Swelling-Based Distributed Chemical Sensing with Standard Acrylate Coated Optical Fibers
Abstract
1. Introduction
2. Materials and Methods
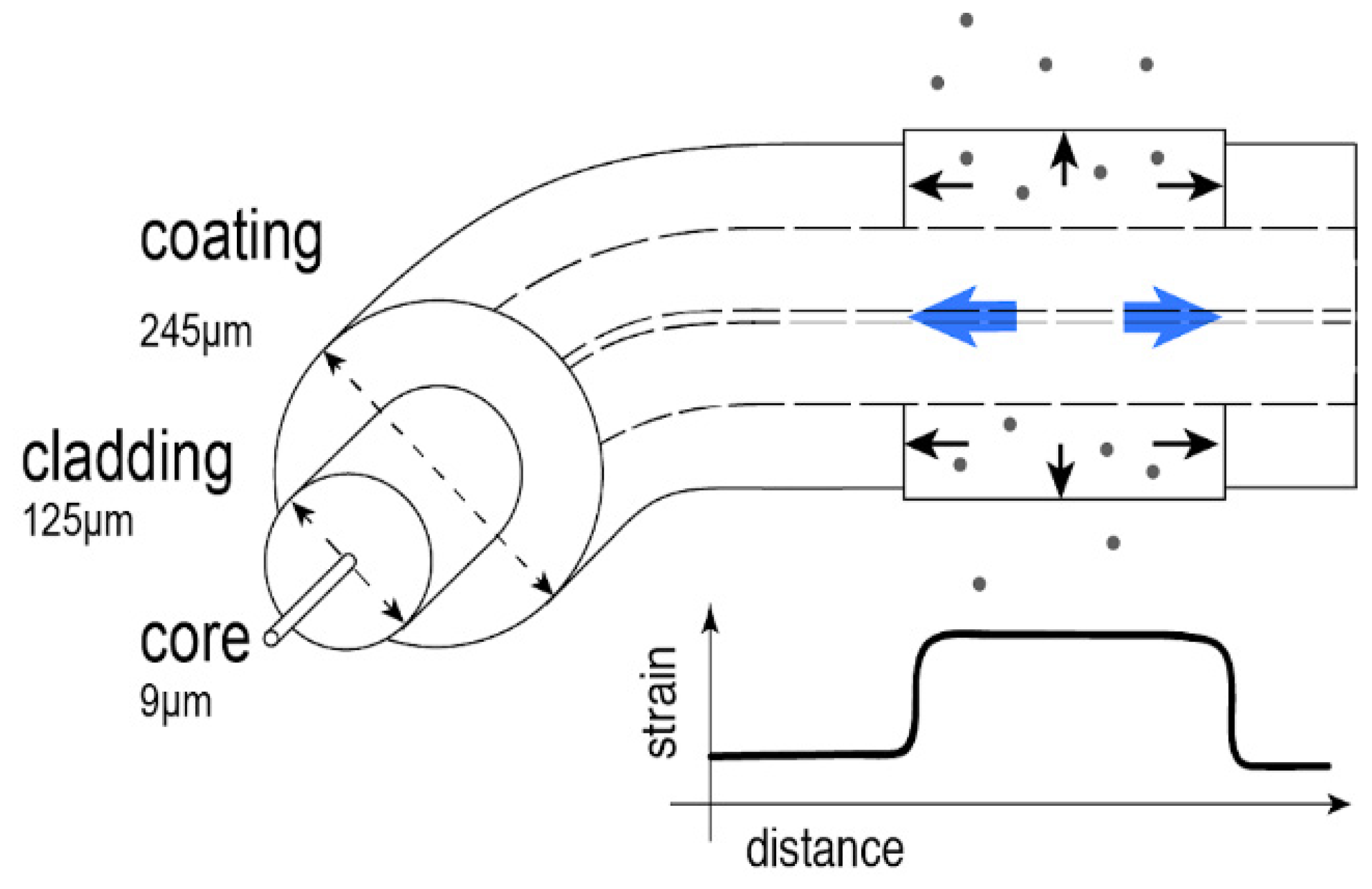
2.1. Chemical Sensing-Based on Swelling Mechanism
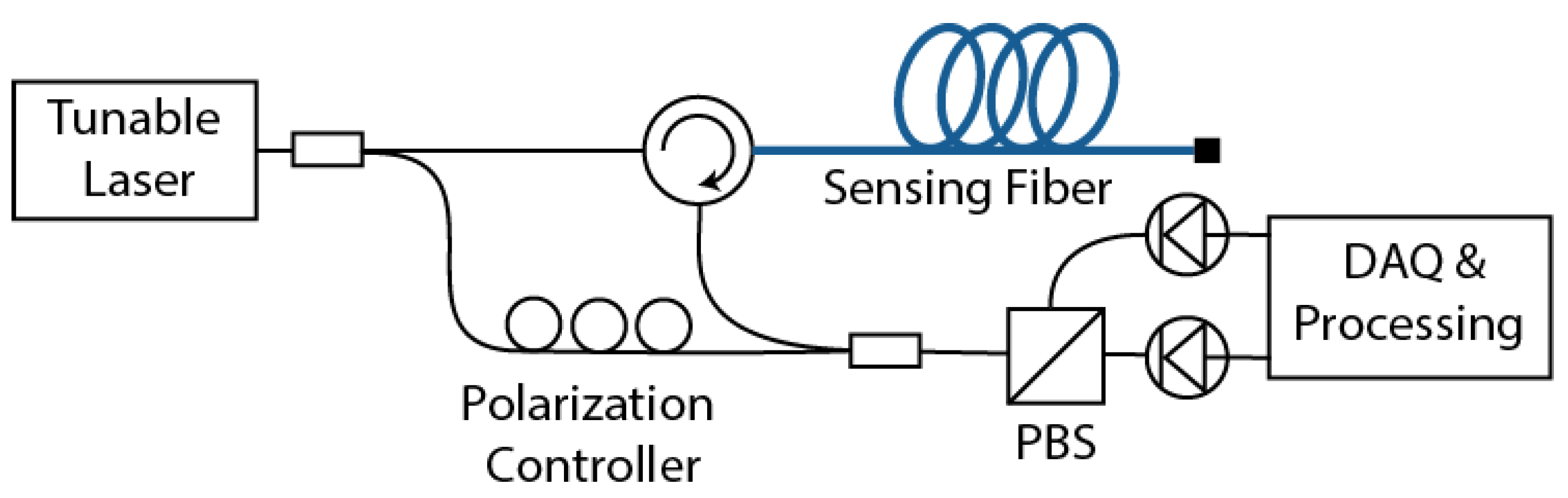
2.2. Optical Frequency-Domain Reflectometry (OFDR)
3. Results
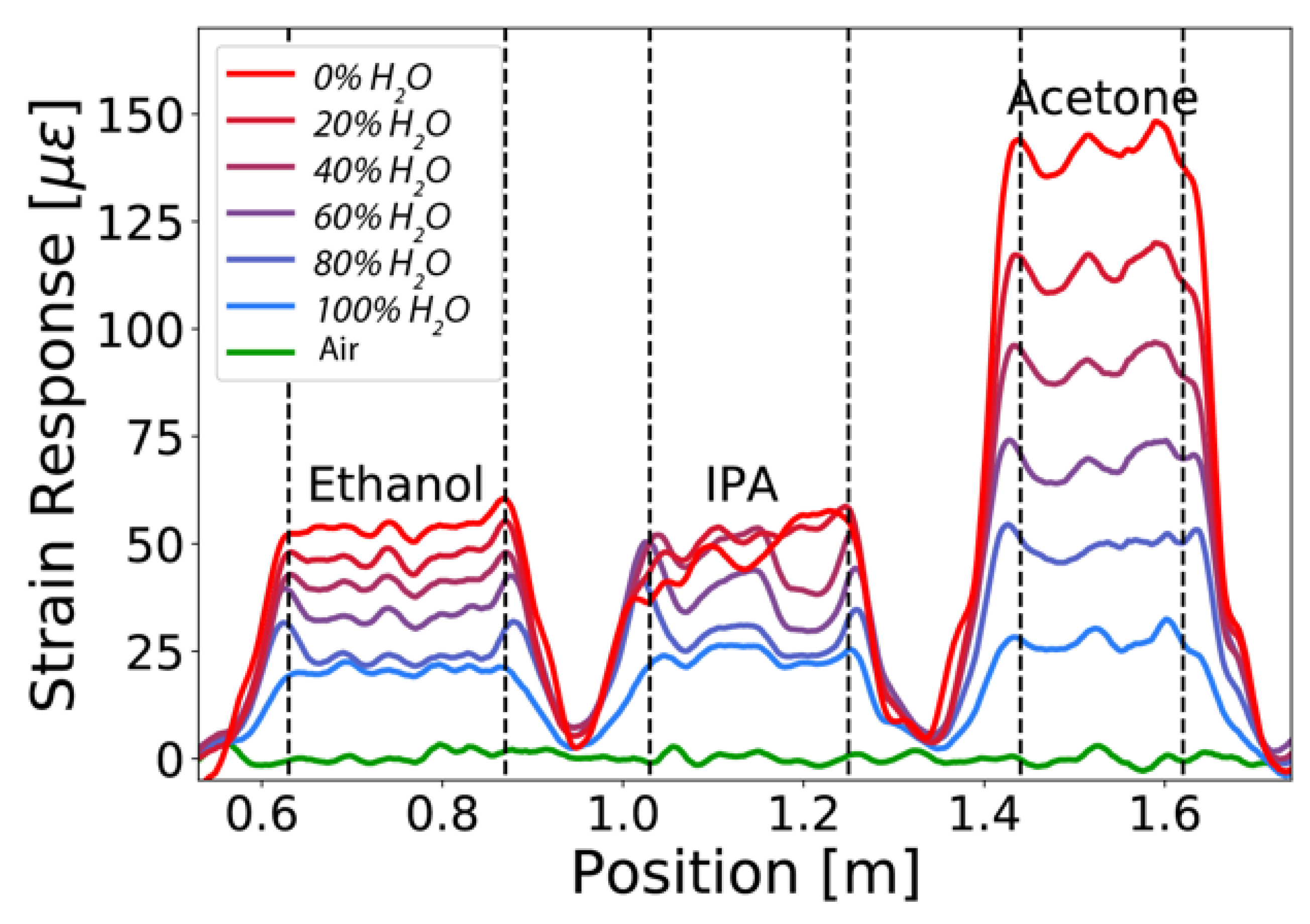
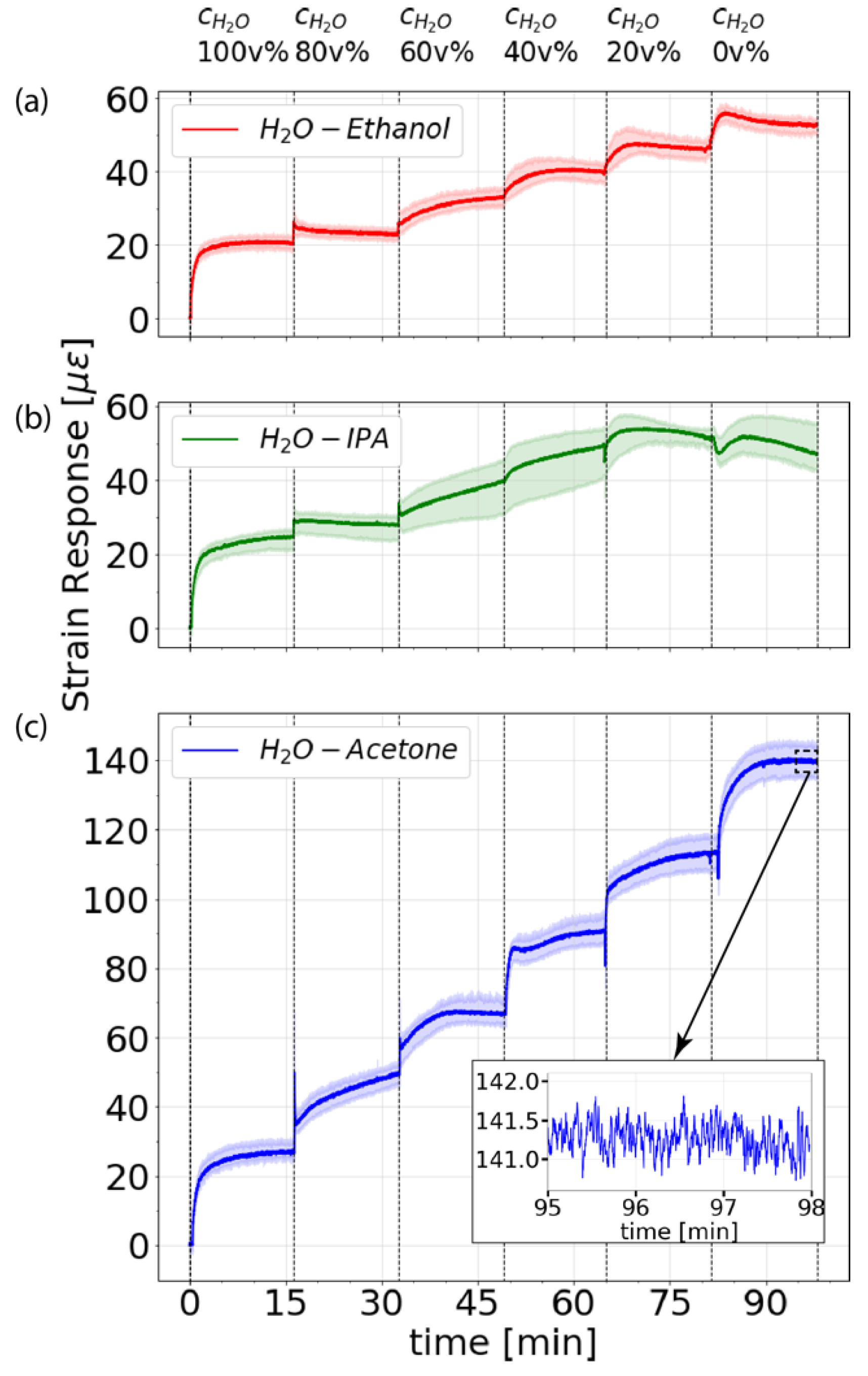
3.1. Distributed Measurements of Water-Alcohol Mixtures
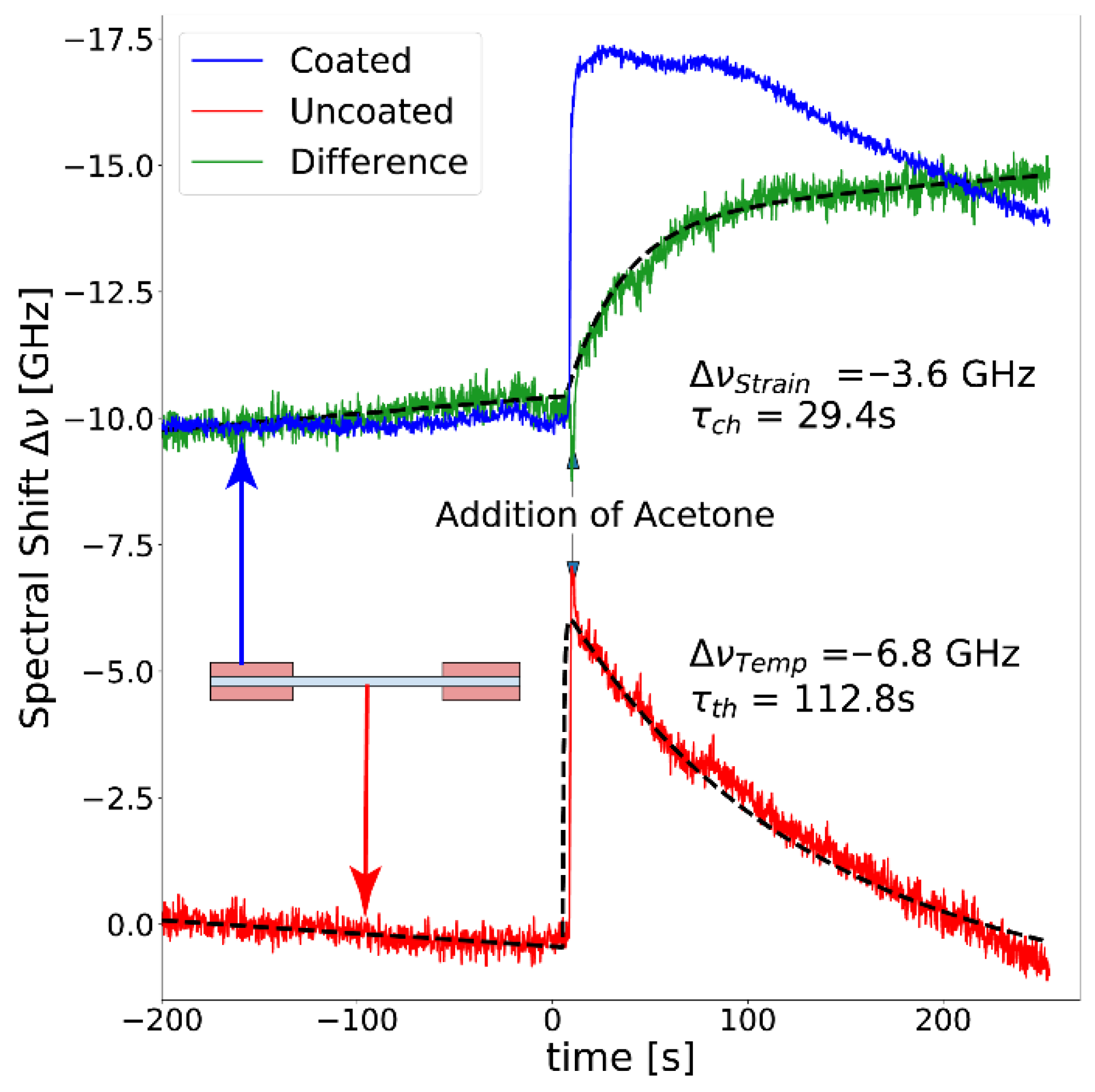
3.2. Monitoring Thermal Effects due to Excess Enthalpy of Mixing of Acetone and Water
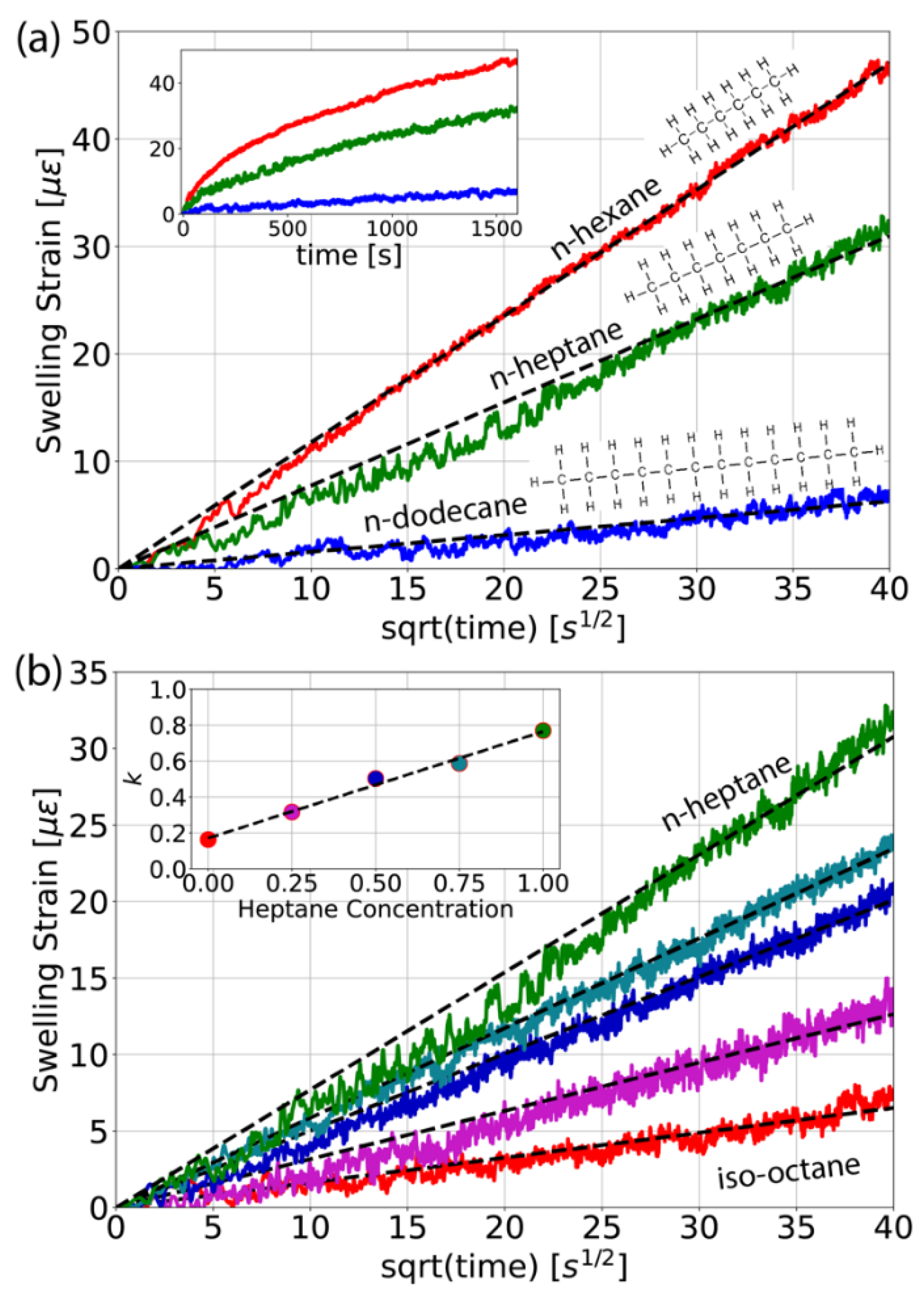
3.3. Measuring n-Alkanes Blends
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Grattan, L.S.; Meggitt, B.T. Optical Fiber Sensor Technology: Fundamentals; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar] [CrossRef]
- Hartog, A.H. An Introduction to Distributed Optical Fibre Sensors; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- Soto, M.A.; Di Pasquale, F. Distributed Raman Sensing. In Handbook of Optical Fibers; Springer Nature: Singapore, 2018. [Google Scholar] [CrossRef]
- Soto, M.A. Distributed Brillouin Sensing: Time-Domain Techniques. In Handbook of Optical Fibers; Springer Nature: Singapore, 2018. [Google Scholar] [CrossRef]
- Fan, X. Distributed Rayleigh Sensing. In Handbook of Optical Fibers; Springer Nature: Singapore, 2018. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kwon, H.; Kim, J.; Song, K.Y. Distributed measurement of hydrostatic pressure based on Brillouin dynamic grating in polarization maintaining fibers. Opt. Express 2016, 24, 21399–21406. [Google Scholar] [CrossRef] [PubMed]
- Schenato, L.; Aneesh, R.; Palmieri, L.; Galtarossa, A.; Pasuto, A. Fiber optic sensor for hydrostatic pressure and temperature measurement in riverbanks monitoring. Optics Laser Technol. 2016, 82, 57–62. [Google Scholar] [CrossRef]
- Thomas, P.J.; Hellevang, J.O. A fully distributed fibre optic sensor for relative humidity measurements. Sens. Actuators B Chem. 2017, 247, 284–289. [Google Scholar] [CrossRef]
- Thomas, P.J.; Hellevang, J.O. A high response polyimide fiber optic sensor for distributed humidity measurements. Sens. Actuators B Chem. 2018, 270, 417–423. [Google Scholar] [CrossRef]
- Liehr, S.; Breithaupt, M.; Krebber, K. Distributed Humidity Sensing in PMMA Optical Fibers at 500 nm and 650 nm Wavelengths. Sensors 2017, 17, 738. [Google Scholar] [CrossRef]
- Palmieri, L.; Sarchi, D.; Galtarossa, A. Distributed measurement of high electric current by means of polarimetric optical fiber sensor. Opt. Express 2015, 23, 11073–11079. [Google Scholar] [CrossRef]
- Masoudi, A.; Newson, T.P. Distributed optical fiber dynamic magnetic field sensor based on magnetostriction. Appl. Opt. 2014, 53, 2833–2838. [Google Scholar] [CrossRef]
- Magalhães, R.; Pereira, J.; Tarasenko, O.; Martin-Lopez, S.; González-Herráez, M.; Margulis, W.; Fidalgo-Martins, H. Towards Distributed Measurements of Electric Fields Using Optical Fibers: Proposal and Proof-of-Concept Experiment. Sensors 2020, 20, 4461. [Google Scholar] [CrossRef]
- Qazi, H.H.; Mohammad, A.B.; Akram, M. Recent Progress in Optical Chemical Sensors. Sensors 2012, 12, 16522–16556. [Google Scholar] [CrossRef]
- Pospíšilová, M.; Kuncová, G.; Trögl, J. Fiber-Optic Chemical Sensors and Fiber-Optic Bio-Sensors. Sensors 2015, 15, 25208–25259. [Google Scholar] [CrossRef]
- Rivero, P.J.; Goicoechea, J.; Arregui, F.J. Optical Fiber Sensors Based on Polymeric Sensitive Coatings. Polymers 2018, 10, 280. [Google Scholar] [CrossRef] [PubMed]
- Eklöv, T.; Sundgren, H.; Lundström, I. Distributed chemical sensing. Sens. Actuators B Chem. 1997, 45, 71–77. [Google Scholar] [CrossRef]
- Lu, X.; Thomas, P.J.; Hellevang, J.O. A Review of Methods for Fibre-Optic Distributed Chemical Sensing. Sensors 2019, 19, 2876. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ruiz, A.; Pastor-Graells, J.; Martins, H.F.; Hey Tow, K.; Thévenaz, L.; Martin-Lopez, S.; Gonzalez-Herraez, M. Distributed photothermal spectroscopy in microstructured optical fibers: Towards high-resolution mapping of gas presence over long distances. Opt. Express 2017, 25, 1789–1805. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Liu, F.; He, X.; Jin, W.; Zhang, M.; Yang, F.; Lut Ho, H.; Tan, Y.; Gu, L. Distributed gas sensing with optical fibre photothermal interferometry. Opt. Express 2017, 25, 31568–31585. [Google Scholar] [CrossRef]
- Yeo, T.L.; Sun, T.; Grattan, K.T.V.; Parry, D.; Lade, R.; Powell, B.D. Polymer-Coated fiber Bragg grating for relative humidity sensing. IEEE Sensors J. 2005, 5, 1082–1089. [Google Scholar] [CrossRef]
- Boland, P.; Sethuraman, G.; Mendez, A.; Graver, T.; Pestov, D.; Tait, G. Fiber Bragg grating multichemical sensor. Proc. Photonic Sens. Technol. 2006, 637109. [Google Scholar] [CrossRef]
- Michie, W.C.; Culshaw, B.; Konstantaki, M.; McKenzie, I.; Kelly, S.; Graham, N.B.; Moran, C. Distributed pH and water detection using fiber-optic sensors and hydrogels. IEEE J. Lightwave Technol. 1995, 13, 1415–1420. [Google Scholar] [CrossRef]
- Bashan, G.; Diamandi, H.H.; London, Y.; Preter, E.; Zadok, A. Optomechanical time-domain reflectometry. Nat. Commun. 2018, 9, 2991. [Google Scholar] [CrossRef]
- Chow, D.; Yang, Z.; Soto, M.A.; Thévenaz, L. Distributed forward Brillouin sensor based on local light phase recovery. Nat. Commun. 2018, 9, 2990. [Google Scholar] [CrossRef]
- Diamandi, H.H.; London, Y.; Bashan, G.; Zadok, A. Distributed opto-mechanical analysis of liquids outside standard fibers coated with polyimide. APL Photonics 2019, 4, 016105. [Google Scholar] [CrossRef]
- Lavine, B.K.; Kaval, N.; Westover, D.J.; Oxenford, L. New Approaches to Chemical Sensing-Sensors Based on Polymer Swelling. Anal. Lett. 2006, 39, 1773–1783. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, C.; Liu, K.; Jiang, J.; Yang, D.; Pan, G.; Pu, Z.; Liu, T. Distributed Optical Fiber Sensors Based on Optical Frequency Domain Reflectometry: A review. Sensors 2018, 18, 1072. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Soto, M.A.; Thévenaz, L. Temperature-Strain discrimination in distributed optical fiber sensing using phase-sensitive optical time-domain reflectometry. Opt. Express 2017, 25, 16059–16071. [Google Scholar] [CrossRef]
- Ding, Z.; Yang, D.; Du, Y.; Liu, K.; Zhou, Y.; Zhang, R.; Xu, Z.; Jiang, J.; Liu, T. Distributed Strain and Temperature Discrimination Using Two Types of Fiber in OFDR. IEEE Photonics J. 2016, 8, 1–8. [Google Scholar] [CrossRef]
- Zhou, D.-P.; Li, W.; Chen, L.; Bao, X. Distributed Temperature and Strain Discrimination with Stimulated Brillouin Scattering and Rayleigh Backscatter in an Optical Fiber. Sensors 2013, 13, 1836–1845. [Google Scholar] [CrossRef]
- Evchuk, I.Y.; Musii, R.I.; Makitra, R.G.; Pristanskii, R.E. Solubility of Polymethyl Methacrylate in Organic Solvents. Russ. J. Appl. Chem. 2005, 78, 1576–1580. [Google Scholar] [CrossRef]
- Benedetti, A.V.; Cilense, M.; Vollet, D.R.; Montone, R.C. Thermodynamic properties of liquid mixtures. III. Acetone—Water. Thermochimica Acta 1983, 66, 219–223. [Google Scholar] [CrossRef]
- Rondinella, V.V.; Matthewson, M.J. Effect of chemical stripping on the strength and surface morphology of fused silica optical fiber. In Proceedings of the Fiber Optics Reliability and Testing: Benign and Adverse Environments, Boston, MA, USA, 1 March 1994. [Google Scholar] [CrossRef]
- Angell, J.; Bernstein, J.; Gu, R.; Miller, P.; Ogura, G. Laser removal of fiber optic coating. In Proceedings of the International Congress on Applications of Lasers & Electro-Optics, Jacksonville, FL, USA, 15–18 October 2001; pp. 1710–1719. [Google Scholar] [CrossRef]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Academic Press: San Diego, CA, USA, 1991; pp. 9–28. [Google Scholar] [CrossRef]
- Papanu, J.S.; Hess, D.W.; Soane, D.S.; Bell, A.T. Swelling of poly(methyl methacrylate) thin films in low molecular weight alcohols. J. Appl. Polym. Sci. 1990, 39, 803–823. [Google Scholar] [CrossRef]
- Stamatialis, D.F.; Sanopoulou, M.; Raptis, I. Swelling and dissolution behavior of poly(methyl methacrylate) films in methyl ethyl ketone/methyl alcohol mixtures studied by optical techniques. J. Appl. Polym. Sci. 2002, 83, 2823–2834. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedighi, S.; Soto, M.A.; Jderu, A.; Dorobantu, D.; Enachescu, M.; Ziegler, D. Swelling-Based Distributed Chemical Sensing with Standard Acrylate Coated Optical Fibers. Sensors 2021, 21, 718. https://doi.org/10.3390/s21030718
Sedighi S, Soto MA, Jderu A, Dorobantu D, Enachescu M, Ziegler D. Swelling-Based Distributed Chemical Sensing with Standard Acrylate Coated Optical Fibers. Sensors. 2021; 21(3):718. https://doi.org/10.3390/s21030718
Chicago/Turabian StyleSedighi, Sina, Marcelo A. Soto, Alin Jderu, Dorel Dorobantu, Marius Enachescu, and Dominik Ziegler. 2021. "Swelling-Based Distributed Chemical Sensing with Standard Acrylate Coated Optical Fibers" Sensors 21, no. 3: 718. https://doi.org/10.3390/s21030718
APA StyleSedighi, S., Soto, M. A., Jderu, A., Dorobantu, D., Enachescu, M., & Ziegler, D. (2021). Swelling-Based Distributed Chemical Sensing with Standard Acrylate Coated Optical Fibers. Sensors, 21(3), 718. https://doi.org/10.3390/s21030718




