The Protraction and Retraction Angles of Horse Limbs: An Estimation during Trotting Using Inertial Sensors
Abstract
1. Introduction
2. Materials and Methods
2.1. Horses
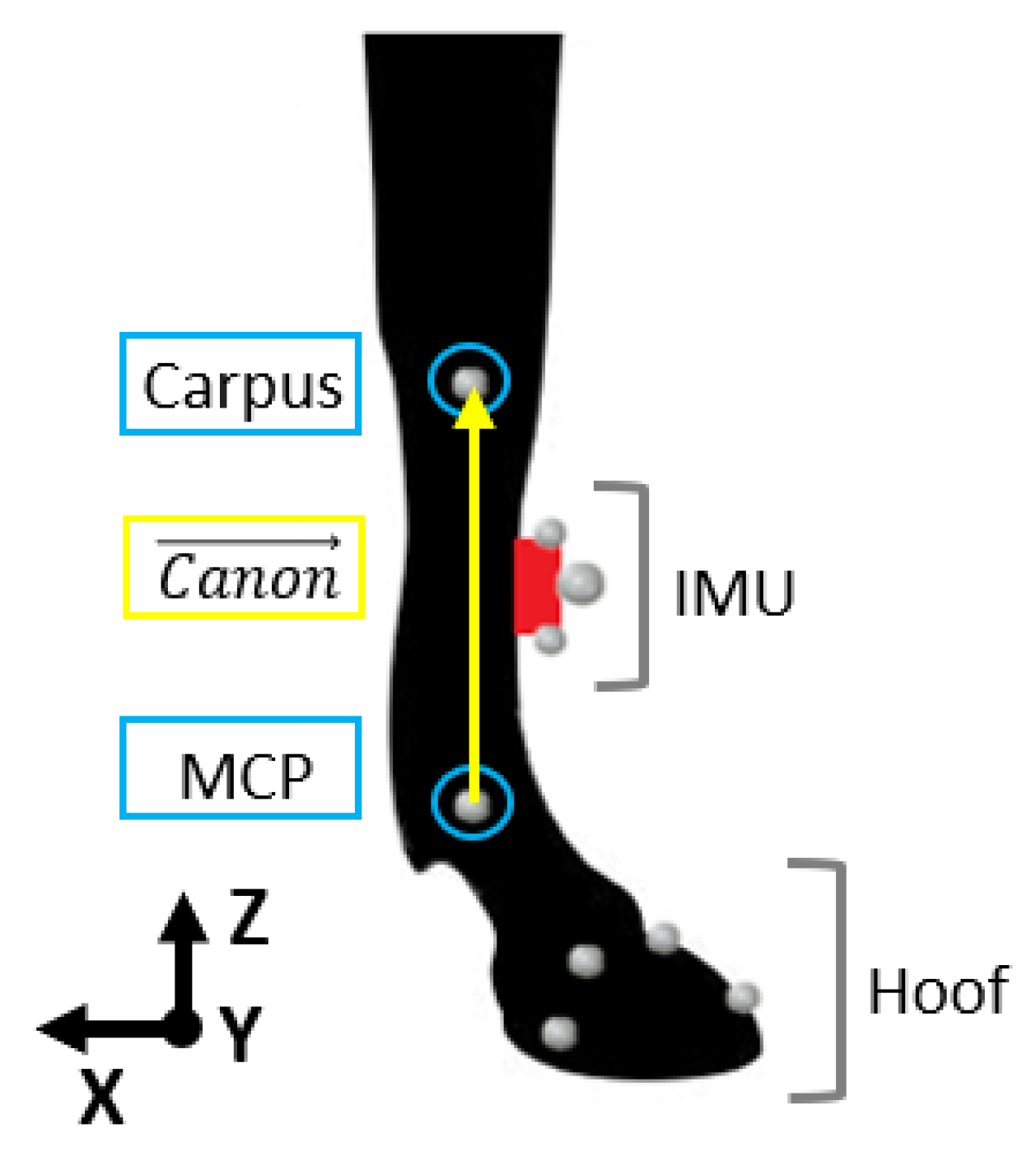
2.2. Data Acquisition
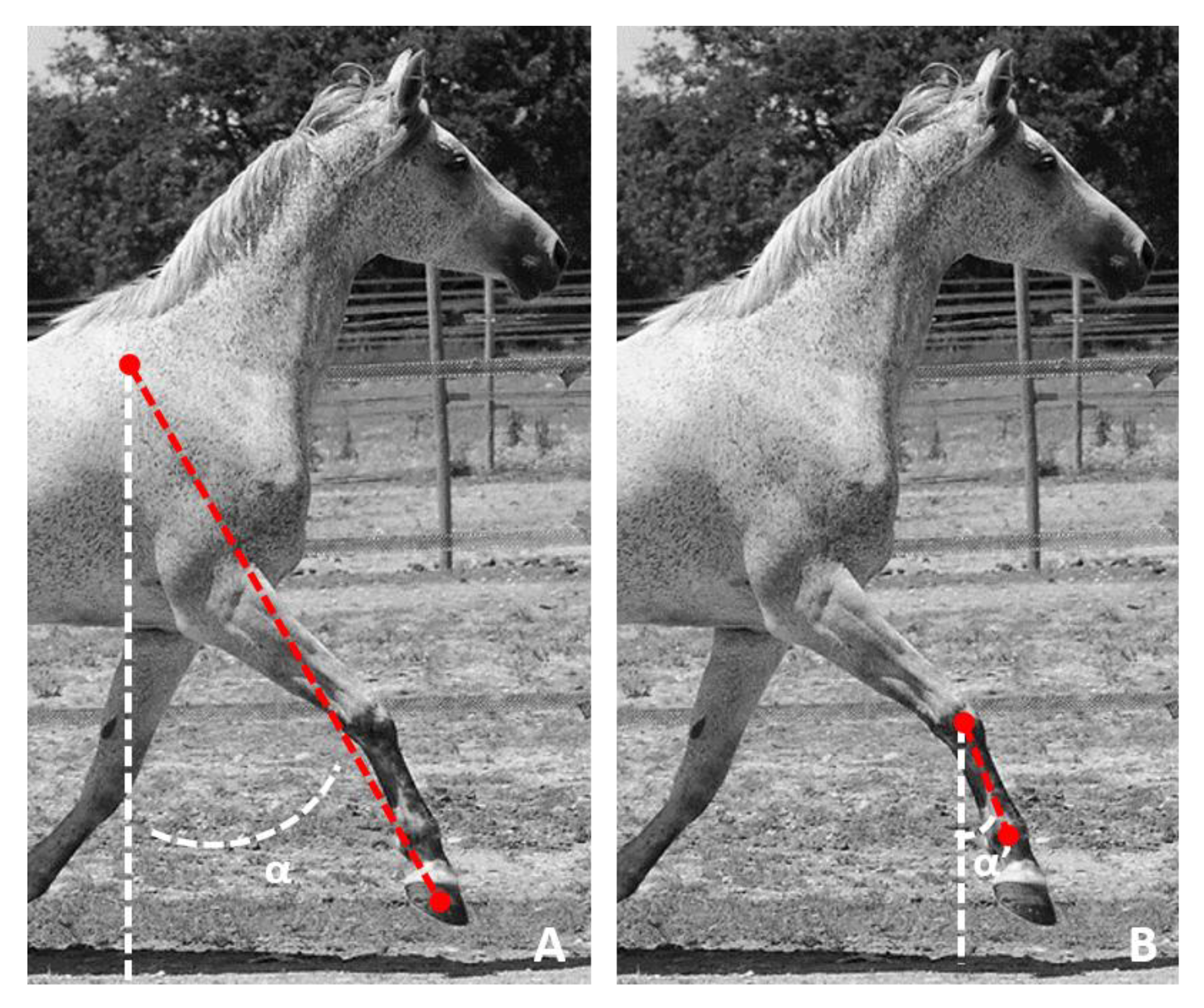
2.3. MOCAP Data Processing
- (1)
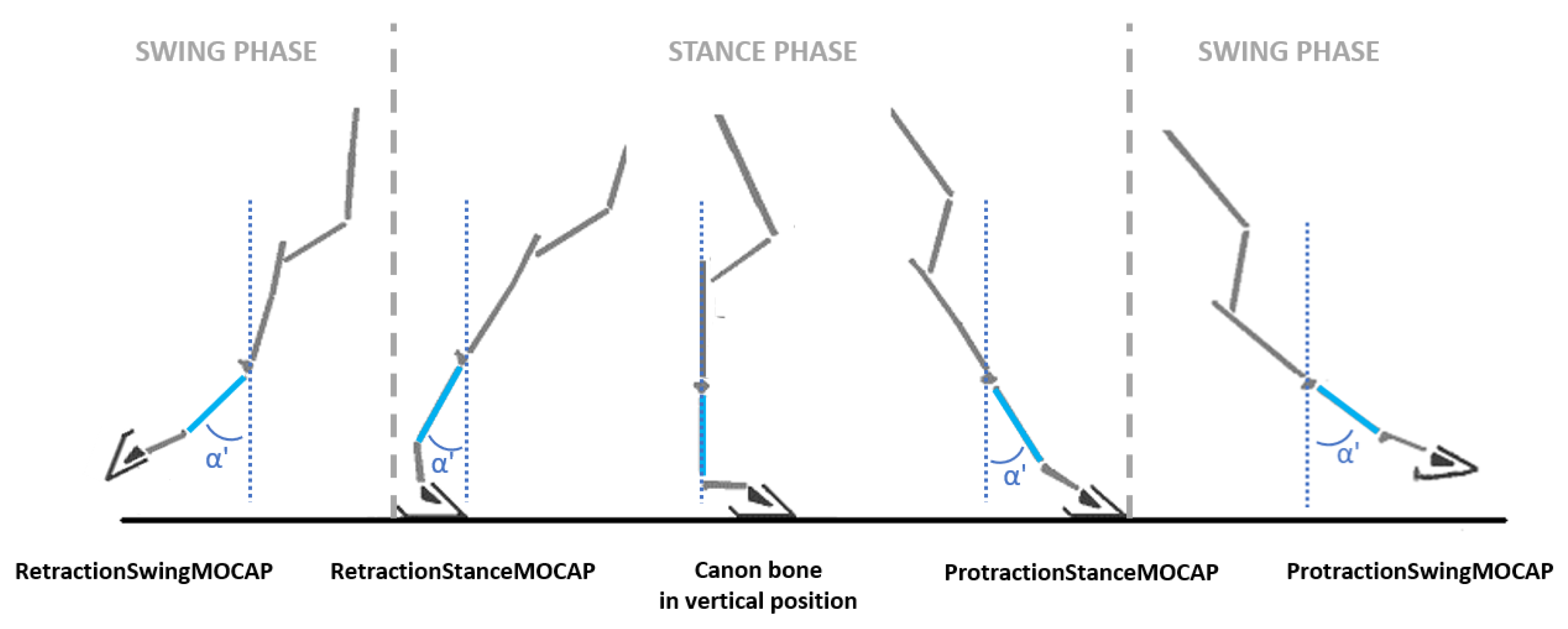
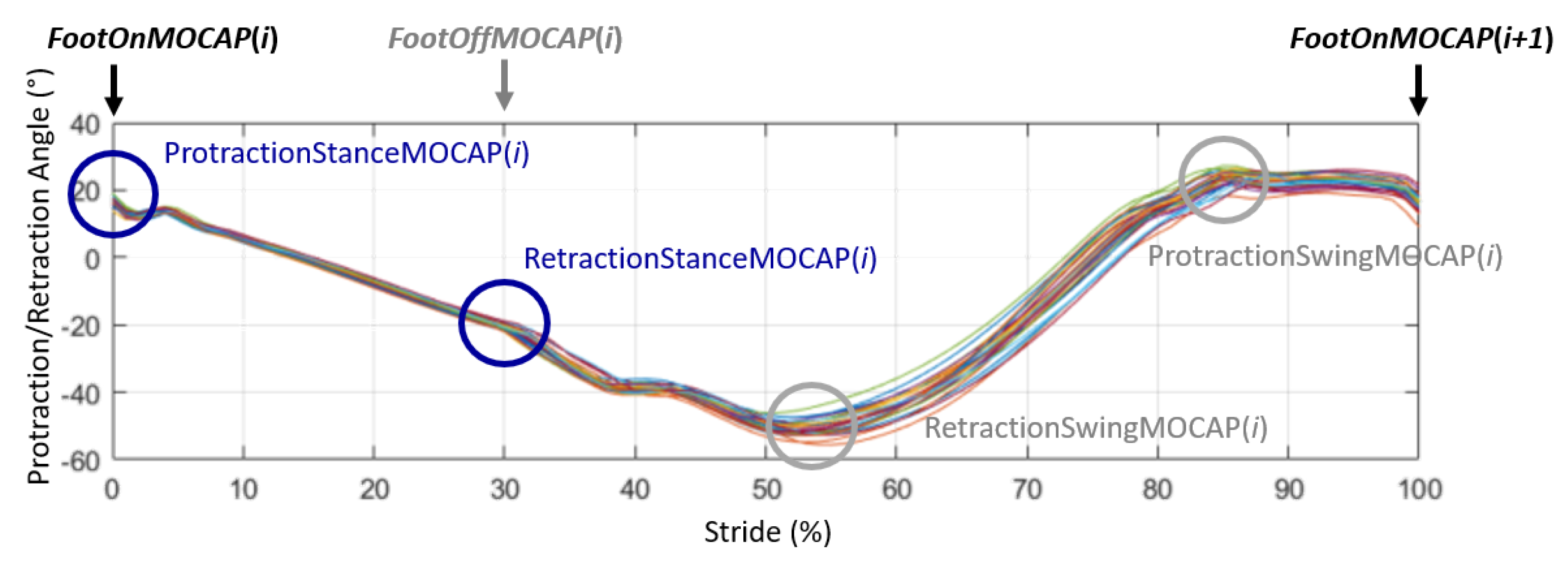
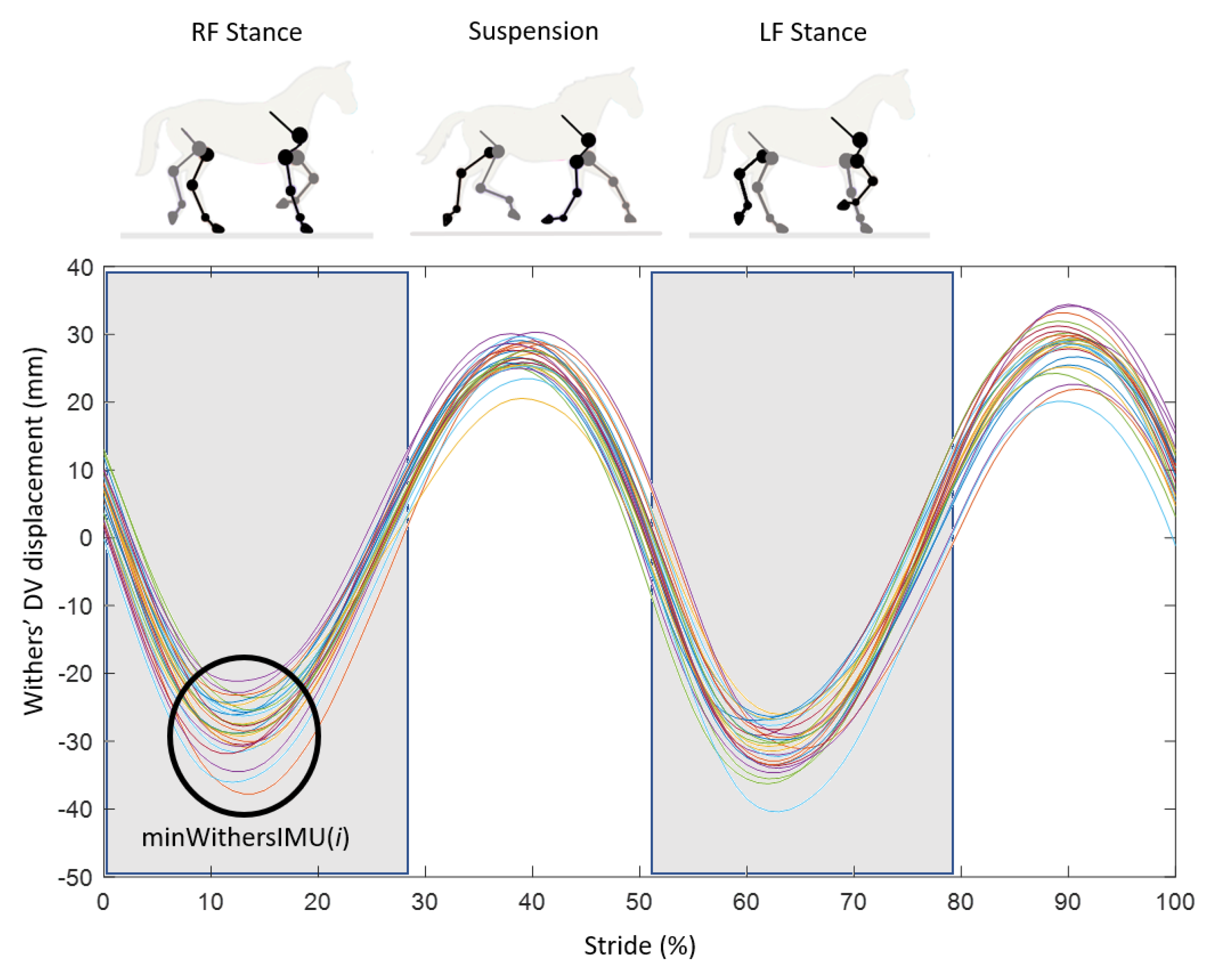
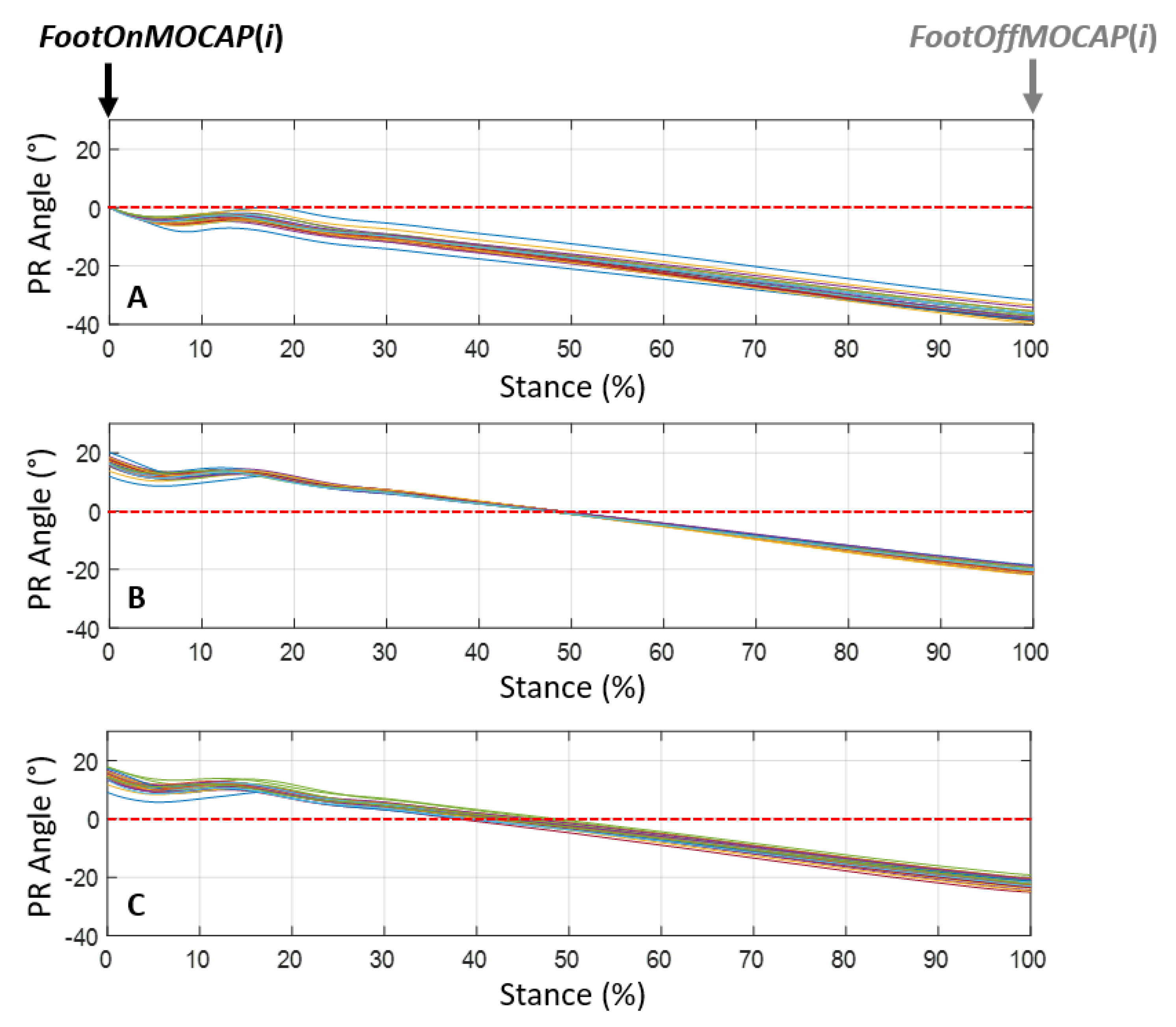
- During the stance phase when the limb was in protraction at the start of the stance phase (initial contact of the hoof flat on the ground) and when the limb was in retraction at the end of the stance phase, at the moment preceding the breakover of the hoof. These events were called, respectively, ProtractionStanceMOCAP(i) and RetractionStanceMOCAP(i) with i corresponding with the stride cycle ranging from 1 to n (n > 25) for each recorded trial (Figure 3).
- (2)
- During the swing phase, when the limb was in maximum extension, which corresponded with the maximum protraction and when the limb was in the maximum retraction. These events were called, respectively, ProtractionSwingMOCAP(i) and RetractionSwingMOCAP(i) with i corresponding with the stride cycle ranging from 1 to n (n > 25) for each recorded trial (Figure 3).
2.4. Data Processing of the IMUs
- (i)
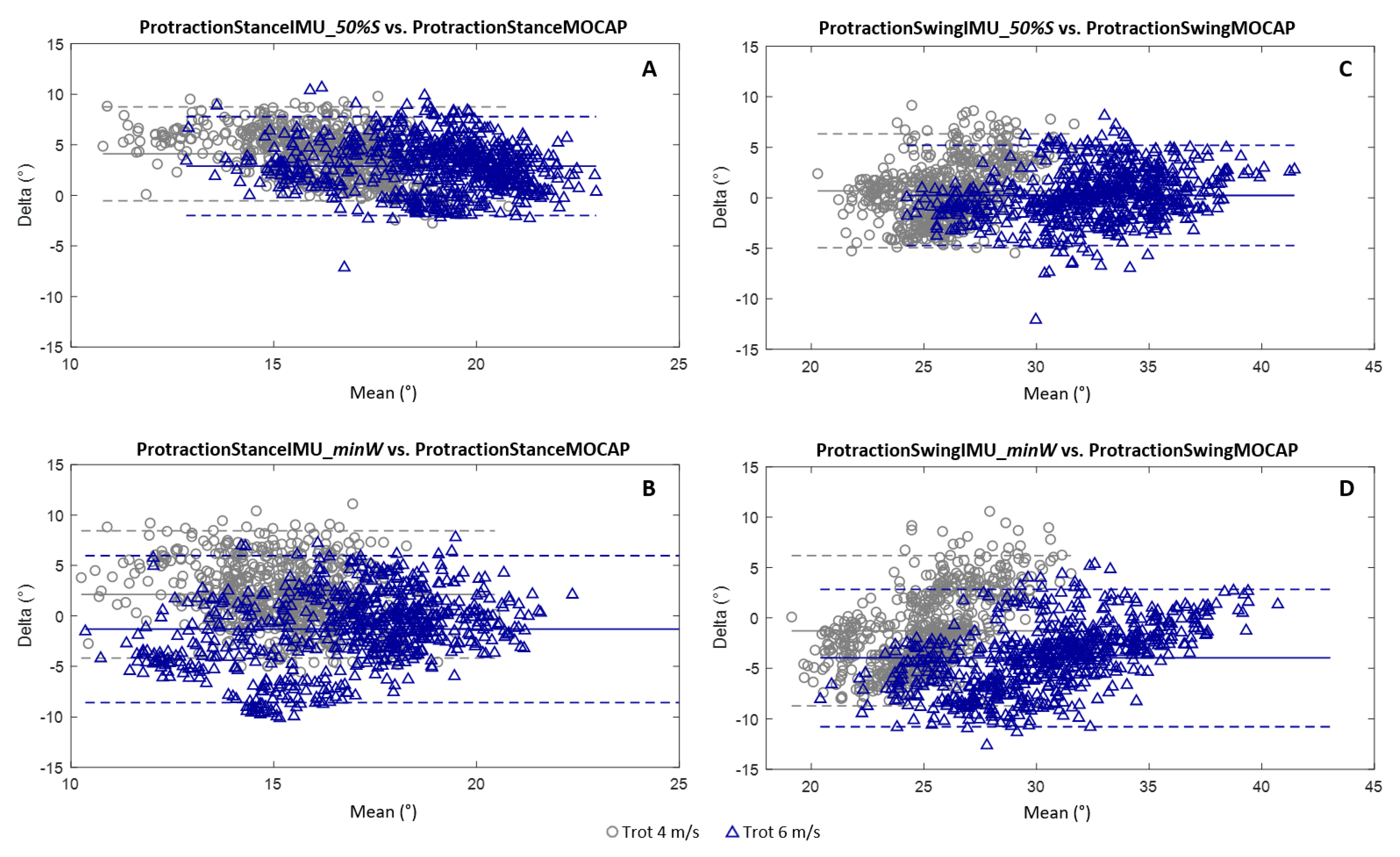
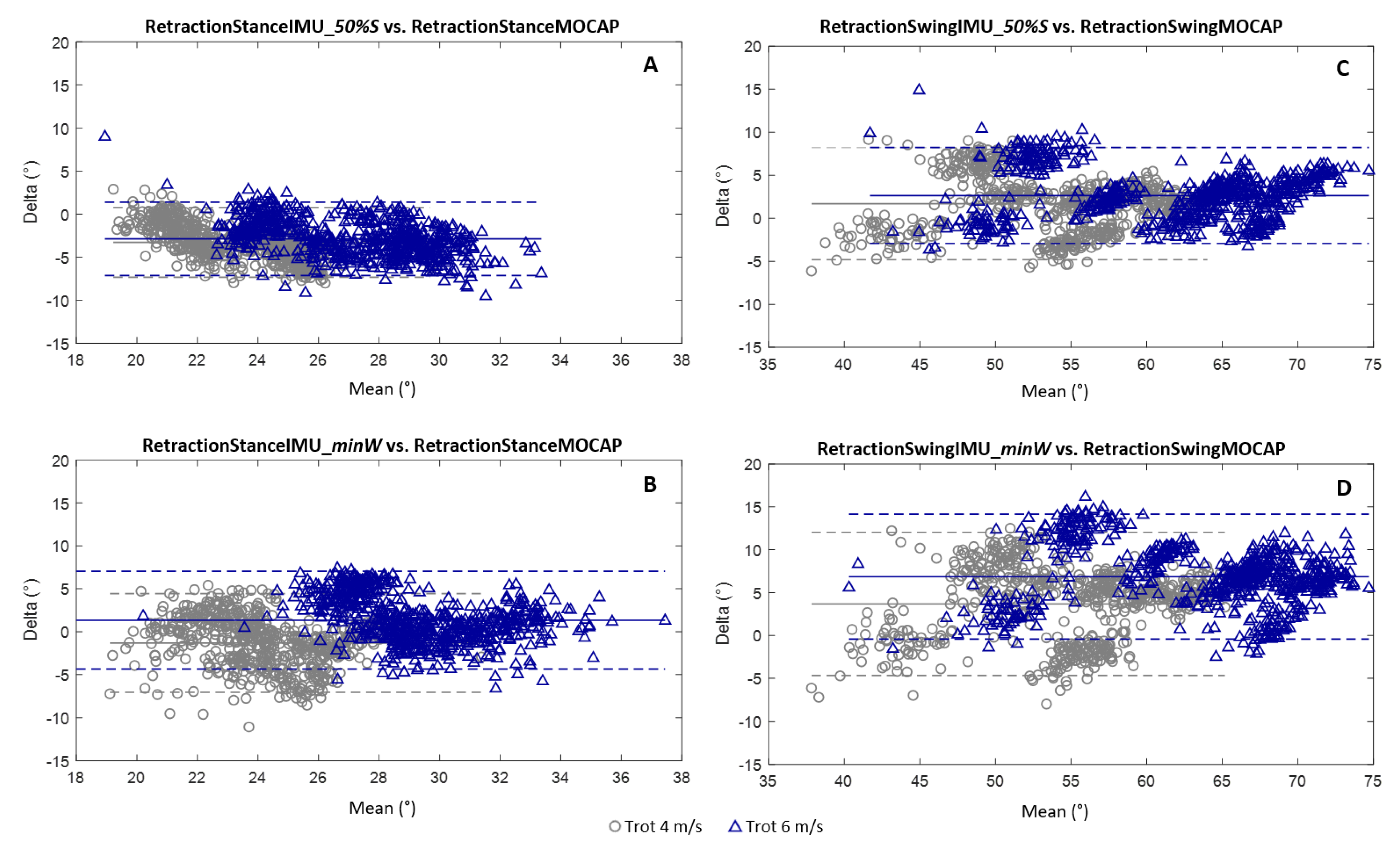
- The canon bone was considered in a vertical position at 50% of the stance phase [12] (“50%_Stance” method).
- (ii)
- The canon bone was considered in a vertical position at the moment when the withers reached their lowest point (“minWithers” method).
2.5. Statistical Analysis
3. Results
3.1. Measurement of the Protraction Angles
3.2. Measurement of the Retraction Angles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Biewener, A.A.; Thomason, J.; Goodship, A.; Lanyon, L.E. Bone stress in the horse forelimb during locomotion at different gaits: A comparison of two experimental methods. J. Biomech. 1983, 16, 565–576. [Google Scholar] [CrossRef]
- Crevier-Denoix, N.; Falala, S.; Holden-Douilly, L.; Camus, M.; Martino, J.; Ravary-Plumioen, B.; Vergari, C.; Desquilbet, L.; Denoix, J.-M.; Chateau, H. Comparative kinematic analysis of the leading and trailing forelimbs of horses cantering on a turf and a synthetic surface: Forelimb kinematics at the canter on turf and synthetic surfaces. Equine Vet. J. 2013, 45, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.C.; Dyson, S.J.; Tranquille, C.; Adams, V. Association of type of sport and performance level with anatomical site of orthopaedic injury diagnosis. Equine Vet. J. 2006, 38, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Meershoek, L.S.; Roepstorff, L.; Schamhardt, H.C.; Johnston, C.; Bobbert, M.F. Joint moments in the distal forelimbs of jumping horses during landing. Equine Vet. J. 2010, 33, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Singer, E.R.; Barnes, J.; Saxby, F.; Murray, J.K. Injuries in the event horse: Training versus competition. Vet. J. 2008, 175, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Audigié, F.; Pourcelot, P.; Degueurce, C.; Geiger, D.; Denoix, J.M. Fourier analysis of trunk displacements: A method to identify the lame limb in trotting horses. J. Biomech. 2002, 35, 1173–1182. [Google Scholar] [CrossRef]
- Benoit, P. Examen clinique du cheval de saut d’obstacles en situation. Bull. l’Académie Vétérinaire Fr. 2008, 161, 365–370. [Google Scholar] [CrossRef]
- Denoix, J.M.; Dyson, S.J. The thoracolumbar spine. In Diagnosis and Lameness in Horses; WB Saunders: Philadelphia, PA, USA, 2003; pp. 509–521. [Google Scholar]
- Boswell, R.; Mitchell, R.; Dyson, S. Lameness in the show hunter and show jumper. In Diagnosis and Lameness in Horses; WB Saunders: Philadelphia, PA, USA, 2003; pp. 965–975. [Google Scholar]
- Weishaupt, M.A.; Wiestner, T.; Hogg, H.P.; Jordan, P.; Auer, J.A. Compensatory load redistribution of horses with induced weightbearing hindlimb lameness trotting on a treadmill. Equine Vet. J. 2004, 36, 727–733. [Google Scholar] [CrossRef]
- Back, W.; Clayton, H. Equine Locomotion, 2nd ed.; Elsevier Health: London, UK, 2013. [Google Scholar]
- Bosch, S.; Serra Bragança, F.; Marin-Perianu, M.; Marin-Perianu, R.; van der Zwaag, B.; Voskamp, J.; Back, W.; van Weeren, R.; Havinga, P. EquiMoves: A Wireless Networked Inertial Measurement System for Objective Examination of Horse Gait. Sensors 2018, 18, 850. [Google Scholar] [CrossRef] [PubMed]
- Buchner, H.H.F.; Savelberg, H.H.C.M.; Schamhardt, H.C.; Merkens, H.W.; Barneveld, A. Kinematics of treadmill versus overground locomotion in horses. Vet. Q. 1994, 16, 87–90. [Google Scholar] [CrossRef]
- Hodson, E.; Clayton, H.M.; Lanovaz, J.L. The forelimb in walking horses: 1. Kinematics and ground reaction forces. Equine Vet. J. 2000, 32, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Roepstorff, L.; Wiestner, T.; Weishaupt, M.A.; Egenvall, E. Comparison of microgyro-based measurements of equine metatarsal/metacarpal bone to a high speed video locomotion analysis system during treadmill locomotion. Vet. J. 2013, 198 (Suppl. 1), e157–e160. [Google Scholar] [CrossRef]
- Muñoz, A.; Cuesta, I.; Riber, C.; Gata, J.; Trigo, P.; Castejón, F.M. Trot asymmetry in relation to physical performance and metabolism in equine endurance rides. Equine Vet. J. 2006, 38, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Keegan, K.G.; Wilson, D.A.; Smith, B.K.; Wilson, D.J. Changes in kinematic variables observed during pressure-induced forelimb lameness in adult horses trotting on a treadmill. Am. J. Vet. Res. 2000, 61, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Weishaupt, M.A.; Wiestner, T.; Hogg, H.P.; Jordan, P.; Auer, J.A. Compensatory load redistribution of horses with induced weight-bearing forelimb lameness trotting on a treadmill. Vet. J. 2006, 171, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Clayton, H.M. Cinematographic analysis of the gait of lame horses. J. Equine Vet. Sci. 1986, 6, 70–78. [Google Scholar] [CrossRef]
- Clayton, H.M. Cinematographic analysis of the gait of lame horses II: Chronic sesamoiditis. J. Equine Vet. Sci. 1986, 6, 310–320. [Google Scholar] [CrossRef]
- Dyson, S.; Pollard, D. Application of a Ridden Horse Pain Ethogram and Its Relationship with Gait in a Convenience Sample of 60 Riding Horses. Animals 2020, 10, 1044. [Google Scholar] [CrossRef]
- Sapone, M.; Martin, P.; Chateau, H.; Parmentier, J.; Ben Mansour, K.; Marin, F. Sizing of inertial sensors adapted to measurement of locomotor parameters in horses using motion capture. Comput. Methods Biomech. Biomed. Eng. 2019, 22 (Suppl. S1), S105–S106. [Google Scholar] [CrossRef]
- Sapone, M.; Martin, P.; Ben Mansour, K.; Château, H.; Marin, F. Comparison of Trotting Stance Detection Methods from an Inertial Measurement Unit Mounted on the Horse’s Limb. Sensors 2020, 20, 2983. [Google Scholar] [CrossRef]
- Nankervis, K.J.; Lefrancois, K. A comparison of protraction-retraction of the distal limb during treadmill and water treadmill walking in horses. J. Equine Vet. Sci. 2018, 70, 57–62. [Google Scholar] [CrossRef]
- Martin, P.; Chateau, H.; Pourcelot, P.; Duray, L.; Chèze, L. Effects of a prototype saddle (short panels) on the biomechanics of the equine back: Preliminary results. Comput. Methods Biomech. Biomed. Eng. 2015, 18 (Suppl. S1), 1990–1991. [Google Scholar] [CrossRef]
- Murray, R.; Guire, R.; Fisher, M.; Fairfax, V. Girth pressure measurements reveal high peak pressures that can be avoided using an alternative girth design that also results in increased limb protraction and flexion in the swing phase. Vet. J. 2013, 198, 92–97. [Google Scholar] [CrossRef]
- De Cocq, P.D.; Van Weeren, P.R.; Back, W. Effects of girth, saddle and weight on movements of the horse. Equine Vet. J. 2004, 36, 758–763. [Google Scholar] [CrossRef]
- Marin, F. Human and Animal Motion Tracking Using Inertial Sensors. Sensors 2020, 20, 6074. [Google Scholar] [CrossRef]
- Luinge, H.J.; Veltink, P.H. Inclination measurement of human movement using a 3-D accelerometer with autocalibration. IEEE Trans. Neural Syst. Rehabil. Eng. 2004, 12, 112–121. [Google Scholar] [CrossRef]
- Rehbinder, H.; Hu, X. Drift-free attitude estimation for accelerated rigid bodies. Automatica 2004, 40, 653–659. [Google Scholar] [CrossRef]
- Zhu, R.; Zhou, Z. A real-time articulated human motion tracking using tri-axis inertial/magnetic sensors package. IEEE Trans. Neural Syst. Rehabil. Eng. 2004, 12, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Luinge, H.J.; Veltink, P.H. Measuring orientation of human body segments using miniature gyroscopes and accelerometers. Med. Biol. Eng. Comput. 2005, 43, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.; Sheret, I.; McMillian, L.; Siliverdis, K.; Sha, N.; Hodgins, D.; Kenney, L.P.J.; Howard, D. Inertial sensor-based knee flexion/extension angle estimation. J. Biomech. 2009, 42, 2678–2685. [Google Scholar] [CrossRef] [PubMed]
- Dejnabadi, H.; Jolles, B.M.; Casanova, E.; Fua, P.; Aminian, K. Estimation and visualization of sagittal kinematics of lower limbs orientation using body-fixed sensors. IEEE Trans. Biomed. Eng. 2006, 53, 1385–1393. [Google Scholar] [CrossRef]
- Wilson, A.M.; Watson, J.C.; Lichtwark, G.A. Biomechanics: A catapult action for rapid limb protraction. Nature 2003, 421, 35–36. [Google Scholar] [CrossRef]
- Nez, A.; Fradet, L.; Laguillaumie, P.; Monnet, T.; Lacouture, P. Simple and efficient thermal calibration for MEMS gyroscopes. Med. Eng. Phys. 2018, 55, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Lepetit, K.; Ben Mansour, K.; Boudaoud, S.; Kinugawa-Bourron, K.; Marin, F. Evaluation of the kinetic energy of the torso by magneto-inertial measurement unit during the sit-to-stand movement. J. Biomech. 2018, 67, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Denoix, J.M. Biomécanique et gymnastique du cheval; Unithèque: Paris, France, 2014. [Google Scholar]
- Robert, C.; Valette, J.P.; Denoix, J.M. The effects of treadmill inclination and speed on the activity of two hindlimb muscles in the trotting horse. Equine Vet. J. 2000, 32, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Valette, J.P.; Pourcelot, P.; Audigie, F.; Denoix, J.M. Effects of trotting speed on muscle activity and kinematics in saddlehorses. Equine Vet. J. 2002, 34, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Merkens, H.W.; Schamhardt, H.C. Relationships between ground reaction force patterns and kinematics in the walking and trotting horse. Equine Vet. J. 1994, 26, 67–70. [Google Scholar] [CrossRef]
- Bigomar. Schéma de la Cinétique d’un Membre de Cheval Lors D’une Foulée. 2006. Available online: https://fr.wikipedia.org/wiki/Fichier:Cinetique_membre_cheval.PNG (accessed on 30 May 2021).
- Viry, S.; Sleimen-Malkoun, R.; Temprado, J.J.; Frances, J.P.; Berton, E.; Laurent, M.; Nicol, C. Patterns of horse-rider coordination during endurance race: A dynamical system approach. PLoS ONE 2013, 8, e71804. [Google Scholar] [CrossRef] [PubMed]
- Viry, S.; De Graaf, J.B.; Frances, J.P.; Berton, E.; Laurent, M.; Nicol, C. Combined influence of expertise and fatigue on riding strategy and horse–rider coupling during the time course of endurance races. Equine Vet. J. 2015, 47, 78–82. [Google Scholar] [CrossRef]
- Barrey, E.; Hermelin, M.; Vaudelin, J.L.; Poirel, D.; Valette, J.P. Utilisation of an accelerometric device in equine gait analysis. Equine Vet. J. 1994, 26 (Suppl. S17), 7–12. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Agreement between Methods of Measurement with Multiple Observations Per Individual. J. Biopharm. Stat. 2007, 17, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Titterton, D.H.; Weston, J.L. Strapdown Inertial Navigation Technology, 2nd ed.; The Institution of Electrical Engineers: Stevenage, UK, 2004. [Google Scholar]
- Šlajpah, S.; Kamnik, R.; Munih, M. Kinematics based sensory fusion for wearable motion assessment in human walking. Comput. Methods Programs Biomed. 2014, 116, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Van Weeren, P.R.; Van Den Bogert, A.J.; Barneveld, A. A quantitative analysis of skin displacement in the trotting horse. Equine Veterinary J. 1990, 22, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Byström, A.; Egenvall, A.; Roepstorff, L.; Rhodin, M.; Bragança, F.S.; Hernlund, E.; Van Weeren, R.; Weishaupt, M.A.; Clayton, H.M. Biomechanical findings in horses showing asymmetrical vertical excursions of the withers at walk. PLoS ONE 2018, 13, e0204548. [Google Scholar] [CrossRef]
- Buchner, H.H.F.; Savelberg, H.H.C.M.; Schamhardt, H.C.; Barneveld, A. Head and trunk movement adaptations in horses with experimentally induced fore-or hindlimb lameness. Equine Vet. J. 1996, 28, 71–76. [Google Scholar] [CrossRef]
- Rhodin, M.; Persson-Sjodin, E.; Egenvall, A.; Serra Bragança, F.M.; Pfau, T.; Roepstorff, L.; Weishaupt, M.A.; Thomsen, M.H.; Van Weeren, P.R.; Hernlund, E. Vertical movement symmetry of the withers in horses with induced forelimb and hindlimb lameness at trot. Equine Vet. J. 2018, 50, 818–824. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapone, M.; Martin, P.; Ben Mansour, K.; Chateau, H.; Marin, F. The Protraction and Retraction Angles of Horse Limbs: An Estimation during Trotting Using Inertial Sensors. Sensors 2021, 21, 3792. https://doi.org/10.3390/s21113792
Sapone M, Martin P, Ben Mansour K, Chateau H, Marin F. The Protraction and Retraction Angles of Horse Limbs: An Estimation during Trotting Using Inertial Sensors. Sensors. 2021; 21(11):3792. https://doi.org/10.3390/s21113792
Chicago/Turabian StyleSapone, Marie, Pauline Martin, Khalil Ben Mansour, Henry Chateau, and Frédéric Marin. 2021. "The Protraction and Retraction Angles of Horse Limbs: An Estimation during Trotting Using Inertial Sensors" Sensors 21, no. 11: 3792. https://doi.org/10.3390/s21113792
APA StyleSapone, M., Martin, P., Ben Mansour, K., Chateau, H., & Marin, F. (2021). The Protraction and Retraction Angles of Horse Limbs: An Estimation during Trotting Using Inertial Sensors. Sensors, 21(11), 3792. https://doi.org/10.3390/s21113792






