Profiles of Accelerometry-Derived Physical Activity Are Related to Perceived Physical Fatigability in Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Assessment of Exposure, Outcome, Covariates
2.3. Statistical Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eldadah, B.A. Fatigue and fatigability in older adults. PM&R 2010, 2, 406–413. [Google Scholar]
- Schrack, J.A.; Simonsick, E.M.; Glynn, N.W. Fatigability: A prognostic indicator of phenotypic aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2020, 75, e63–e66. [Google Scholar] [CrossRef] [PubMed]
- LaSorda, K.R.; Gmelin, T.; Kuipers, A.L.; Boudreau, R.M.; Santanasto, A.J.; Christensen, K.; Renner, S.W.; Wojczynski, M.K.; Andersen, S.L.; Cosentino, S.; et al. Epidemiology of perceived physical fatigability in older adults: The Long Life Family Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2020, 75, e81–e88. [Google Scholar] [CrossRef] [PubMed]
- Simonsick, E.M.; Glynn, N.W.; Jerome, G.J.; Shardell, M.; Schrack, J.A.; Ferrucci, L. Fatigued, but Not Frail: Perceived Fatigability as a Marker of Impending Decline in Mobility-Intact Older Adults. J. Am. Geriatr. Soc. 2016, 64, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Simonsick, E.M.; Schrack, J.A.; Santanasto, A.J.; Studenski, S.A.; Ferrucci, L.; Glynn, N.W. Pittsburgh Fatigability Scale: One-Page Predictor of Mobility Decline in Mobility-Intact Older Adults. J. Am. Geriatr. Soc. 2018, 66, 2092–2096. [Google Scholar] [CrossRef] [PubMed]
- Schrack, J.A.; Kuo, P.-L.; Wanigatunga, A.A.; Di, J.; Simonsick, E.M.; Spira, A.P.; Ferrucci, L.; Zipunnikov, V. Active-to-Sedentary Behavior Transitions, Fatigability, and Physical Functioning in Older Adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2019, 74, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Wanigatunga, A.A.; Varadhan, R.; Simonsick, E.M.; Carlson, O.D.; Studenski, S.; Ferrucci, L.; Schrack, J.A. Longitudinal Relationship Between Interleukin-6 and Perceived Fatigability Among Well-Functioning Adults in Mid-to-Late Life. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2019, 74, 720–725. [Google Scholar] [CrossRef]
- Wanigatunga, A.A.; Simonsick, E.M.; Zipunnikov, V.; Spira, A.P.; Studenski, S.; Ferrucci, L.; Schrack, J.A. Perceived Fatigability and Objective Physical Activity in Mid- to Late-Life. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 630–635. [Google Scholar] [CrossRef]
- Qiao, Y.; Gmelin, T.; Renner, S.W.; Boudreau, R.M.; Martin, S.; Wojczynski, M.K.; Christensen, K.; Andersen, S.L.; Cosentino, S.; Santanasto, A.J.; et al. Evaluation of the Bidirectional Relations of Perceived Physical Fatigability and Physical Activity on Slower Gait Speed. J. Gerontol. Ser. A 2020. [CrossRef]
- Touitou, Y.; Reinberg, A.; Touitou, D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci. 2017, 173, 94–106. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Liu, R.-Y.; Wang, Q.-S.; Van Someren, E.J.W.; Xu, H.; Zhou, J.-N. Age-associated difference in circadian sleep-wake and rest-activity rhythms. Physiol. Behav. 2002, 76, 597–603. [Google Scholar] [CrossRef]
- Neikrug, A.B.; Chen, I.Y.; Palmer, J.R.; McCurry, S.M.; Von Korff, M.; Perlis, M.; Vitiello, M.V. Characterizing behavioral activity rhythms in older adults using actigraphy. Sensors 2020, 20, 549. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Palacios-Bois, J.; Schwartz, G.; Iskandar, H.; Thakur, M.; Quirion, R.; Nair, N.P. Circadian rhythms of melatonin and cortisol in aging. Biol. Psychiatry 1989, 25, 305–319. [Google Scholar] [CrossRef]
- Van Someren EJ Circadian and sleep disturbances in the elderly. Exp. Gerontol. 2000, 35, 1229–1237. [CrossRef]
- Paudel, M.L.; Taylor, B.C.; Ancoli-Israel, S.; Blackwell, T.; Stone, K.L.; Tranah, G.; Redline, S.; Cummings, S.R.; Ensrud, K.E. Osteoporotic Fractures in Men (MrOS) Study Rest/activity rhythms and mortality rates in older men: MrOS Sleep Study. Chronobiol. Int. 2010, 27, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.S.; Blackwell, T.L.; Lane, N.E.; Tranah, G.; Orwoll, E.S.; Cauley, J.A.; Ancoli-Israel, S.; Stone, K.L.; Cummings, S.R.; Cawthon, P.M. Rest-activity patterns and falls and fractures in older men. Osteoporos. Int. 2017, 28, 1313–1322. [Google Scholar] [CrossRef]
- Rogers-Soeder, T.S.; Blackwell, T.; Yaffe, K.; Ancoli-Israel, S.; Redline, S.; Cauley, J.A.; Ensrud, K.E.; Paudel, M.; Barrett-Connor, E.; LeBlanc, E.; et al. Research Group Rest-Activity Rhythms and Cognitive Decline in Older Men: The Osteoporotic Fractures in Men Sleep Study. J. Am. Geriatr. Soc. 2018, 66, 2136–2143. [Google Scholar] [CrossRef]
- Tranah, G.J.; Blackwell, T.; Ancoli-Israel, S.; Paudel, M.L.; Ensrud, K.E.; Cauley, J.A.; Redline, S.; Hillier, T.A.; Cummings, S.R.; Stone, K.L. Study of Osteoporotic Fractures Research Group Circadian activity rhythms and mortality: The study of osteoporotic fractures. J. Am. Geriatr. Soc. 2010, 58, 282–291. [Google Scholar] [CrossRef]
- Watson, K.B.; Carlson, S.A.; Gunn, J.P.; Galuska, D.A.; O’Connor, A.; Greenlund, K.J.; Fulton, J.E. Physical Inactivity Among Adults Aged 50 Years and Older—United States, 2014. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Urbanek, J.K.; Zipunnikov, V.; Harris, T.; Crainiceanu, C.; Harezlak, J.; Glynn, N.W. Validation of gait characteristics extracted from raw accelerometry during walking against measures of physical function, mobility, fatigability, and fitness. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 676–681. [Google Scholar] [CrossRef]
- Venditti, E.M.; Zgibor, J.C.; Vander Bilt, J.; Kieffer, L.A.; Boudreau, R.M.; Burke, L.E.; Glynn, N.W.; Jakicic, J.M.; Smith, K.J.; Semler, L.N.; et al. Mobility and vitality lifestyle program (MOVE UP): A community health worker intervention for older adults with obesity to improve weight, health, and physical function. Innov. Aging 2018, 2, igy012. [Google Scholar] [CrossRef] [PubMed]
- Lange-Maia, B.S.; Newman, A.B.; Strotmeyer, E.S.; Harris, T.B.; Caserotti, P.; Glynn, N.W. Performance on fast- and usual-paced 400-m walk tests in older adults: Are they comparable? Aging Clin. Exp. Res. 2015, 27, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Glynn, N.W.; Santanasto, A.J.; Simonsick, E.M.; Boudreau, R.M.; Beach, S.R.; Schulz, R.; Newman, A.B. The Pittsburgh Fatigability Scale for older adults: Development and validation. J. Am. Geriatr. Soc. 2015, 63, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Wasson, E.; Rosso, A.L.; Santanasto, A.J.; Rosano, C.; Butters, M.A.; Rejeski, W.J.; Boudreau, R.M.; Aizenstein, H.; Gmelin, T.; Glynn, N.W. LIFE Study Group Neural correlates of perceived physical and mental fatigability in older adults: A pilot study. Exp. Gerontol. 2019, 115, 139–147. [Google Scholar] [CrossRef]
- Cooper, R.; Popham, M.; Santanasto, A.J.; Hardy, R.; Glynn, N.W.; Kuh, D. Are BMI and inflammatory markers independently associated with physical fatigability in old age? Int. J. Obes. 2019, 43, 832–841. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Ferrucci, L.; Pieper, C.F.; Leveille, S.G.; Markides, K.S.; Ostir, G.V.; Studenski, S.; Berkman, L.F.; Wallace, R.B. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2000, 55, M221–M231. [Google Scholar] [CrossRef]
- Stewart, A.L.; Mills, K.M.; King, A.C.; Haskell, W.L.; Gillis, D.; Ritter, P.L. CHAMPS physical activity questionnaire for older adults: Outcomes for interventions. Med. Sci. Sports Exerc. 2001, 33, 1126–1141. [Google Scholar] [CrossRef]
- Radloff, L.S. The CES-D Scale:A Self-Report DepressionScale for Research in the General Population. Appl. Psychol. Meas 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Choi, L.; Ward, S.C.; Schnelle, J.F.; Buchowski, M.S. Assessment of wear/nonwear time classification algorithms for triaxial accelerometer. Med. Sci. Sports Exerc. 2012, 44, 2009–2016. [Google Scholar] [CrossRef]
- Marler, M.R.; Gehrman, P.; Martin, J.L.; Ancoli-Israel, S. The sigmoidally transformed cosine curve: A mathematical model for circadian rhythms with symmetric non-sinusoidal shapes. Stat. Med. 2006, 25, 3893–3904. [Google Scholar] [CrossRef]
- Walsh, C.M.; Blackwell, T.; Tranah, G.J.; Stone, K.L.; Ancoli-Israel, S.; Redline, S.; Paudel, M.; Kramer, J.H.; Yaffe, K. Weaker circadian activity rhythms are associated with poorer executive function in older women. Sleep 2014, 37, 2009–2016. [Google Scholar] [CrossRef]
- Paudel, M.L.; Taylor, B.C.; Ancoli-Israel, S.; Stone, K.L.; Tranah, G.; Redline, S.; Barrett-Connor, E.; Stefanick, M.L.; Ensrud, K.E. Rest/activity rhythms and cardiovascular disease in older men. Chronobiol. Int. 2011, 28, 258–266. [Google Scholar] [CrossRef]
- Smagula, S.F.; Krafty, R.T.; Taylor, B.J.; Martire, L.M.; Schulz, R.; Hall, M.H. Rest-activity rhythm and sleep characteristics associated with depression symptom severity in strained dementia caregivers. J. Sleep Res. 2017, 26, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.L.; Buysse, D.J.; Redline, S.; Stone, K.L.; Ensrud, K.; Leng, Y.; Ancoli-Israel, S.; Hall, M.H. Multidimensional Sleep and Mortality in Older Adults: A Machine-Learning Comparison With Other Risk Factors. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2019, 74, 1903–1909. [Google Scholar] [CrossRef]
- Smagula, S.F.; Krafty, R.T.; Thayer, J.F.; Buysse, D.J.; Hall, M.H. Rest-activity rhythm profiles associated with manic-hypomanic and depressive symptoms. J. Psychiatr. Res. 2018, 102, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Krafty, R.T.; Fu, H.; Graves, J.L.; Bruce, S.A.; Hall, M.H.; Smagula, S.F. Measuring Variability in Rest-Activity Rhythms from Actigraphy with Application to Characterizing Symptoms of Depression. Stat. Biosci. 2019, 11, 314–333. [Google Scholar] [CrossRef] [PubMed]
- Van Someren, E.J.; Lijzenga, C.; Mirmiran, M.; Swaab, D.F. Long-term fitness training improves the circadian rest-activity rhythm in healthy elderly males. J. Biol. Rhythms 1997, 12, 146–156. [Google Scholar] [CrossRef]
- Scrucca, L.; Fop, M.; Murphy, T.B.; Raftery, A.E. mclust 5: Clustering, Classification and Density Estimation Using Gaussian Finite Mixture Models. R J. 2016, 8, 289–317. [Google Scholar] [CrossRef]
- Alfini, A.J.; Schrack, J.A.; Urbanek, J.K.; Wanigatunga, A.A.; Wanigatunga, S.K.; Zipunnikov, V.; Ferrucci, L.; Simonsick, E.M.; Spira, A.P. Associations of actigraphic sleep parameters with fatigability in older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2020, 75, e95–e102. [Google Scholar] [CrossRef]
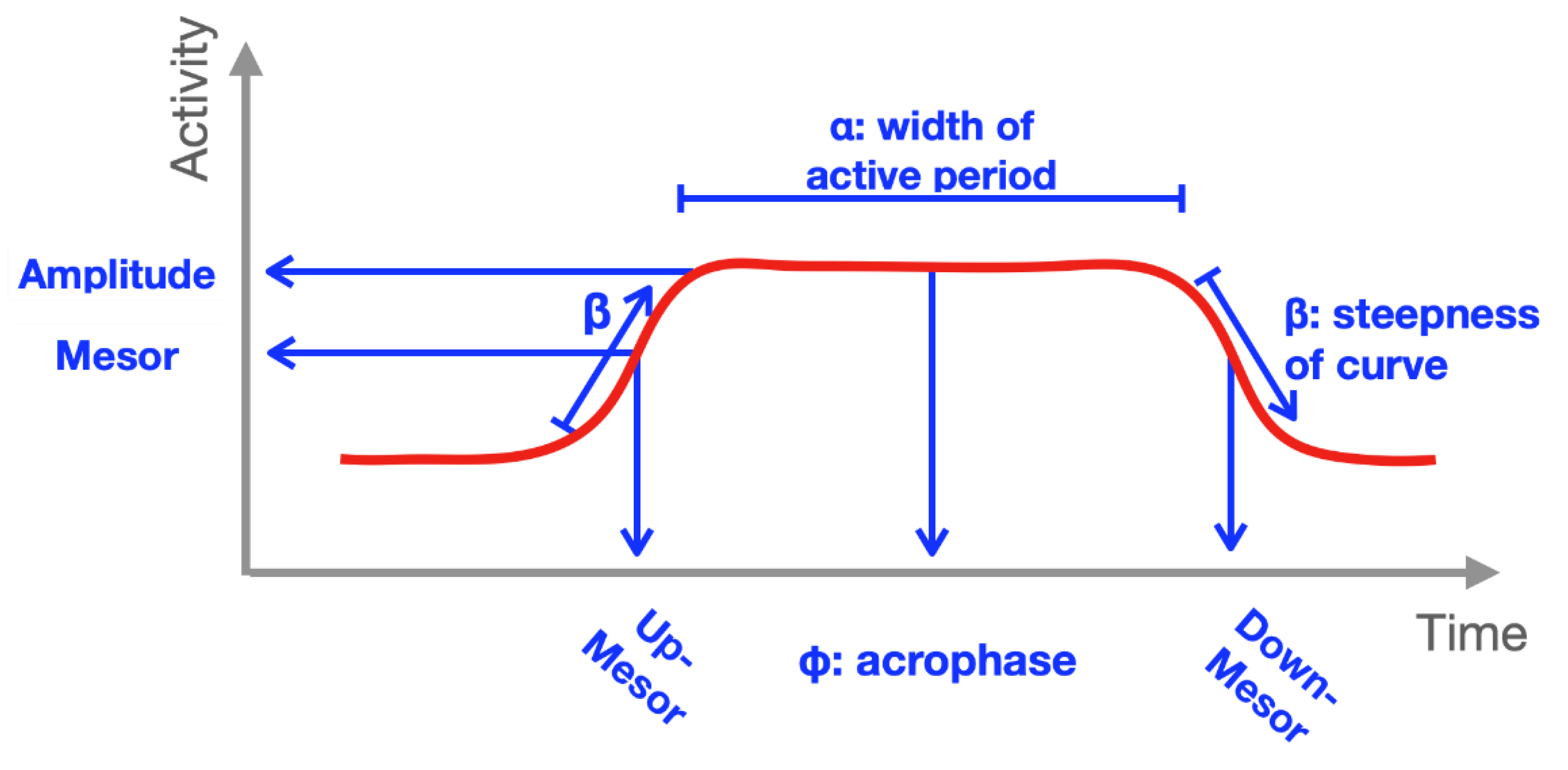

| Characteristics | Overall (n = 181) + | PFS Physical Scores ≥15 (n = 111) + | PFS Physical Scores <15 (n = 70) + | p Value |
|---|---|---|---|---|
| MOVEUP Study | 127 (70.2) | 88 (79.3) | 39 (55.7) | <0.001 *,1 |
| Age, years | 71.3 ± 6.7 | 70.9 ± 6.5 | 72.1 ± 6.9 | 0.20 *,1 |
| Sex, female | 143 (79.0) | 92 (82.9) | 51 (72.9) | 0.11 *,3 |
| Race, White | 127 (70.2) | 82 (73.9) | 45 (64.3) | 0.17 *,3 |
| Education, ≥High School | 140 (77.3) | 88 (79.3) | 52 (74.3) | 0.43 *,3 |
| Short Physical Performance Battery, 0–12 | 10.5 ± 1.8 | 10.3 ± 2.0 | 10.9 ± 1.5 | 0.02 *,1 |
| Body mass index, kg/m2 | 32.3 ± 6.0 | 33.6 ± 5.6 | 30.3 ± 6.1 | <0.001 *,2 |
| Usual Gait Speed, m/s | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.1 ± 0.2 | <0.001 *,1 |
| Physical activity (CHAMPS) 4, MET-min/day | 320.8 ± 251.7 | 292.7 ± 245.3 | 365.3 ± 257.0 | 0.03 *,1 |
| Depression symptomology (CES-D) 5, 0–30 | 6.9 ± 5.9 | 8.0 ± 6.1 | 5.0 ± 5.2 | <0.001 *,1 |
| Unadjusted Associations | Multivariable Adjusted Quantile Regression 3 | |||||
|---|---|---|---|---|---|---|
| Characteristics | Overall (n = 181)+ | PFS Physical Scores ≥ 15 (n = 111)+ | PFS Physical Scores < 15 (n = 70)+ | p Value | β Estimate (95% CI) | Standardized β (95% CI) |
| Alpha | −0.3 ± 0.3 | −0.3 ± 0.3 | −0.4 ± 0.2 | 0.380 1 | 2.70 (−3.83, 9.23) | 0.67 (−0.96, 2.30) |
| Beta | 22.2 ± 50.1 | 26.0 ± 58.1 | 16.4 ± 33.5 | 0.120 1 | 0.03 (0.01, 0.05) * | 1.48 (0.10, 2.86) * |
| Acrophase | 14.8 ± 1.3 | 14.9 ± 1.4 | 14.6 ± 1.1 | 0.020 *,1 | 1.29 (0.31, 2.27) * | 1.67 (0.41, 2.92) * |
| Amplitude | 5.9 ± 2.8 | 5.6 ± 2.7 | 6.4 ± 3.0 | 0.030 *,1 | −0.09 (−0.58, 0.40) | −0.24 (−1.63, 1.15) |
| Mesor | 2.8 ± 0.6 | 2.8 ± 0.6 | 2.9 ± 0.6 | 0.110 1 | −0.50 (−3.05, 2.05) | −0.30 (−1.86, 1.25) |
| Up Mesor (hours) | 7.5 ± 1.3 | 7.7 ± 1.3 | 7.2 ± 1.2 | 0.004 *,2 | 1.38 (0.40, 2.36) * | 1.76 (0.50, 3.01) * |
| Down Mesor (hours) | 22.0 ± 2.0 | 22.1 ± 2.3 | 22.0 ± 1.6 | 0.250 1 | 0.82 (−0.02, 1.66) | 1.66 (−0.06, 3.37) |
| Pseudo-F Statistic | 995.5 ± 479.6 | 962.8 ± 499.1 | 1047.4 ± 445.3 | 0.070 1 | 0.00 (0.00, 0.00) | −0.17 (−1.56, 1.23) |
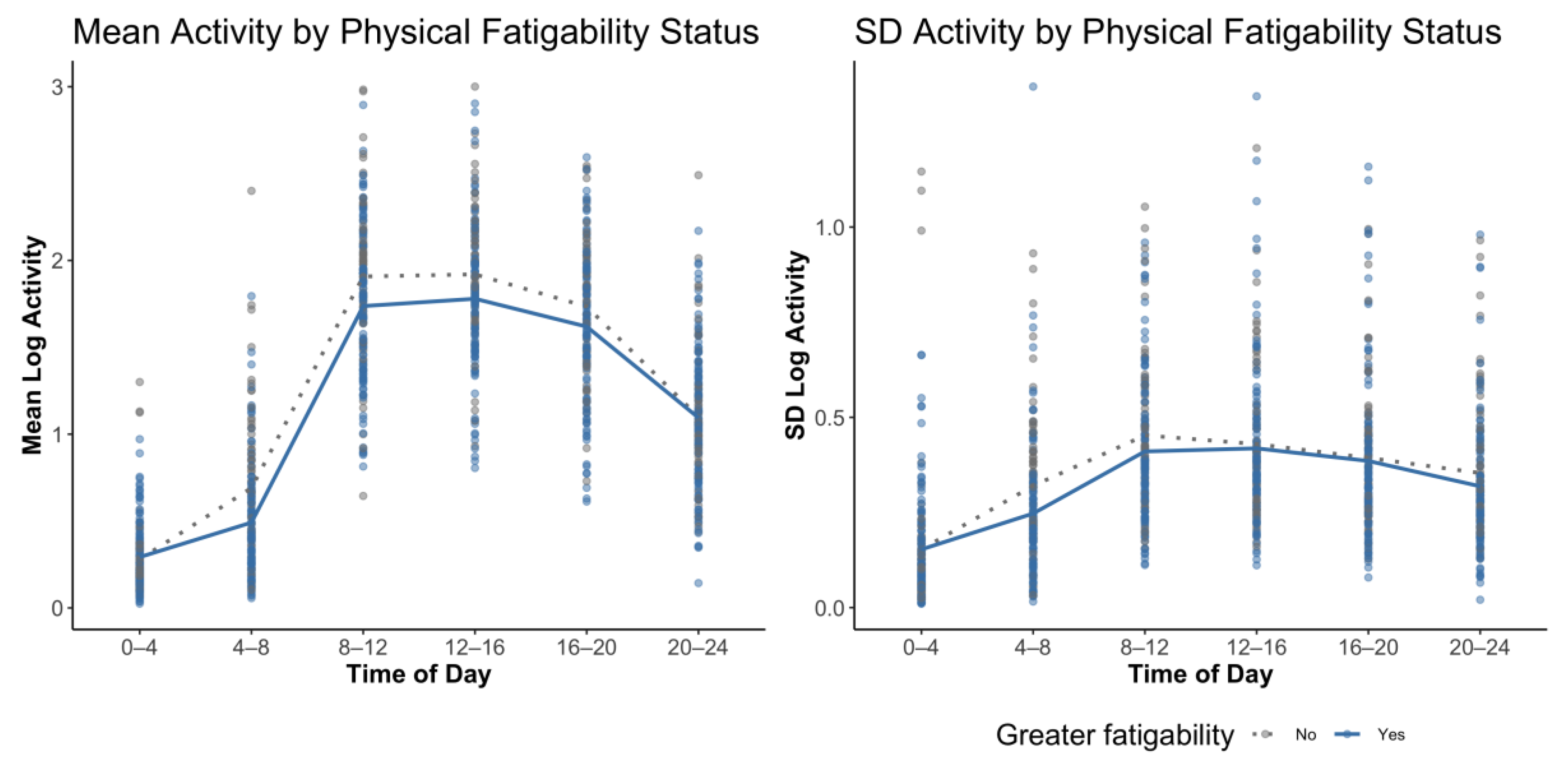
| Mean of Activity | Standard Deviation of Activity | |||||
|---|---|---|---|---|---|---|
| Clock Time | β Estimate | 95% Confidence Interval | p Value | β Estimate | 95% Confidence Interval | p Value |
| 00:00–04:00 | 2.42 | (−6.72, 11.56) | 0.60 | 1.12 | (−12.52, 14.76) | 0.87 |
| 04:00–08:00 | −4.50 | (−8.39, −0.61) | 0.03 | −5.75 | (−15.83, 4.33) | 0.27 |
| 08:00–12:00 | −3.05 | (−6.98, 0.89) | 0.13 | −0.22 | (−8.07, 7.64) | 0.96 |
| 12:00–16:00 | −1.96 | (−5.83, 1.91) | 0.32 | −1.01 | (−8.98, 6.95) | 0.80 |
| 16:00–20:00 | −1.53 | (−5.82, 2.77) | 0.49 | −1.02 | (−9.39, 7.35) | 0.81 |
| 20:00–24:00 | 2.39 | (−3.07, 7.84) | 0.39 | −7.42 | (−16.64, 1.8) | 0.12 |
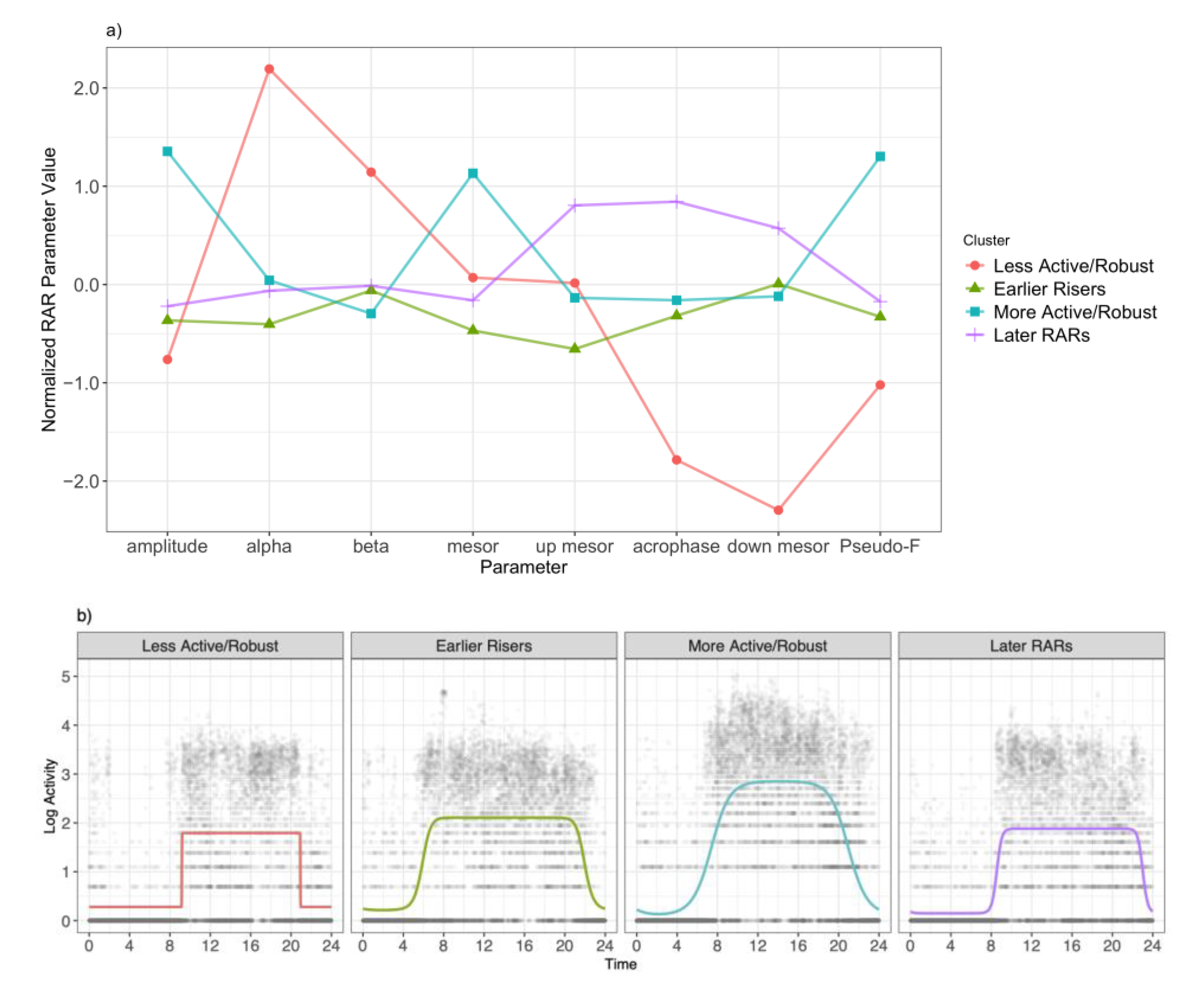
| β Coefficient | 95% Confidence Interval | p-Value | LRT + | |
|---|---|---|---|---|
| Model 1 | ||||
| Earlier Risers | ref | 0.03 | ||
| More Active/Robust | 0.45 | (−2.78, 3.68) | 0.79 | |
| Later RAR | 3.53 | (0.30, 6.76) | 0.03 | |
| Less Active/Robust | 6.14 | (−0.01, 12.29) | 0.05 | |
| Model 2 | ||||
| Earlier Risers or More Active/Robust | ref | |||
| Less Active/Robust or Later RAR | 3.71 | (0.99, 6.43) | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graves, J.L.; Qiao, Y.; Moored, K.D.; Boudreau, R.M.; Venditti, E.M.; Krafty, R.T.; Shiroma, E.J.; Harezlak, J.; Glynn, N.W. Profiles of Accelerometry-Derived Physical Activity Are Related to Perceived Physical Fatigability in Older Adults. Sensors 2021, 21, 1718. https://doi.org/10.3390/s21051718
Graves JL, Qiao Y, Moored KD, Boudreau RM, Venditti EM, Krafty RT, Shiroma EJ, Harezlak J, Glynn NW. Profiles of Accelerometry-Derived Physical Activity Are Related to Perceived Physical Fatigability in Older Adults. Sensors. 2021; 21(5):1718. https://doi.org/10.3390/s21051718
Chicago/Turabian StyleGraves, Jessica L., Yujia (Susanna) Qiao, Kyle D. Moored, Robert M. Boudreau, Elizabeth M. Venditti, Robert T. Krafty, Eric J. Shiroma, Jaroslaw Harezlak, and Nancy W. Glynn. 2021. "Profiles of Accelerometry-Derived Physical Activity Are Related to Perceived Physical Fatigability in Older Adults" Sensors 21, no. 5: 1718. https://doi.org/10.3390/s21051718
APA StyleGraves, J. L., Qiao, Y., Moored, K. D., Boudreau, R. M., Venditti, E. M., Krafty, R. T., Shiroma, E. J., Harezlak, J., & Glynn, N. W. (2021). Profiles of Accelerometry-Derived Physical Activity Are Related to Perceived Physical Fatigability in Older Adults. Sensors, 21(5), 1718. https://doi.org/10.3390/s21051718








