Immunoglobulin E Detection Method Based on Cascade Enzymatic Reaction Utilizing Portable Personal Glucose Meter
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Capture Particle
2.3. Characterization of Capture Particle
2.4. Formation of a Magnetic Bead-Based Sandwich Complex
2.5. Human IgE Assay Based on CER Utilizing PGM
2.6. Human IgE Assay Based on Colorimetric p-NPP
2.7. Commercialized ELISA Kit
2.8. Zeta Potential Analysis of Capture Particles and Proteins
2.9. Recovery Test
3. Results and Discussion
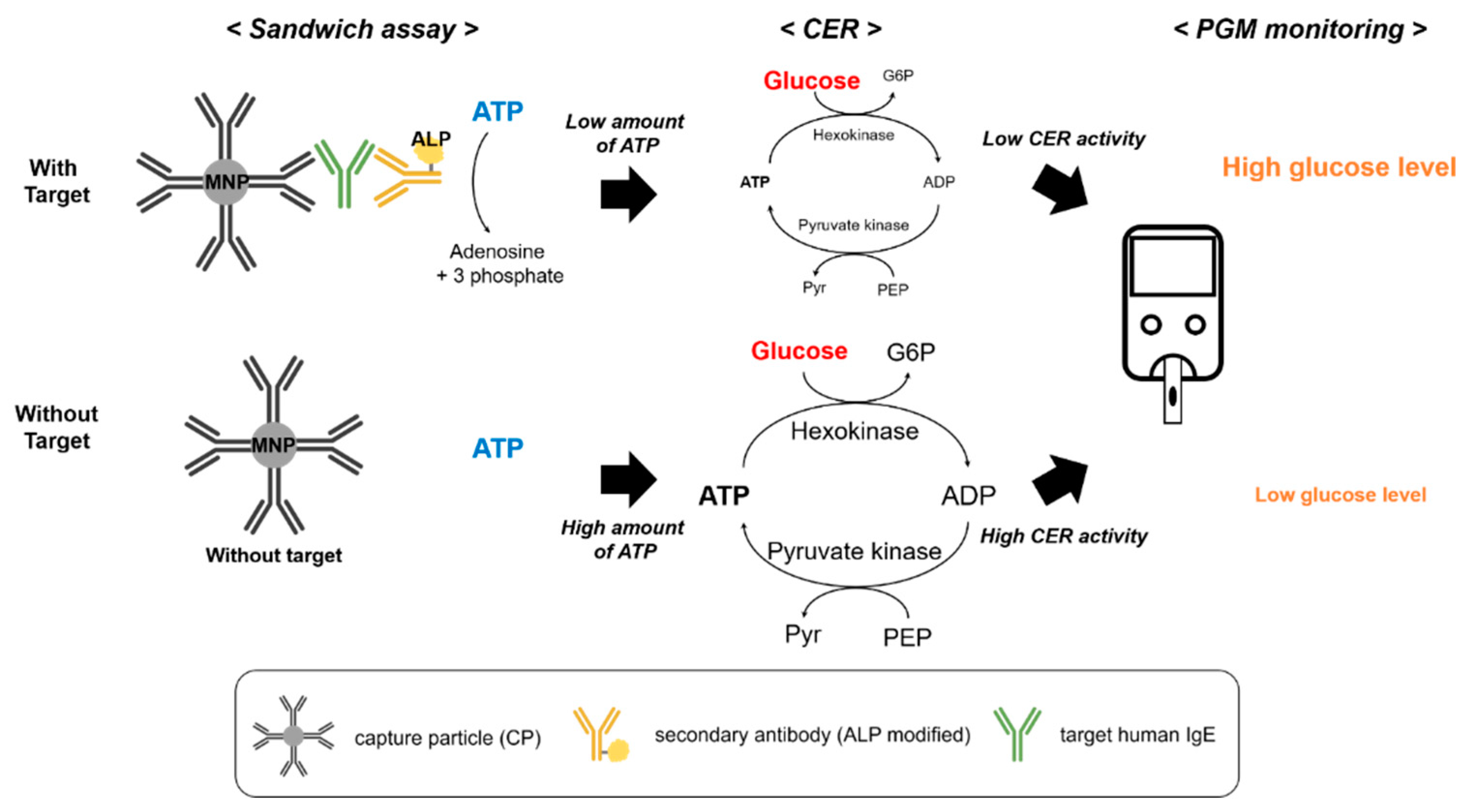
3.1. Detection Principle of the PGM-Based Human IgE Assay
3.2. Characterization of Capture Particle
3.3. Detection Feasibility of the PGM-Based Human IgE Assay
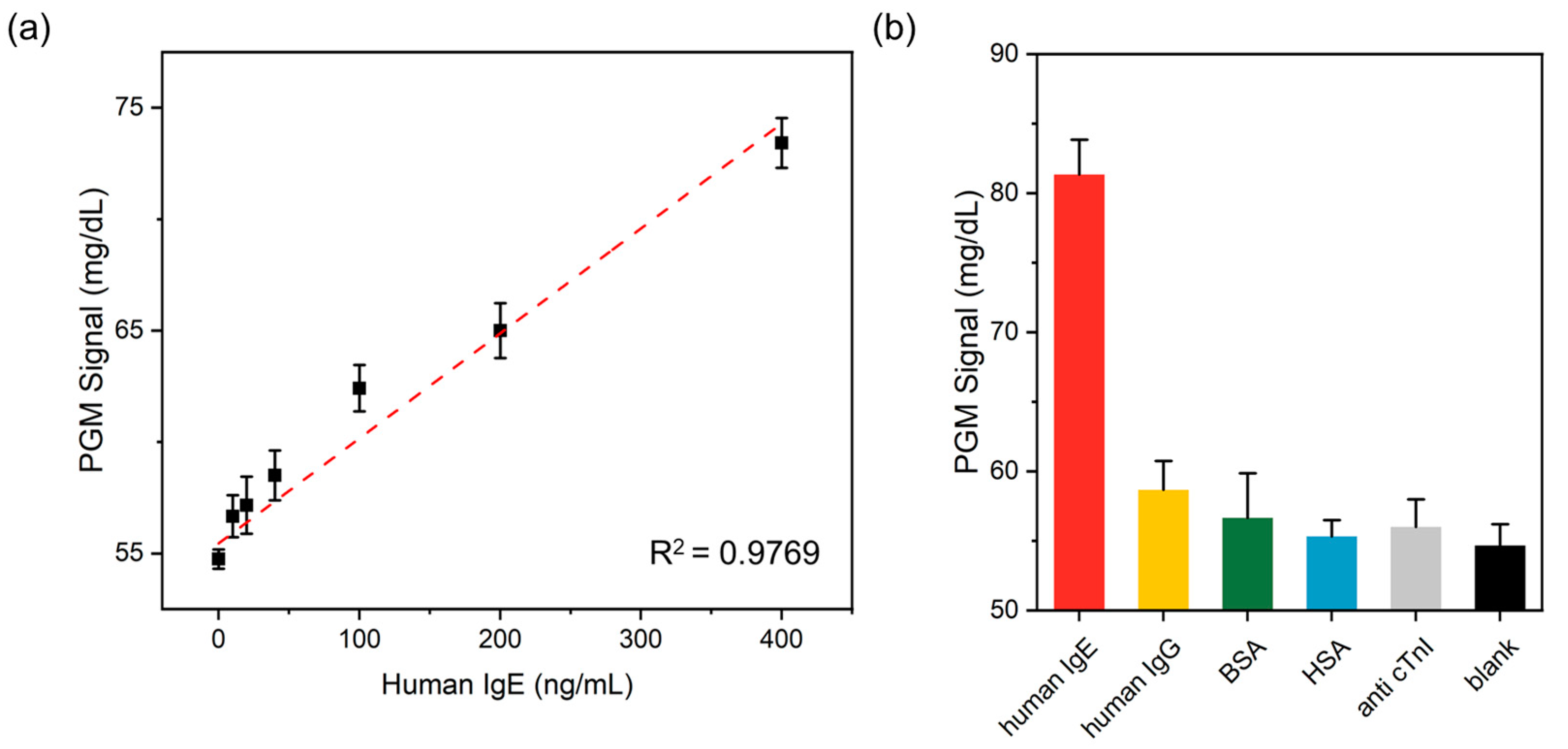
3.4. Sensitivity of the PGM-Based Human IgE Assay
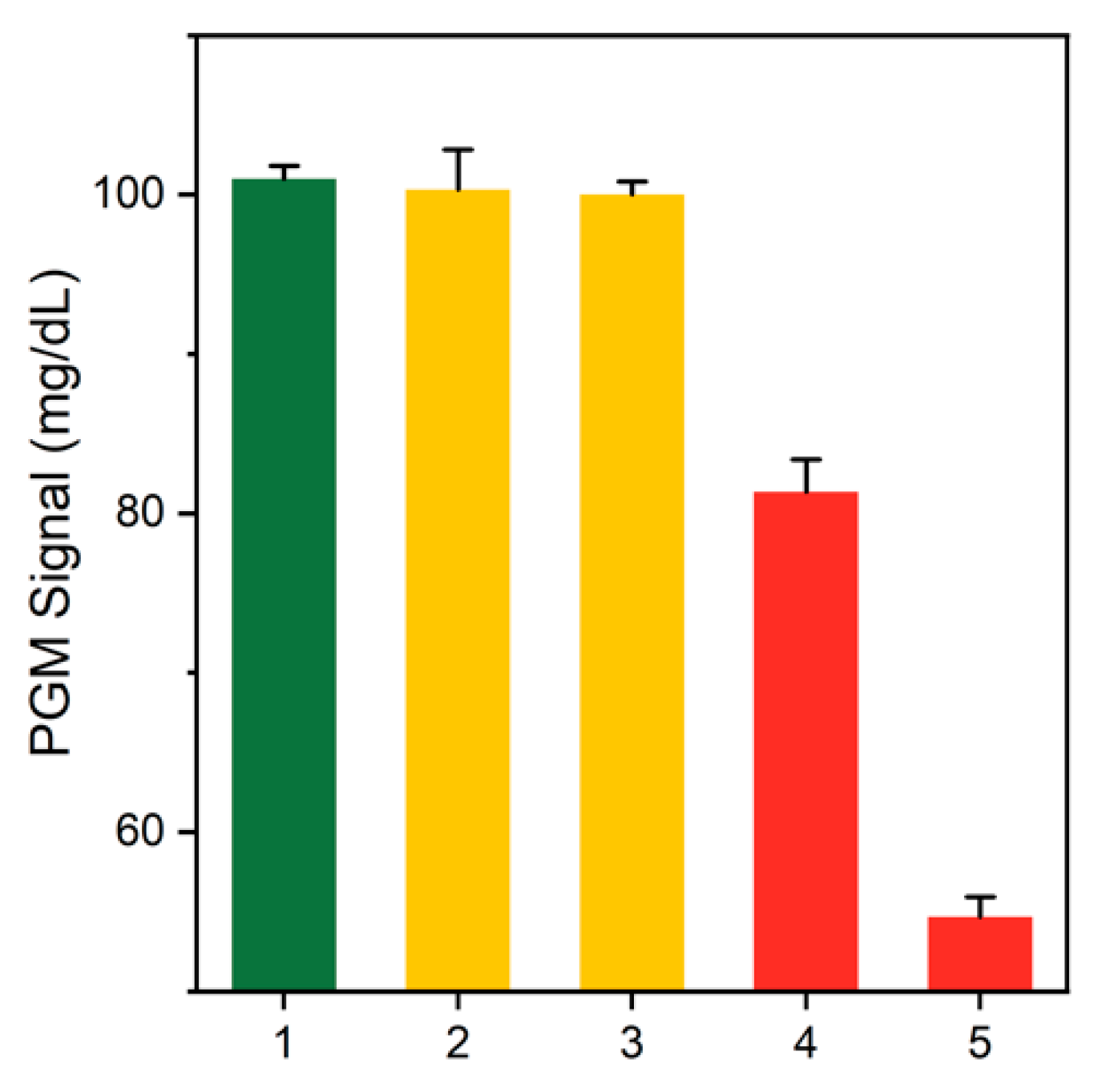
3.5. Selectivity of the PGM-Based Human IgE Assay
3.6. Practical Applicability of the PGM-Based Human IgE Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; duToit, G.; Eigenmann, P.; et al. EAACI Food Allergy and Anaphylaxis Guidelines: Diagnosis and management of food allergy. Allergy 2014, 69, 1008–1025. [Google Scholar] [CrossRef]
- Morais, S.; Tortajada-Genaro, L.A.; Maquieira, Á.; Martinez, M.Á.G. Biosensors for food allergy detection according to specific IgE levels in serum. Trends Anal. Chem. 2020, 127, 115904. [Google Scholar] [CrossRef]
- Holgate, S.T.; Broide, D. New targets for allergic rhinitis—A disease of civilization. Nat. Rev. Drug Discov. 2003, 2, 903–915. [Google Scholar] [CrossRef]
- Winter, W.E.; Hardt, N.S.; Fuhrman, S. Immunoglobulin E: Importance in parasitic infections and hypersensitivity responses. Arch. Pathol. Lab. Med. 2000, 124, 1382–1385. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, J.P.; Han, S.J.; Sim, S.J. Aptamer biosensor for lable-free detection of human immunoglobulin E based on surface plasmon resonance. Sens. Actuators B Chem. 2009, 139, 471–475. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, M.; Jiao, M.; Luo, X. Antifouling and ultrasensitive biosensing interface based on self-assembled peptide and aptamer on macroporous gold for electrochemical detection of immunoglobulin E in serum. Anal. Bioanal. Chem. 2018, 410, 5871–5878. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.X.; Busnel, J.M.; Peltre, G.; Zhang, X.X.; Girault, H.H. Magnetic beads based immunoaffinity capillary electrophoresis of total serum IgE with laser-induced fluorescence detection. Anal. Chem. 2008, 80, 9583–9588. [Google Scholar] [PubMed]
- Proczek, G.; Gassner, A.L.; Busnel, J.M.; Girault, H.H. Total serum IgE quantification by microfluidic ELISA using magnetic beads. Anal. Bioanal. Chem. 2012, 402, 2645–2653. [Google Scholar]
- King, C.L.; Poindexter, R.W.; Ragunathan, J.; Fleisher, T.A.; Ottesen, E.A.; Nutman, T.B. Frequency analysis of IgE-secreting B lymphocytes in persons with normal or elevated serum IgE levels. J. Immunol. 1991, 146, 1478–1483. [Google Scholar] [PubMed]
- Peavy, R.D.; Metcalfe, D.D. Understanding the mechanisms of anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 310. [Google Scholar] [PubMed]
- Oh, S.J.; Ahn, J.K.; Park, H.; Song, Y.; Kwon, S.J.; Shin, H.B. An electrochemical immunosensing system on patterned electrodes for immunoglobulin E detection. Anal. Methods 2019, 11, 4410–4415. [Google Scholar] [CrossRef]
- Ebo, D.G.; Hagendorens, M.M.; Bridts, C.H.; Schuerwegh, A.J.; De Clerck, L.S.; Stevens, W.J. In vitro allergy diagnosis: Should we follow the flow? Clin. Exp. Allergy 2004, 34, 332–339. [Google Scholar] [CrossRef]
- Johansson, S.G. Radioimmunoassay of IgE and IgE antibody and its clinical application. J. Clin. Pathol. 1975, 6, 33. [Google Scholar] [CrossRef][Green Version]
- Grange, R.D.; Thompson, J.P.; Lambert, D.G. Radioimmunoassay, enzyme and non-enzyme-based immunoassays. Br. J. Anaesth. 2014, 112, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Guilloux, L.; Ricard-Blum, S.; Ville, G.; Motin, J. A new radioimmunoassay using a commercially available solid support for the detection of IgE antibodies against muscle relaxants. J. Allergy Clin. Immunol. 1992, 90, 153–159. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, X.; Chen, Y.; Woods, R.; Lee, N.; Yang, H.; Chowdhury, P.S.; Roskos, L.K.; White, W.I. An electrochemiluminescence (ECL)-based assay for the specific detection of anti-drug antibodies of the IgE isotype. J. Pharm. Biomed. Anal. 2013, 86, 73–81. [Google Scholar] [CrossRef]
- Shi, G.F.; Cao, J.T.; Zhang, J.J.; Liu, Y.M.; Chen, Y.H.; Ren, S.W. An electrochemiluminescence aptasensor based on flowerlike CdS–MoS2 composites and DNAzyme for detection of immunoglobulin E. Sens. Actuators B Chem. 2015, 220, 340–346. [Google Scholar] [CrossRef]
- Cao, J.; Wang, H.; Liu, Y. Petal-like CdS nanospheres-based electrochemiluminescence aptasensor for detection of IgE with gold nanoparticles amplification. Spectrochim. Acta A 2015, 151, 274–279. [Google Scholar] [CrossRef]
- Ghosh, S.; Aggarwal, K.; Vinitha, T.U.; Nguyen, T.; Han, J.; Ahn, C.H. A new microchannel capillary flow assay (MCFA) platform with lyophilized chemiluminescence reagents for a smartphone-based POCT detecting malaria. Microsyst. Nanoeng. 2020, 6, 1–18. [Google Scholar] [CrossRef]
- Liu, D.; Li, X.; Zhou, J.; Liu, S.; Tian, T.; Song, Y.; Zhu, Z.; Zhou, L.; Ji, T.H.; Yang, C. A fully integrated distance readout ELISA-Chip for point-of-care testing with sample-in-answer-out capability. Biosens. Bioelectron. 2017, 96, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Lu, Y. Using personal glucose meters and functional DNA sensors to quantify a variety of analytical targets. Nat. Chem. 2011, 3, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Lu, Y. An invasive DNA approach toward a general method for portable quantification of metal ions using a personal glucose meter. Chem. Commun. 2013, 49, 585–587. [Google Scholar] [CrossRef]
- Xiang, Y.; Lu, Y. Portable and quantitative detection of protein biomarkers and small molecular toxins using antibodies and ubiquitous personal glucose meters. Anal. Chem. 2012, 84, 4174–4178. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Lu, Y. Using commercially available personal glucose meters for portable quantification of DNA. Anal. Chem. 2012, 84, 1975–1980. [Google Scholar] [CrossRef]
- Zhou, Z.; Xiang, Y.; Tong, A.; Lu, Y. Simple and efficient method to purify DNA–protein conjugates and its sensing applications. Anal. Chem. 2014, 86, 3869–3875. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Lan, T.; Shi, H.; Lu, Y. Portable detection of melamine in milk using a personal glucose meter based on an in vitro selected structure-switching aptamer. Anal. Chem. 2015, 87, 7676–7682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Luan, Y.; Xiong, M.; Zhang, J.; Lake, R.; Lu, Y. DNAzyme Amplified Aptasensing Platform for Ochratoxin A Detection Using a Personal Glucose Meter. ACS Appl. Mater. Interfaces 2021, 13, 9472–9481. [Google Scholar] [CrossRef]
- Xu, J.; Qiao, X.; Zhang, J.; Cheng, N.; Sheng, Q.; Zheng, J.; Cao, W.; Yue, T.; Lu, Y. Point-of-care monitoring of intracellular glutathione and serum triglyceride levels using a versatile personal glucose meter. Anal. Methods 2019, 11, 1849–1856. [Google Scholar] [CrossRef]
- Shan, Y.; Zhang, Y.; Kang, W.; Wang, B.; Li, J.; Wu, X.; Wang, S.; Liu, F. Quantitative and selective DNA detection with portable personal glucose meter using loop-based DNA competitive hybridization strategy. Sens. Actuators B Chem. 2019, 282, 197–203. [Google Scholar] [CrossRef]
- Wu, T.; Yang, Y.; Cao, Y.; Song, Y.; Xu, L.P.; Zhang, X.; Wang, S. Bioinspired DNA–inorganic hybrid nanoflowers combined with a personal glucose meter for onsite detection of miRNA. ACS Appl. Mater. Interfaces 2018, 10, 42050–42057. [Google Scholar] [CrossRef] [PubMed]
- Lisi, F.; Peterson, J.R.; Gooding, J.J. The application of personal glucose meters as universal point-of-care diagnostic tools. Biosens. Bioelectron. 2020, 148, 111835. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, T.; Xu, L.P.; Zhang, X. Portable detection of Staphylococcus aureus using personal glucose meter based on hybridization chain reaction strategy. Talanta 2021, 226, 122132. [Google Scholar] [CrossRef]
- Ahn, J.K.; Kim, H.Y.; Park, K.S.; Park, H.G. A personal glucose meter for label-free and washing-free biomolecular detection. Anal. Chem. 2018, 90, 11340–11343. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.K.; Kim, H.Y.; Lee, C.Y.; Park, K.S.; Park, H.G. Label-free and washing-free alkaline phosphatase assay using a personal glucose meter. J. Biol. Eng. 2019, 13, 1–5. [Google Scholar] [CrossRef]
- Kim, H.Y.; Ahn, J.K.; Park, K.S.; Park, H.G. Portable glucose meter-based label-free strategy for target DNA detection. Sens. Actuators B Chem. 2020, 310, 127808. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, C.Y.; Kim, H.; Park, K.S.; Park, H.G. Portable glucose meter-utilized label-free and washing-free telomerase assay. Analyst. 2020, 145, 5578–5583. [Google Scholar] [CrossRef] [PubMed]
- Monroe, M.R.; Reddington, A.P.; Collins, A.D.; LaBoda, C.; Cretich, M.; Chiari, M.; Little, F.F.; UÜnlü, M.S. Multiplexed method to calibrate and quantitate fluorescence signal for allergen-specific IgE. Anal. Chem. 2011, 83, 9485–9491. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, K.I.; Kreuzer, M.P.; Guilbault, G.G. Viability of allergy (IgE) detection using an alternative aptamer receptor and electrochemical means. Sens. Actuators B Chem. 2007, 121, 178–186. [Google Scholar] [CrossRef]
- Chinnasamy, T.; Segerink, L.I.; Nystrand, M.; Gantelius, J.; Svahn, H.A. A lateral flow paper microarray for rapid allergy point of care diagnostics. Analyst 2014, 139, 2348–2354. [Google Scholar] [CrossRef]
- Reuterswärd, P.; Gantelius, J.; Svahn, H.A. An 8 min colorimetric paper-based reverse phase vertical flow serum microarray for screening of hyper IgE syndrome. Analyst 2015, 140, 7327–7334. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Lee, S.J.; Lee, H.J. Ultra-sensitive detection of IgE using biofunctionalized nanoparticle-enhanced SPR. Talanta 2010, 81, 1755–1759. [Google Scholar] [CrossRef]
- Shyur, S.D.; Jan, R.L.; Webster, J.R.; Chang, P.; Lu, Y.J.; Wang, J.Y. Determination of multiple allergen-specific IgE by microfluidic immunoassay cartridge in clinical settings. Pediatr. Allergy Immunol. 2010, 21, 623–633. [Google Scholar] [CrossRef]
- Tai, L.W.; Tseng, K.Y.; Wang, S.T.; Chiu, C.C.; Kow, C.H.; Chang, P.; Chen, C.; Wang, J.Y.; Webster, J.R. An automated microfluidic-based immunoassay cartridge for allergen screening and other multiplexed assays. Anal. Biochem. 2009, 391, 98–105. [Google Scholar] [CrossRef] [PubMed]
- González, T.Y.; Leonard, A.; Gaude, V.; Delplanque, A.; Barre, A.; Rougé, P.; Garnier, L.; Bienvenu, F.; Bienvenu, J.; Zelsmann, M.; et al. IgE detection in allergic patient’s serum by absorption analysis of biofunctionalised microparticles. Microelectron. Eng. 2019, 207, 27–32. [Google Scholar] [CrossRef]
- Platt, G.W.; Damin, F.; Swann, M.J.; Metton, I.; Skorski, G.; Cretich, M.; Chiari, M. Allergen immobilisation and signal amplification by quantum dots for use in a biosensor assay of IgE in serum. Biosens. Bioelectron. 2014, 52, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Z.; Niu, Q.; Li, H.; Vuki, M.; Xu, D. Visual microarray detection for human IgE based on silver nanoparticles. Sens. Actuators B Chem. 2017, 239, 45–51. [Google Scholar] [CrossRef]
- Teste, B.; Malloggi, F.; Siaugue, J.M.; Varenne, A.; Kanoufi, F.; Descroix, S. Microchip integrating magnetic nanoparticles for allergy diagnosis. Lab. Chip 2011, 11, 4207–4213. [Google Scholar] [CrossRef] [PubMed]
- Unal, D.; Gelincik, A.; Elitok, A.; Demir, S.; Olgac, M.; Coskun, R.; Kocaaga, M.; Colakoglu, B.; Buyukozturk, S. Impact of high serum Immunoglobulin E levels on the risk of atherosclerosis in humans. Asia Pac. Allergy 2017, 7, 74–81. [Google Scholar] [CrossRef]
- Ahn, J.K.; Park, K.S.; Won, B.Y.; Park, H.G. A novel electrochemical method to detect theophylline utilizing silver ions captured within abasic site-incorporated duplex DNA. Biosens. Bioelectron. 2015, 67, 590–594. [Google Scholar] [CrossRef]
- Ahn, J.K.; Kim, H.Y.; Baek, S.; Park, H.G. A new s-adenosylhomocysteine hydrolase-linked method for adenosine detection based on DNA-templated fluorescent Cu/Ag nanoclusters. Biosens. Bioelectron. 2017, 93, 330–334. [Google Scholar] [CrossRef]

| Method | Key Elements | LOD (ng/mL) | Sample | Ref. |
|---|---|---|---|---|
| Arrays | Indirect assay with bound allergens | 49.3 | Non-diluted serum | [37] |
| Electrochemical | Aptasensor | 300 | Non-diluted serum | [38] |
| Immunochemical | Paper-based assay | 2.4 | Non-diluted serum | [39] |
| Vertical flow assays | 1900 | Diluted serum (10%) | [40] | |
| Label-free | SPR | 190 | Buffer solution | [41] |
| Microfluidics | Miniaturized array | 27 | Non-diluted serum | [42] |
| 2.4 | Non-diluted serum | [43] | ||
| Miniaturized immunodiffusion | 1 | Diluted serum (20%) | [43] | |
| Nanomaterial-based | Magnetic capture | 24 | Diluted serum | [44] |
| Quantum dots | 84 | Diluted serum (2%) | [45] | |
| Silver particle | 20 | Diluted serum (20%) | [46] | |
| PGM | Cascade enzymatic reaction | 29.6 | Non-diluted serum | This work |
| Added IgE (μg/mL) | Measured IgE (μg/mL) | SD | CV (%) | Recovery (%) |
|---|---|---|---|---|
| 1.0 | 1.05 | 0.082 | 7.75 | 105.21 |
| 0.5 | 0.49 | 0.036 | 7.35 | 99.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, H.; Park, J.; Ahn, J.K. Immunoglobulin E Detection Method Based on Cascade Enzymatic Reaction Utilizing Portable Personal Glucose Meter. Sensors 2021, 21, 6396. https://doi.org/10.3390/s21196396
Han H, Park J, Ahn JK. Immunoglobulin E Detection Method Based on Cascade Enzymatic Reaction Utilizing Portable Personal Glucose Meter. Sensors. 2021; 21(19):6396. https://doi.org/10.3390/s21196396
Chicago/Turabian StyleHan, Hyogu, Junhyun Park, and Jun Ki Ahn. 2021. "Immunoglobulin E Detection Method Based on Cascade Enzymatic Reaction Utilizing Portable Personal Glucose Meter" Sensors 21, no. 19: 6396. https://doi.org/10.3390/s21196396
APA StyleHan, H., Park, J., & Ahn, J. K. (2021). Immunoglobulin E Detection Method Based on Cascade Enzymatic Reaction Utilizing Portable Personal Glucose Meter. Sensors, 21(19), 6396. https://doi.org/10.3390/s21196396







