Monitoring of Dynamic Plantar Foot Temperatures in Diabetes with Personalised 3D-Printed Wearables
Abstract
1. Introduction
2. Materials and Methods
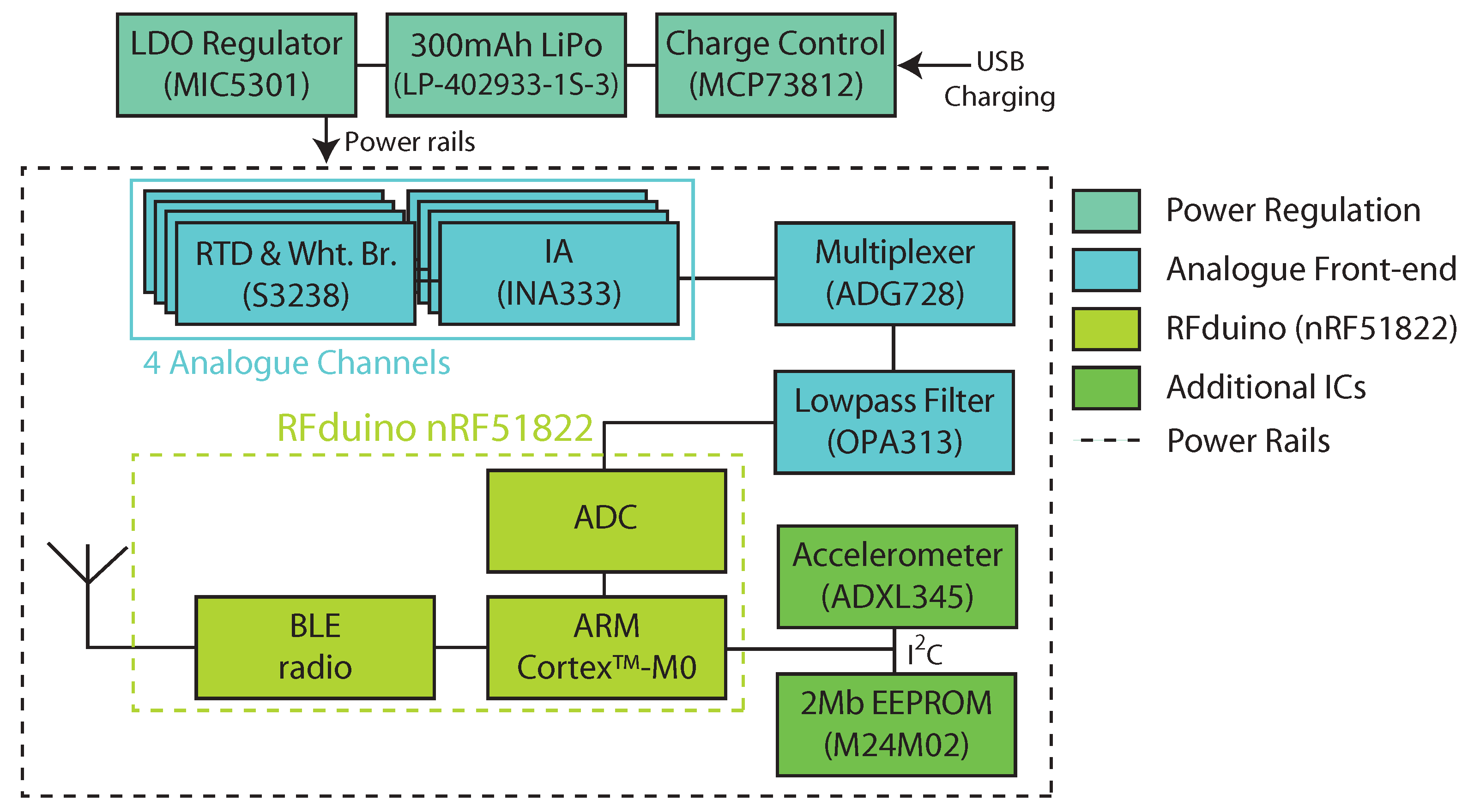
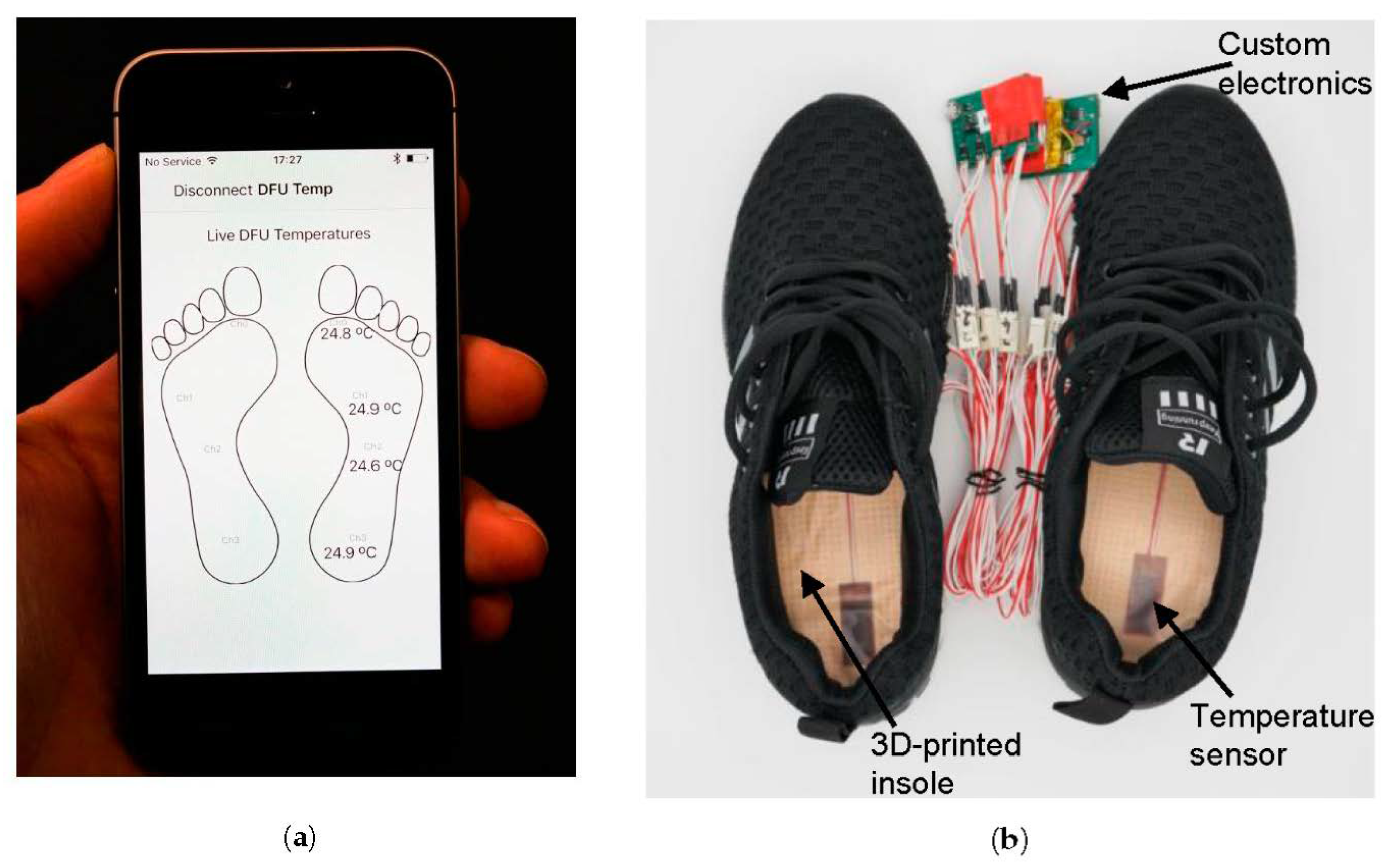
2.1. Study Device
2.2. Participants
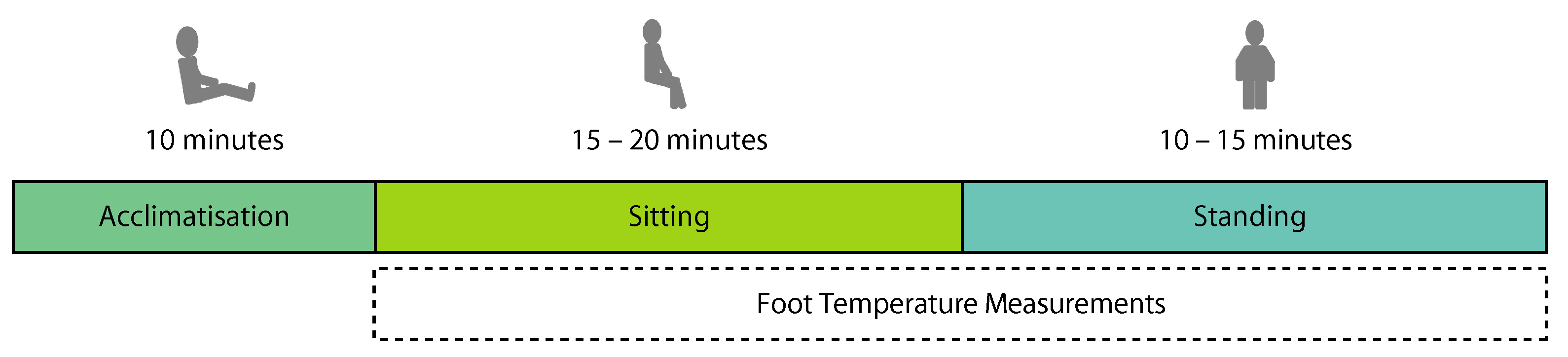
2.3. Experimental Protocol
2.4. Data Analysis and Model Fitting
2.5. Statistical Analysis
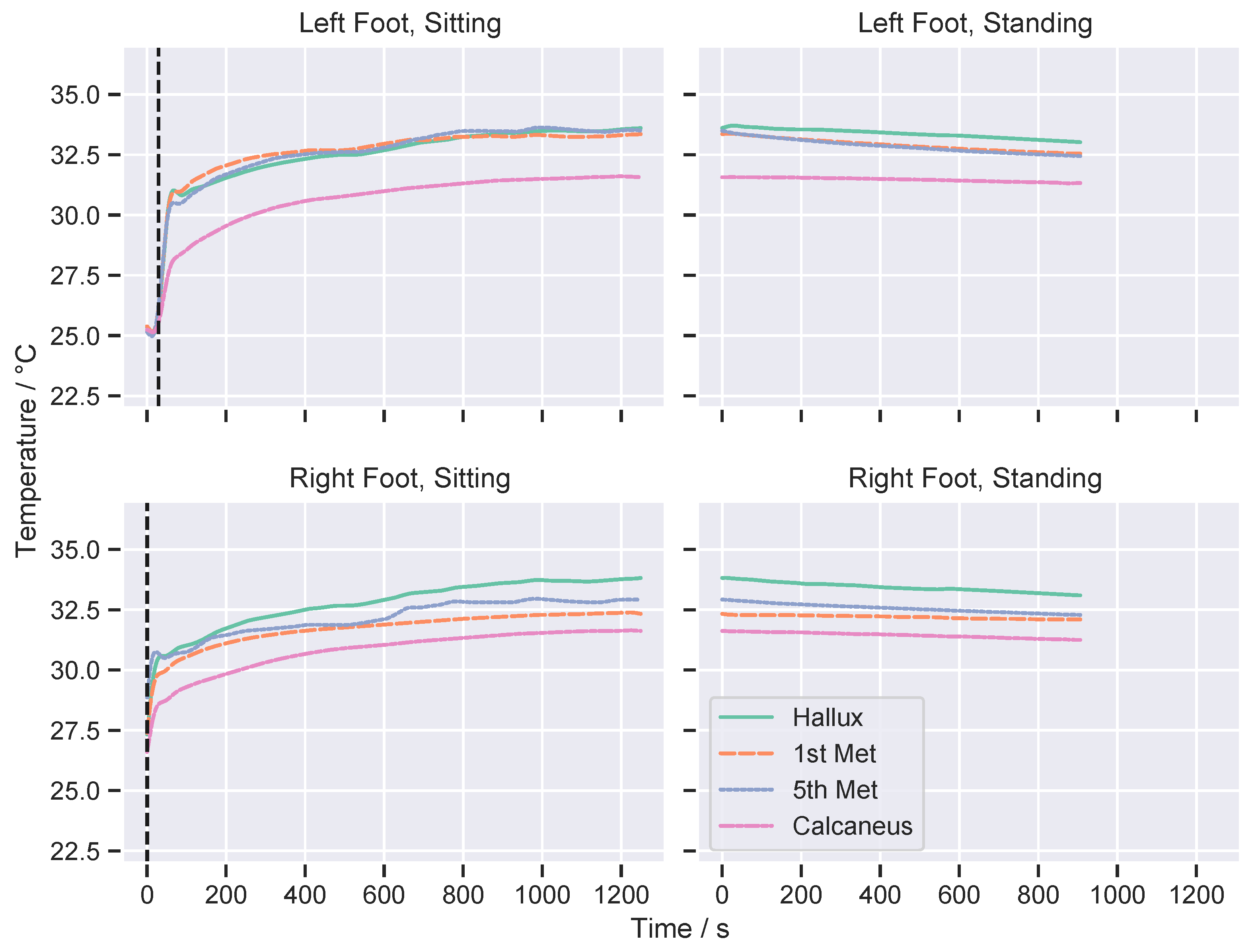
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADC | Analogue-to-Digital Converter |
| BLE | Bluetooth Low Energy |
| BMI | Body-Mass Index |
| DFU | Diabetic Foot Ulcer |
| EEPROM | Electrically Erasable Programmable Read-Only Memory |
| IA | Instrumentation Amplifier |
| LDO | Low Dropout |
| RTD | Resistance Temperature Detector |
| SD | Standard Deviation |
References
- Armstrong, D.G.; Boulton, A.J.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef]
- Kerr, M.; Barron, E.; Chadwick, P.; Evans, T.; Kong, W.; Rayman, G.; Sutton-Smith, M.; Todd, G.; Young, B.; Jeffcoate, W. The cost of diabetic foot ulcers and amputations to the National Health Service in England. Diabet. Med. 2019, 36, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.J.M.; Vileikyte, L.; Ragnarson-Tennvall, G.; Apelqvist, J. The global burden of diabetic foot disease. Lancet 2005, 366, 1719–1724. [Google Scholar] [CrossRef]
- Singh, N.; Armstrong, D.G.; Lipsky, B.A. Preventing foot ulcers in patients with diabetes. JAMA 2005, 293, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Bus, S.A.; van Netten, J.J. A shift in priority in diabetic foot care and research: 75% of foot ulcers are preventable. Diabetes Metab. Res. Rev. 2016, 32, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Dagher, L.; Shi, H.; Zhao, Y.; Marrouche, N.F. Wearables in cardiology: Here to stay. Heart Rhythm 2020, 17, 889–895. [Google Scholar] [CrossRef]
- Najafi, B.; Reeves, N.D.; Armstrong, D.G. Leveraging smart technologies to improve the management of diabetic foot ulcers and extend ulcer-free days in remission. Diabetes Metab. Res. Rev. 2020, 36, e3239. [Google Scholar] [CrossRef] [PubMed]
- Basatneh, R.; Najafi, B.; Armstrong, D.G. Health sensors, smart home devices, and the internet of medical things: An opportunity for dramatic improvement in care for the lower extremity complications of diabetes. J. Diabetes Sci. Technol. 2018, 12, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Fernando, M.; Lazzarini, P.; Najafi, B.; Armstrong, D.G. The potential role of sensors, wearables and telehealth in the remote management of diabetes-related foot disease. Sensors 2020, 20, 4527. [Google Scholar] [CrossRef] [PubMed]
- Ledoux, W.R.; Shofer, J.B.; Cowley, M.S.; Ahroni, J.H.; Cohen, V.; Boyko, E.J. Diabetic foot ulcer incidence in relation to plantar pressure magnitude and measurement location. J. Diabetes Complicat. 2013, 27, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Abbott, C.; Carrington, A.; Ashe, H.; Bath, S.; Every, L.; Griffiths, J.; Hann, A.; Hussein, A.; Jackson, N.; Johnson, K.; et al. The North-West Diabetes Foot Care Study: Incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet. Med. 2002, 19, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Peters, E.J.; Athanasiou, K.A.; Lavery, L.A. Is there a critical level of plantar foot pressure to identify patients at risk for neuropathic foot ulceration? J. Foot Ankle Res. 1998, 37, 303–307. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Lavery, L.A.; Pham, H.; Harvey, C.; Harkless, L.; Veves, A. Role of neuropathy and high foot pressures in diabetic foot ulceration. Diabetes Care 1998, 21, 1714–1719. [Google Scholar] [CrossRef]
- Owings, T.; Apelqvist, J.; Stenström, A.; Becker, M.; Bus, S.; Kalpen, A.; Ulbrecht, J.; Cavanagh, P. Plantar pressures in diabetic patients with foot ulcers which have remained healed. Diabet. Med. 2009, 26, 1141–1146. [Google Scholar] [CrossRef]
- Melai, T.; IJzerman, T.H.; Schaper, N.C.; de Lange, T.L.; Willems, P.J.; Meijer, K.; Lieverse, A.G.; Savelberg, H.H. Calculation of plantar pressure time integral, an alternative approach. Gait Posture 2011, 34, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Najafi, B.; Ron, E.; Enriquez, A.; Marin, I.; Razjouyan, J.; Armstrong, D.G. Smarter sole survival: Will neuropathic patients at high risk for ulceration use a smart insole-based foot protection system? J. Diabetes Sci. Technol. 2017, 11, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Abbott, C.A.; Chatwin, K.E.; Foden, P.; Hasan, A.N.; Sange, C.; Rajbhandari, S.M.; Reddy, P.N.; Vileikyte, L.; Bowling, F.L.; Boulton, A.J.M.; et al. Innovative intelligent insole system reduces diabetic foot ulcer recurrence at plantar sites: A prospective, randomised, proof-of-concept study. Lancet Digital Health 2019, 1, e308–e318. [Google Scholar] [CrossRef]
- Wang, L.; Jones, D.; Chapman, G.J.; Siddle, H.J.; Russell, D.A.; Nodeh, A.A.; Culmer, P. A Review of Wearable Sensor Systems to Monitor Plantar Loading in the Assessment of Diabetic Foot Ulcers. IEEE Trans. Biomed. Eng. 2020, 67, 1989–2004. [Google Scholar] [CrossRef] [PubMed]
- Chatwin, K.E.; Abbott, C.A.; Boulton, A.J.; Bowling, F.L.; Reeves, N.D. The role of foot pressure measurement in the prediction and prevention of diabetic foot ulceration—A comprehensive review. Diabetes Metab. Res. Rev. 2019, 36, e3258. [Google Scholar] [CrossRef]
- Yavuz, M.; Brem, R.W.; Davis, B.L.; Patel, J.; Osbourne, A.; Matassini, M.R.; Wood, D.A.; Nwokolo, I.O. Temperature as a predictive tool for plantar triaxial loading. J. Biomech. 2014, 47, 3767–3770. [Google Scholar] [CrossRef]
- Yavuz, M.; Master, H.; Garrett, A.; Lavery, L.A.; Adams, L.S. Peak plantar shear and pressure and foot ulcer locations: A call to revisit ulceration pathomechanics. Diabetes Care 2015, 38, e184–e185. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, M.; Ersen, A.; Hartos, J.; Schwarz, B.; Garrett, A.G.; Lavery, L.A.; Wukich, D.K.; Adams, L.S. Plantar shear stress in individuals with a history of diabetic foot ulcer: An emerging predictive marker for foot ulceration. Diabetes Care 2017, 40, e14–e15. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jones, D.; Chapman, G.J.; Siddle, H.J.; Russell, D.A.; Alazmani, A.; Culmer, P. An Inductive Force Sensor for In-Shoe Plantar Normal and Shear Load Measurement. IEEE Sens. J. 2020, 20, 13318–13331. [Google Scholar] [CrossRef]
- Bus, S. Innovations in plantar pressure and foot temperature measurements in diabetes. Diabetes Metab. Res. Rev. 2016, 32, 221–226. [Google Scholar] [CrossRef]
- Lavery, L.A.; Higgins, K.R.; Lanctot, D.R.; Constantinides, G.P.; Zamorano, R.G.; Armstrong, D.G.; Athanasiou, K.A.; Agrawal, C.M. Home monitoring of foot skin temperatures to prevent ulceration. Diabetes Care 2004, 27, 2642–2647. [Google Scholar] [CrossRef]
- Lavery, L.A.; Higgins, K.R.; Lanctot, D.R.; Constantinides, G.P.; Zamorano, R.G.; Athanasiou, K.A.; Armstrong, D.G.; Agrawal, C.M. Preventing Diabetic Foot Ulcer Recurrence in High-Risk Patients: Use of temperature monitoring as a self-assessment tool. Diabetes Care 2007, 30, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G.; Gordon, I.L.; Reyzelman, A.M.; Cazzell, S.M.; Fitzgerald, R.H.; Rothenberg, G.M.; Bloom, J.D.; Petersen, B.J.; Linders, D.R.; Nouvong, A.; et al. Feasibility and Efficacy of a Smart Mat Technology to Predict Development of Diabetic Plantar Ulcers. Diabetes Care 2017, 40, 973–980. [Google Scholar] [CrossRef]
- Reyzelman, A.M.; Koelewyn, K.; Murphy, M.; Shen, X.; Yu, E.; Pillai, R.; Fu, J.; Scholten, H.J.; Ma, R. Continuous Temperature-Monitoring Socks for Home Use in Patients with Diabetes: Observational Study. J. Med. Internet Res. 2018, 20, e12460. [Google Scholar] [CrossRef]
- Martín-Vaquero, J.; Hernández Encinas, A.; Queiruga-Dios, A.; José Bullón, J.; Martínez-Nova, A.; Torreblanca González, J.; Bullón-Carbajo, C. Review on wearables to monitor foot temperature in diabetic patients. Sensors 2019, 19, 776. [Google Scholar] [CrossRef]
- Niemann, U.; Spiliopoulou, M.; Malanowski, J.; Kellersmann, J.; Szczepanski, T.; Klose, S.; Dedonaki, E.; Walter, I.; Ming, A.; Mertens, P.R. Plantar temperatures in stance position: A comparative study with healthy volunteers and diabetes patients diagnosed with sensoric neuropathy. EBioMedicine 2020, 54, 102712. [Google Scholar] [CrossRef]
- Leister, K.R.; Wurdeman, S.R. A walking bout reveals altered foot thermodynamics associated with unilateral transtibial amputation among individuals with type 2 diabetes. Prosthet. Orthot. Int. 2020, 0309364620968642. [Google Scholar] [CrossRef]
- Reddy, P.N.; Cooper, G.; Weightman, A.; Hodson-Tole, E.; Reeves, N.D. Walking cadence affects rate of plantar foot temperature change but not final temperature in younger and older adults. Gait Posture 2017, 52, 272–279. [Google Scholar] [CrossRef]
- La Fontaine, J.; Lavery, L.; Jude, E. Current concepts of Charcot foot in diabetic patients. Foot 2016, 26, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Fitipaldi, H.; McCarthy, M.I.; Florez, J.C.; Franks, P.W. A global overview of precision medicine in type 2 diabetes. Diabetes 2018, 67, 1911–1922. [Google Scholar] [CrossRef]
- Van Netten, J.J.; Woodburn, J.; Bus, S.A. The future for diabetic foot ulcer prevention: A paradigm shift from stratified healthcare towards personalized medicine. Diabetes Metab. Res. Rev. 2020, 36, e3234. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: London, UK, 1988. [Google Scholar]
- van Netten, J.J.; van Baal, J.G.; Liu, C.; van Der Heijden, F.; Bus, S.A. Infrared thermal imaging for automated detection of diabetic foot complications. J. Diabetes Sci. Technol. 2013, 7, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Petrova, N.; Donaldson, N.K.; Tang, W.; MacDonald, A.; Allen, J.; Lomas, C.; Leech, N.; Ainarkar, S.; Bevans, J.; Plassmann, P.; et al. Infrared thermography and ulcer prevention in the high-risk diabetic foot: Data from a single-blind multicentre controlled clinical trial. Diabet. Med. 2020, 37, 95–104. [Google Scholar] [CrossRef]
- Gefen, A.; Megido-Ravid, M.; Azariah, M.; Itzchak, Y.; Arcan, M. Integration of plantar soft tissue stiffness measurements in routine MRI of the diabetic foot. Clin. Biomech. 2001, 16, 921–925. [Google Scholar] [CrossRef]
- Schramm, J.C.; Dinh, T.; Veves, A. Microvascular changes in the diabetic foot. Int. J. Low Extrem. Wounds 2006, 5, 149–159. [Google Scholar] [CrossRef]
- Rahemi, H.; Armstrong, D.G.; Enriquez, A.; Owl, J.; Talal, T.K.; Najafi, B. Lace up for healthy feet: The impact of shoe closure on plantar stress response. J. Diabetes Sci. Technol. 2017, 11, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Weston, A.D.; Hood, L. Systems biology, proteomics, and the future of health care: Toward predictive, preventative, and personalized medicine. J. Proteome Res. 2004, 3, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.; Glusman, G.; Brogaard, K.; Price, N.D.; Hood, L. P4 medicine: How systems medicine will transform the healthcare sector and society. Pers. Med. 2013, 10, 565–576. [Google Scholar] [CrossRef] [PubMed]


| C | D | |
|---|---|---|
| Number of participants | 12 | 13 |
| Gender (female/male) | 5/7 | 7/6 |
| Age (years) | 42.1 ± 17.7 | 44.4 ± 22.8 |
| Height (cm) | 172.1 ± 8.8 | 169.7 ± 7.7 |
| Mass (kg) | 76.9 ± 17.1 | 77.4 ± 16.6 |
| BMI (kg/m2) | 25.6 ± 3.6 | 26.9 ± 5.9 |
| Site | Temperature Difference/°C | p | |
|---|---|---|---|
| C | D | ||
| Hallux | 1.19 ± 0.83 | 1.33 ± 1.39 | 0.393 |
| 1st Metatarsal Head | 0.56 ± 0.49 | 0.60 ± 0.42 | 0.388 |
| 5th Metatarsal Head | 0.76 ± 0.69 | 0.77 ± 0.34 | 0.298 |
| Calcaneus | 0.62 ± 0.75 | 0.57 ± 0.58 | 0.477 |
| Sitting | ||||
| Site | Time Constant (τ)/s | p | Effect Size | |
| C | D | |||
| Hallux | 370.4 ± 172.1 | 279.1 ± 154.1 | 0.032 * | 0.55 |
| 1st Metatarsal Head | 322.3 ± 200.9 | 329.6 ± 134.9 | 0.299 | – |
| 5th Metatarsal Head | 481.9 ± 225.4 | 356.6 ± 165.0 | 0.011 * | 0.62 |
| Calcaneus | 483.9 ± 148.6 | 422.5 ± 189.7 | 0.081 | – |
| Standing | ||||
| Site | Rate of Temperature Change (m)/ | p | Effect Size | |
| C | D | |||
| Hallux | −0.87 ± 1.54 | −0.80 ± 1.50 | 0.430 | – |
| 1st Metatarsal Head | −0.77 ± 1.06 | −0.97 ± 1.22 | 0.324 | – |
| 5th Metatarsal Head | −0.74 ± 1.11 | −0.91 ± 1.18 | 0.299 | – |
| Calcaneus | −0.07 ± 0.79 | 0.00 ± 1.18 | 0.286 | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beach, C.; Cooper, G.; Weightman, A.; Hodson-Tole, E.F.; Reeves, N.D.; Casson, A.J. Monitoring of Dynamic Plantar Foot Temperatures in Diabetes with Personalised 3D-Printed Wearables. Sensors 2021, 21, 1717. https://doi.org/10.3390/s21051717
Beach C, Cooper G, Weightman A, Hodson-Tole EF, Reeves ND, Casson AJ. Monitoring of Dynamic Plantar Foot Temperatures in Diabetes with Personalised 3D-Printed Wearables. Sensors. 2021; 21(5):1717. https://doi.org/10.3390/s21051717
Chicago/Turabian StyleBeach, Christopher, Glen Cooper, Andrew Weightman, Emma F. Hodson-Tole, Neil D. Reeves, and Alexander J. Casson. 2021. "Monitoring of Dynamic Plantar Foot Temperatures in Diabetes with Personalised 3D-Printed Wearables" Sensors 21, no. 5: 1717. https://doi.org/10.3390/s21051717
APA StyleBeach, C., Cooper, G., Weightman, A., Hodson-Tole, E. F., Reeves, N. D., & Casson, A. J. (2021). Monitoring of Dynamic Plantar Foot Temperatures in Diabetes with Personalised 3D-Printed Wearables. Sensors, 21(5), 1717. https://doi.org/10.3390/s21051717









