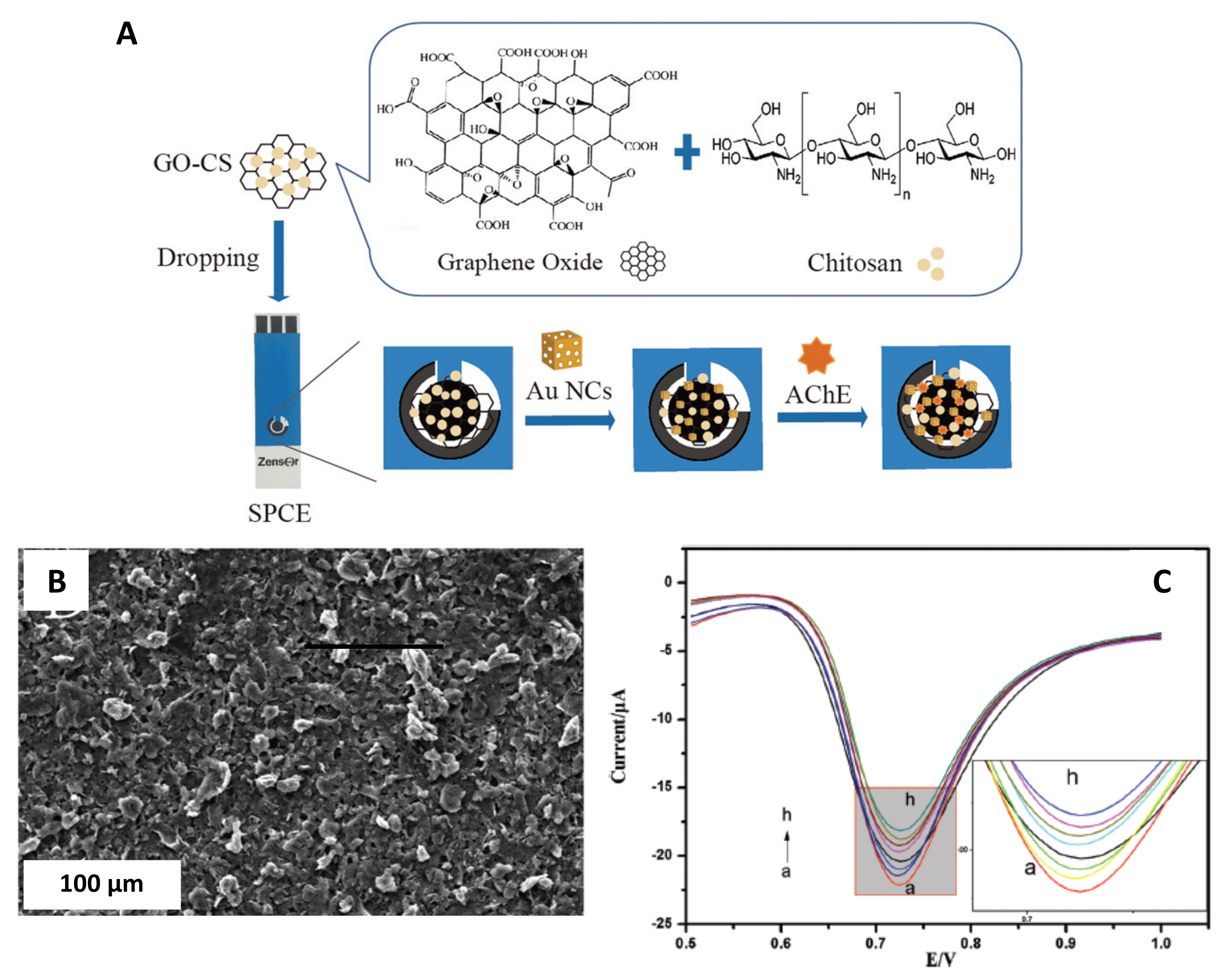
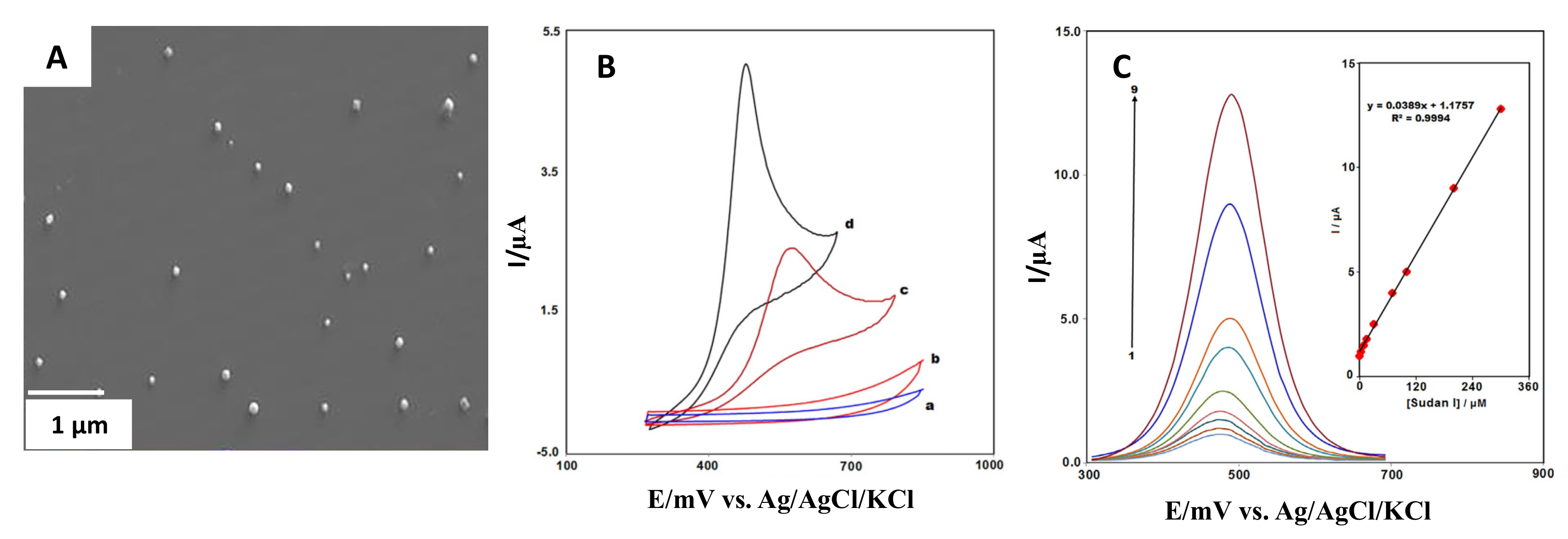
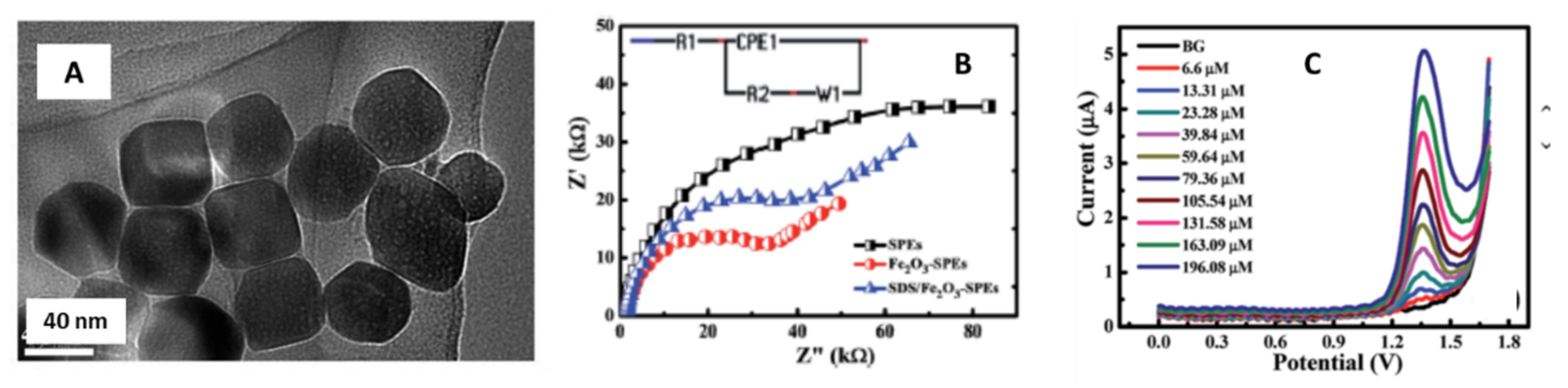
| Metal/Shape. | Size | SPE/Modification Strategy | Analyte | LOD | Real Samples Study | Reference |
|---|---|---|---|---|---|---|
| Pt/Sphere | 15 nm | Reduced graphene screen-printed electrode/Ink mixing | Cysteine | 0.12 µM | NA | Singh et al. [39] |
| Rh/Sphere | Not specified | Screen printed carbon electrode (SPCE)/Electrodeposition | Bromide anion | 39 µM | Seawater, surfactant, pharmaceutical formulation | Cunha-Silva and Arcos-Martinez [40] |
| Ru/Triangles | Approx. 18 nm | Graphene modified screen printed carbon electrode (SPEGPH)/Drop-casting | Biomedicine/ß-Nicotinamide adenine nucleotide (NADH) | 4.0 µM | NA | Baradoke et al. [41] |
| Ag/Triangles | Between 14.25 and 16.46 nm | Screen printed carbon nanofibers electrode (SPCNFE)/Spincoating | Voltammetric determination of As (V) | 1.6–2.5 µg·L−1 | Tap water | Torres-Rivero et al. [42] |
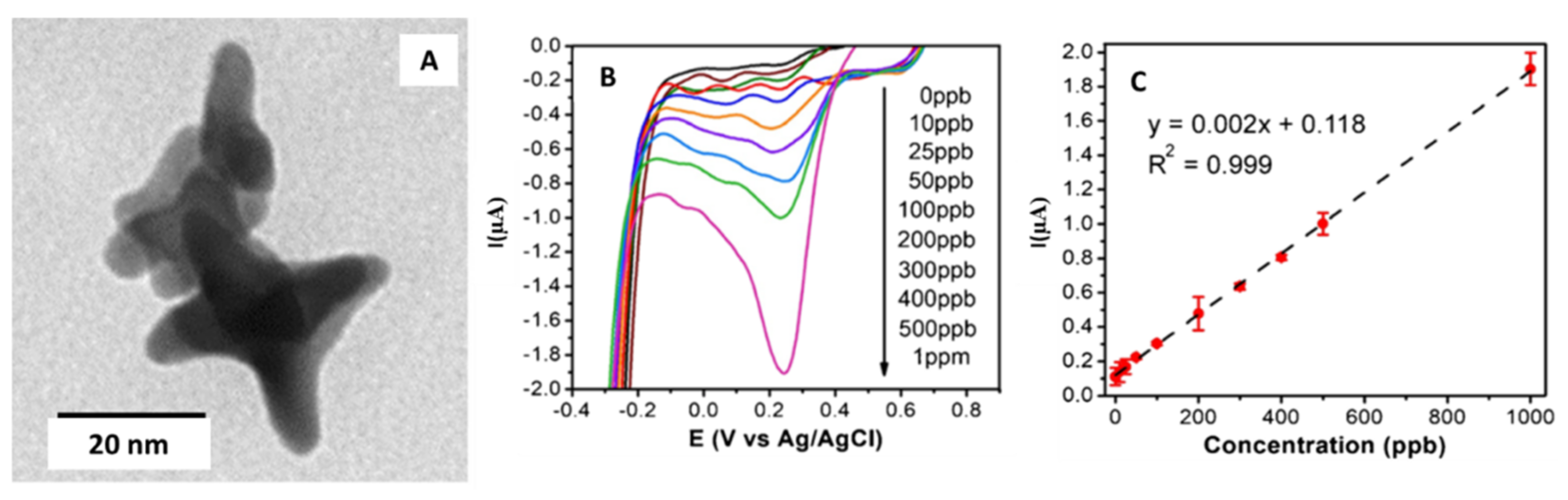
| Gold/Star | Tip-to-tip diameter 49 ± 14 nm, spikes number nanostar ranged from 4 to 10 nm. | Screen printed carbon electrodes (SPCE)/Drop-casting | Simultaneous detection of Cd (II), As (III), and Se (IV) | 1.62, 0.83, 1.57 µg·L−1 for Cd(II), As(III) and Se(IV), respectively | Ground and Surface water | Lu et al. [43] |
| Gold/Star | Star diameter ranged from 30 to 52 nm | Carbon pasted screen printed electrode (CPSPE)/Drop-casting | Detection of Cr (VI) in water | 3.5 µg·L−1 | Groundwater | Dutta et al. [44] |
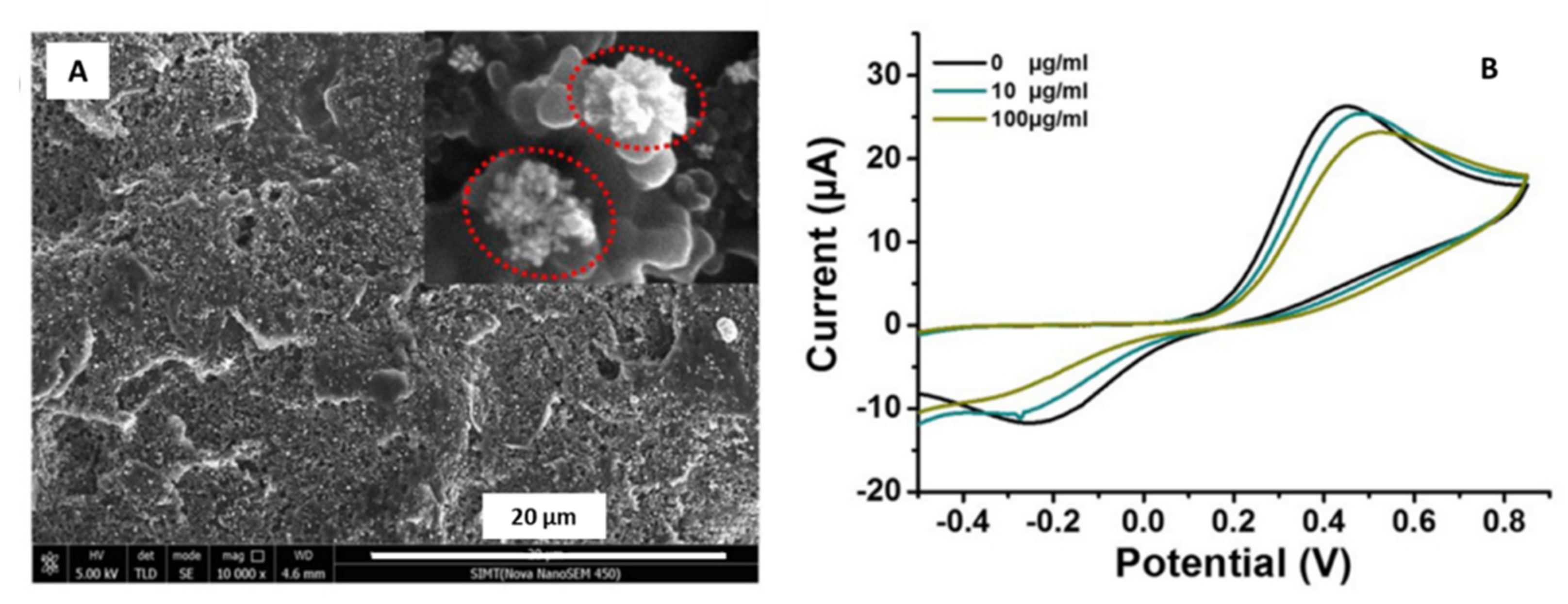
| Gold/Nanoflowers | Not specified | Screen printed carbon electrode (SPCE)/Electrodeposition-drop-casting | Glycated hemoglobin (HbA1c) | 0.65% | Human blood | Wang et al. [45] |
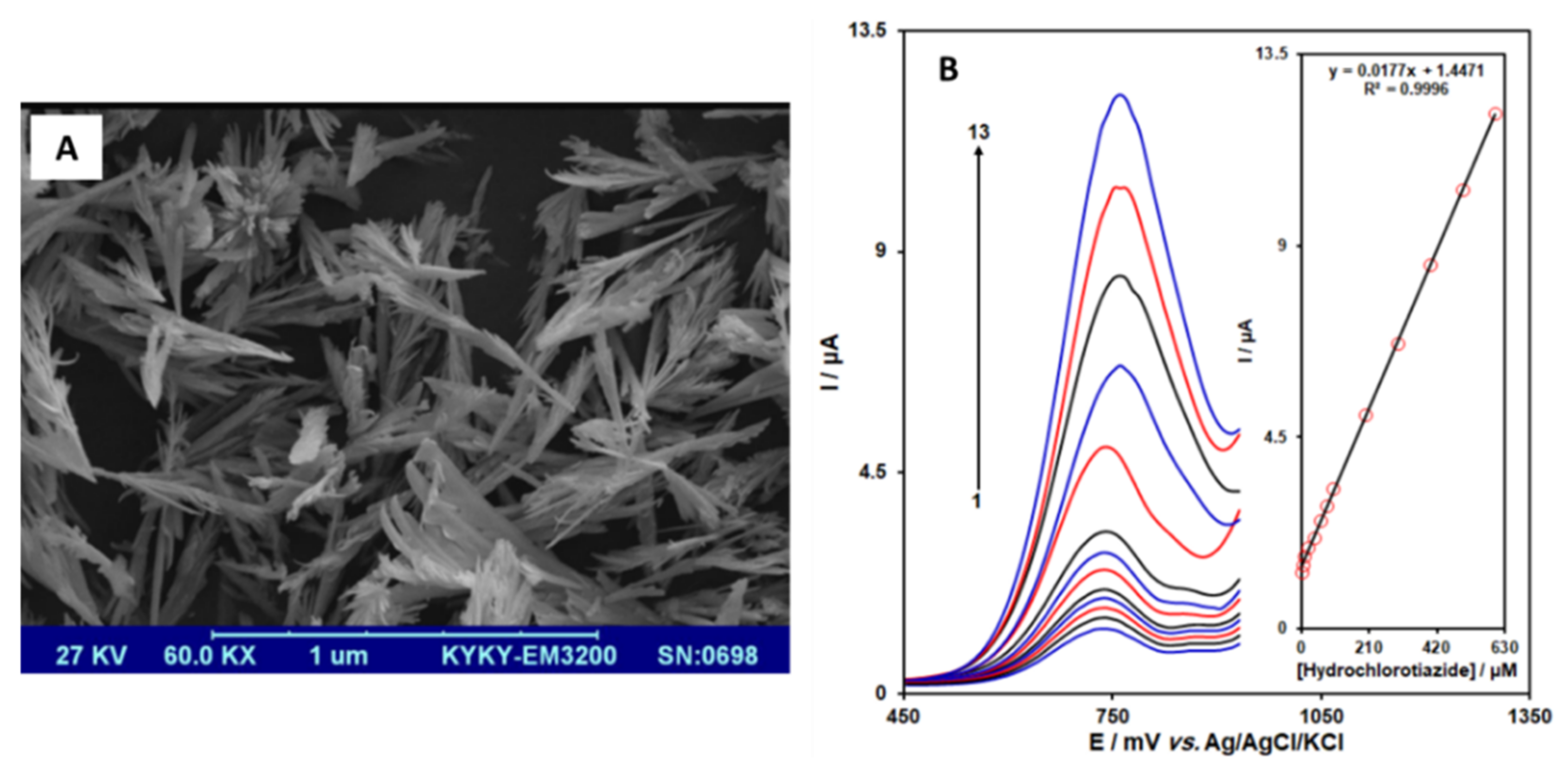
| Lanthanum-doped zinc oxide/Nanoflowers | Not specified | Graphite screen printed electrode/Drop-casting | Hydrochlorothiazide | 0.6 µM | Pharmaceutical formulation and urine | Rezaei et al. [46] |
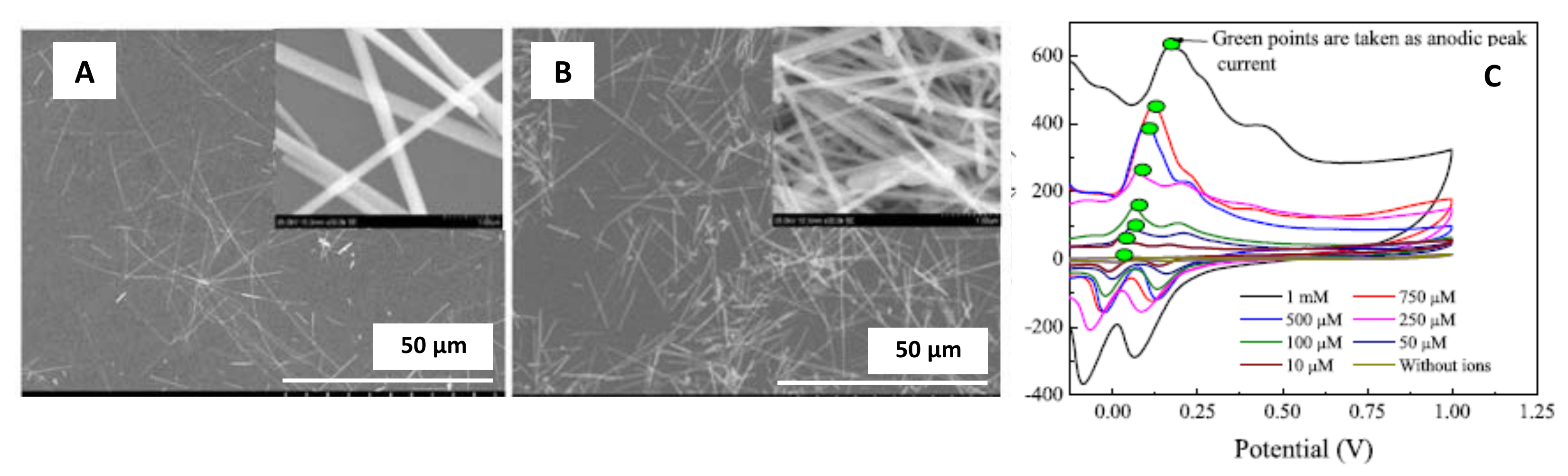
| Ammonium molybdate tetrahydrate silver/Nanowires | Diameter of 100 nm for a reaction time of 10 min | Carbon screen printed electrode/Drop-casting | Phosphate detection | 3 μM | NA | Kabir et al. [47] |
| GO-CS/AChE/Gold/Nano-cages | 20–50 nm, lattice spacing distances along the adjacent fringes were 0.235 nm | Screen printed carbon electrode (SPCE)/Drop-casting | Chlorpyrifos detection | 3 ng·L−1 | Vegetable samples | Yao et al. [48] |
| Lanthanum-doped Co3O4/nanocubes | Not specified | Graphite screen printed electrode/Drop-casting | Sudan I | 0.05 µM | Food samples | Mahmoudi-Moghaddam et al. [49] |
| Fe2O3/nanocubes | 37 nm | Carbon-graphite screen printed electrode/Drop-casting | Meclizine | 1.69 µM | Pharmaceutical formulation | Khorshed et al. [50]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Rivero, K.; Florido, A.; Bastos-Arrieta, J. Recent Trends in the Improvement of the Electrochemical Response of Screen-Printed Electrodes by Their Modification with Shaped Metal Nanoparticles. Sensors 2021, 21, 2596. https://doi.org/10.3390/s21082596
Torres-Rivero K, Florido A, Bastos-Arrieta J. Recent Trends in the Improvement of the Electrochemical Response of Screen-Printed Electrodes by Their Modification with Shaped Metal Nanoparticles. Sensors. 2021; 21(8):2596. https://doi.org/10.3390/s21082596
Chicago/Turabian StyleTorres-Rivero, Karina, Antonio Florido, and Julio Bastos-Arrieta. 2021. "Recent Trends in the Improvement of the Electrochemical Response of Screen-Printed Electrodes by Their Modification with Shaped Metal Nanoparticles" Sensors 21, no. 8: 2596. https://doi.org/10.3390/s21082596
APA StyleTorres-Rivero, K., Florido, A., & Bastos-Arrieta, J. (2021). Recent Trends in the Improvement of the Electrochemical Response of Screen-Printed Electrodes by Their Modification with Shaped Metal Nanoparticles. Sensors, 21(8), 2596. https://doi.org/10.3390/s21082596









