Scanning and Actuation Techniques for Cantilever-Based Fiber Optic Endoscopic Scanners—A Review
Abstract
1. Introduction
2. Overview of Optical Components Used in Endoscopes
2.1. Fiber Bundles in Endoscopes
2.2. Single Fiber Endoscopy
2.3. Graded-Index (GRIN) Lens Scanner
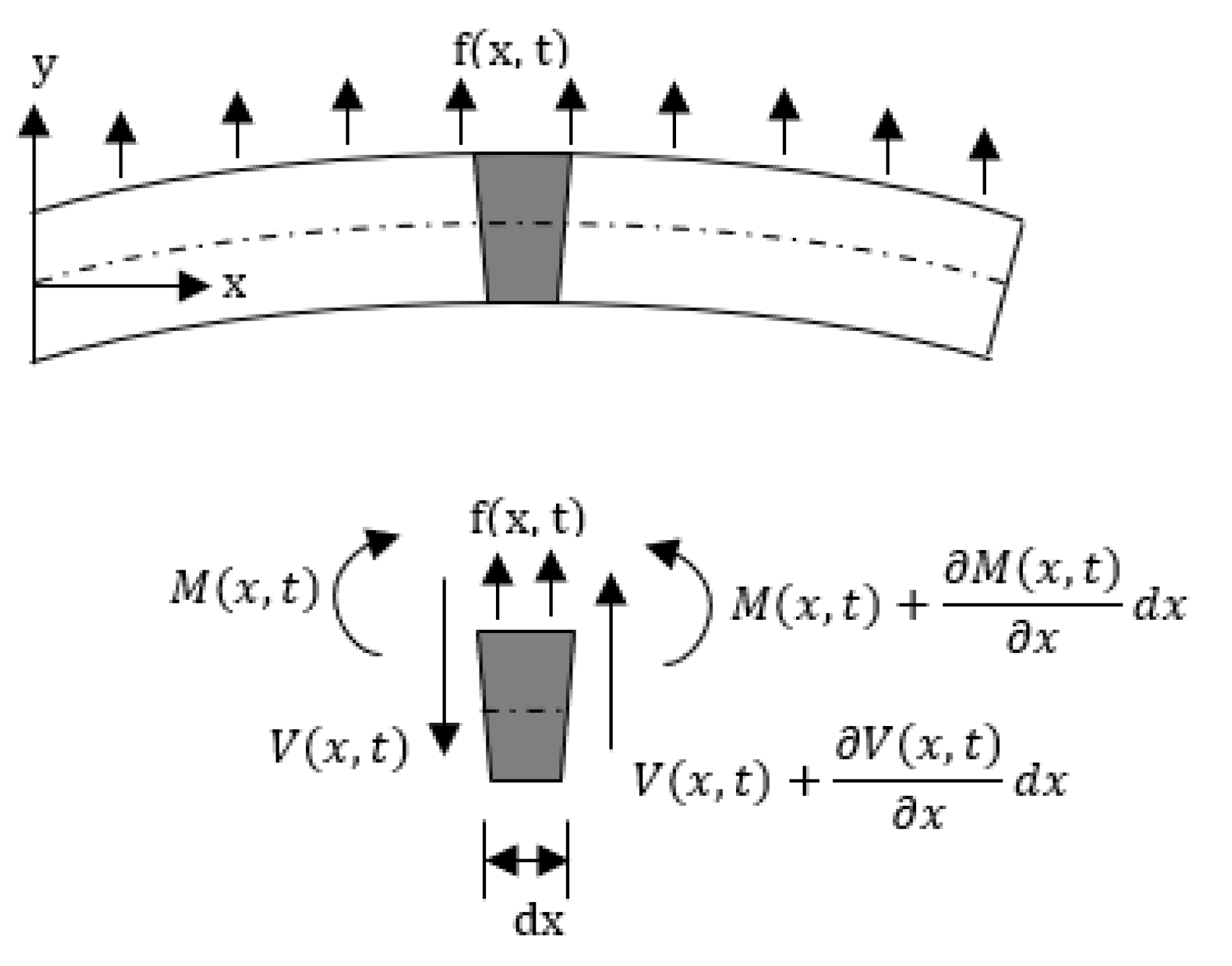
3. Cantilever Beam Mechanics
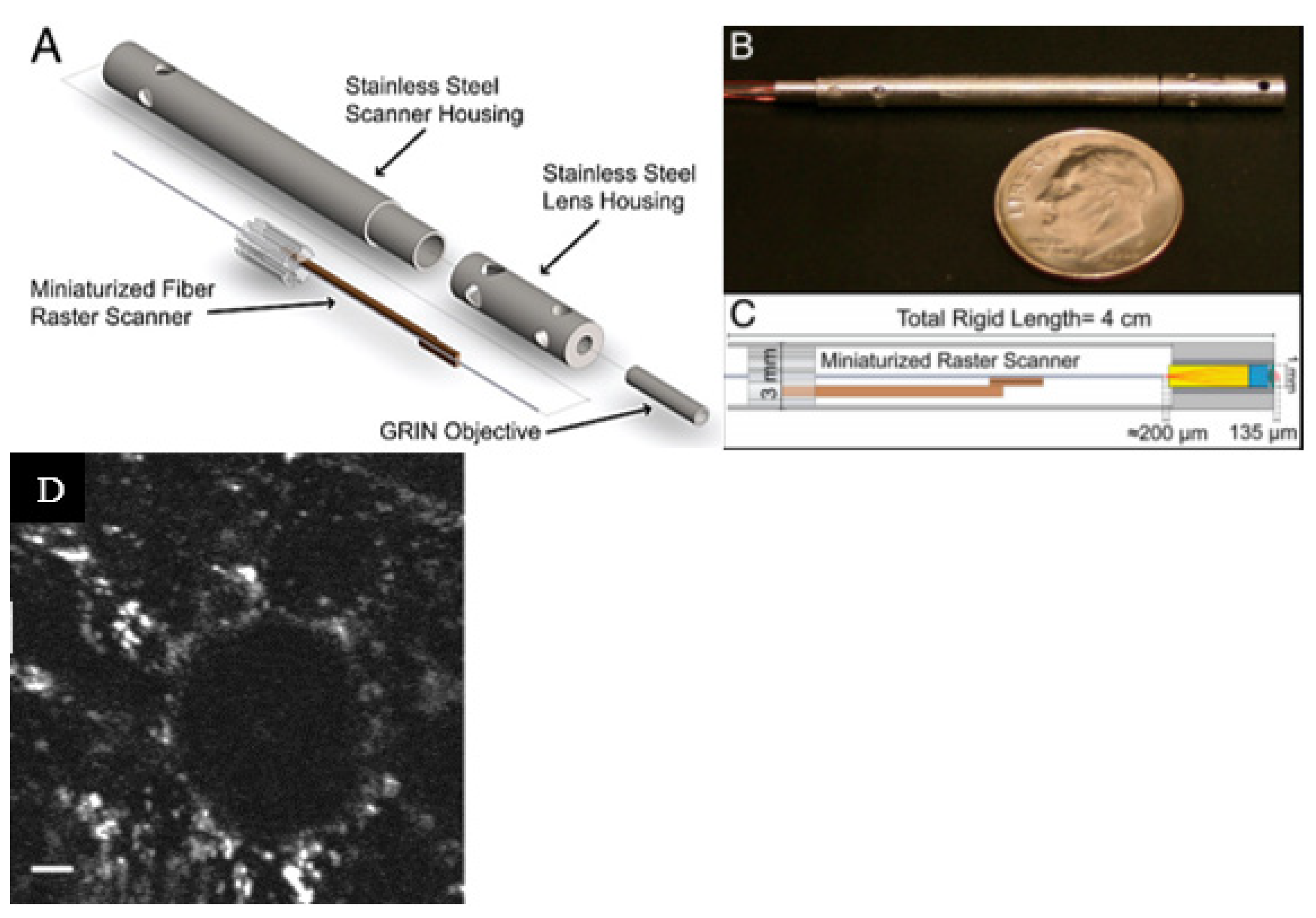
3.1. Resonantly Actuated Scanners
3.2. Non-Resonantly Actuated Scanner
4. Actuators in Cantilever-Based Endoscopic Devices
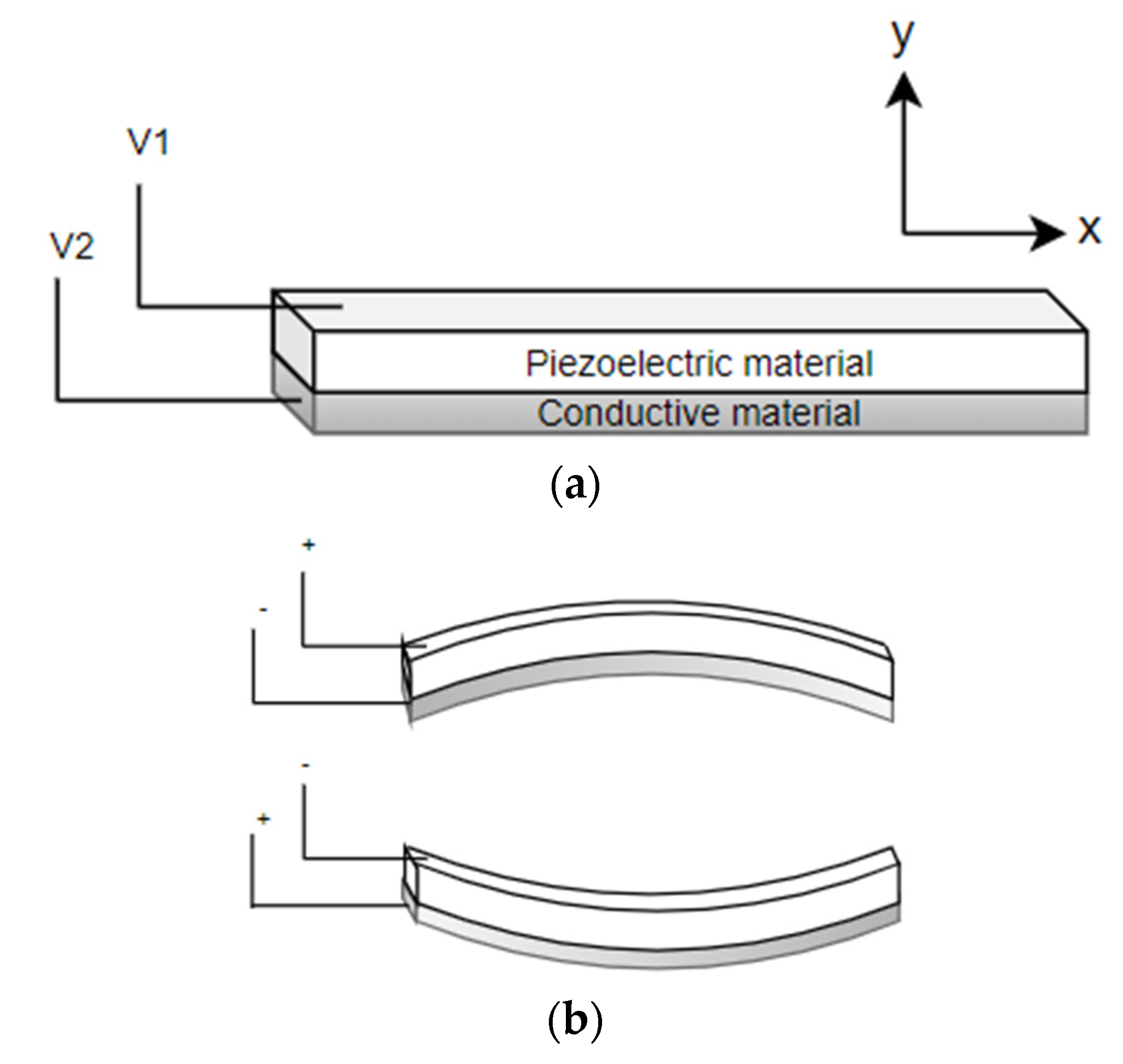
4.1. Piezoelectric Actuators
4.1.1. Disc Piezoelectric Actuator
4.1.2. Tubular Piezoelectric Actuator
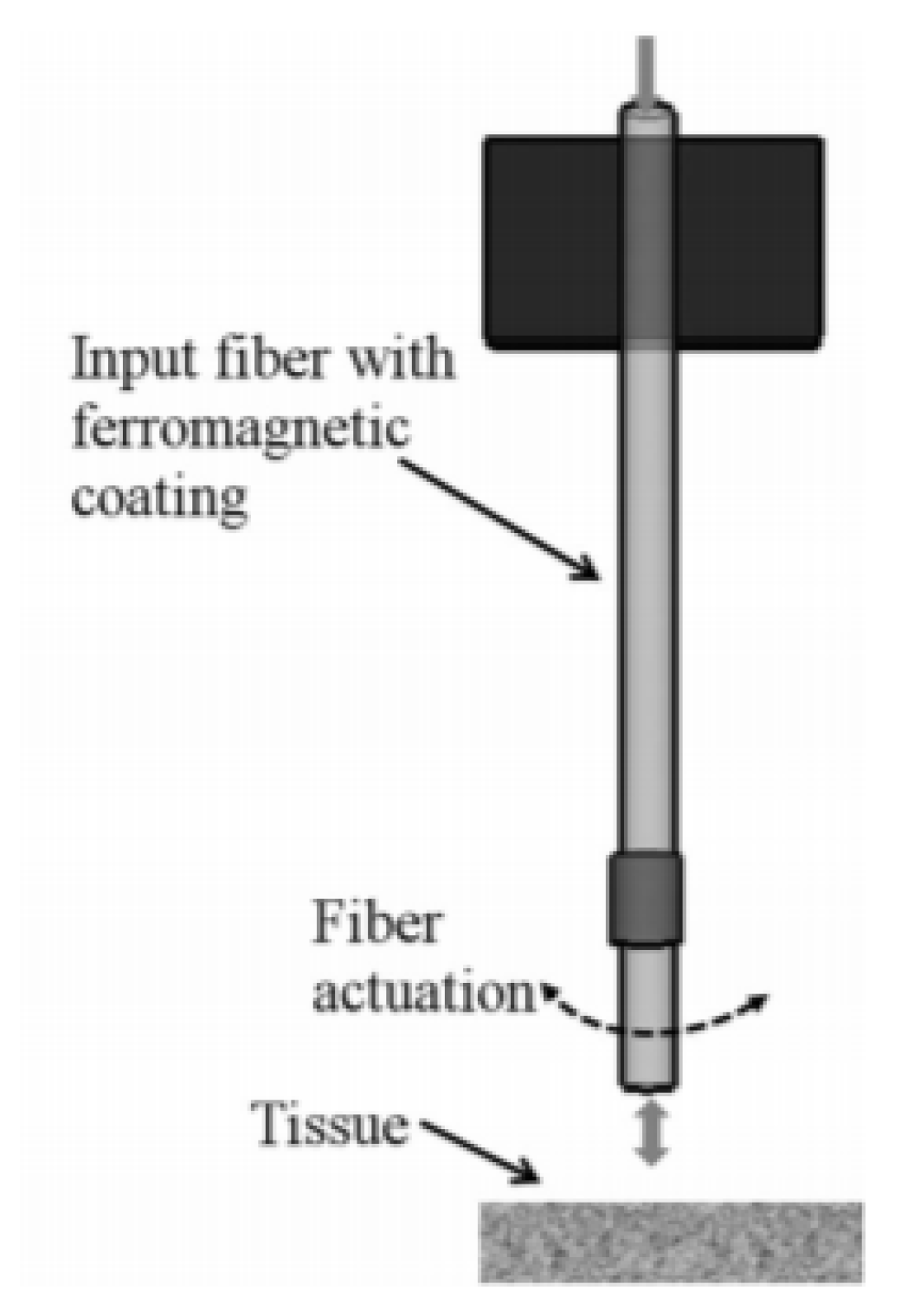
4.2. Electrothermal Actuators
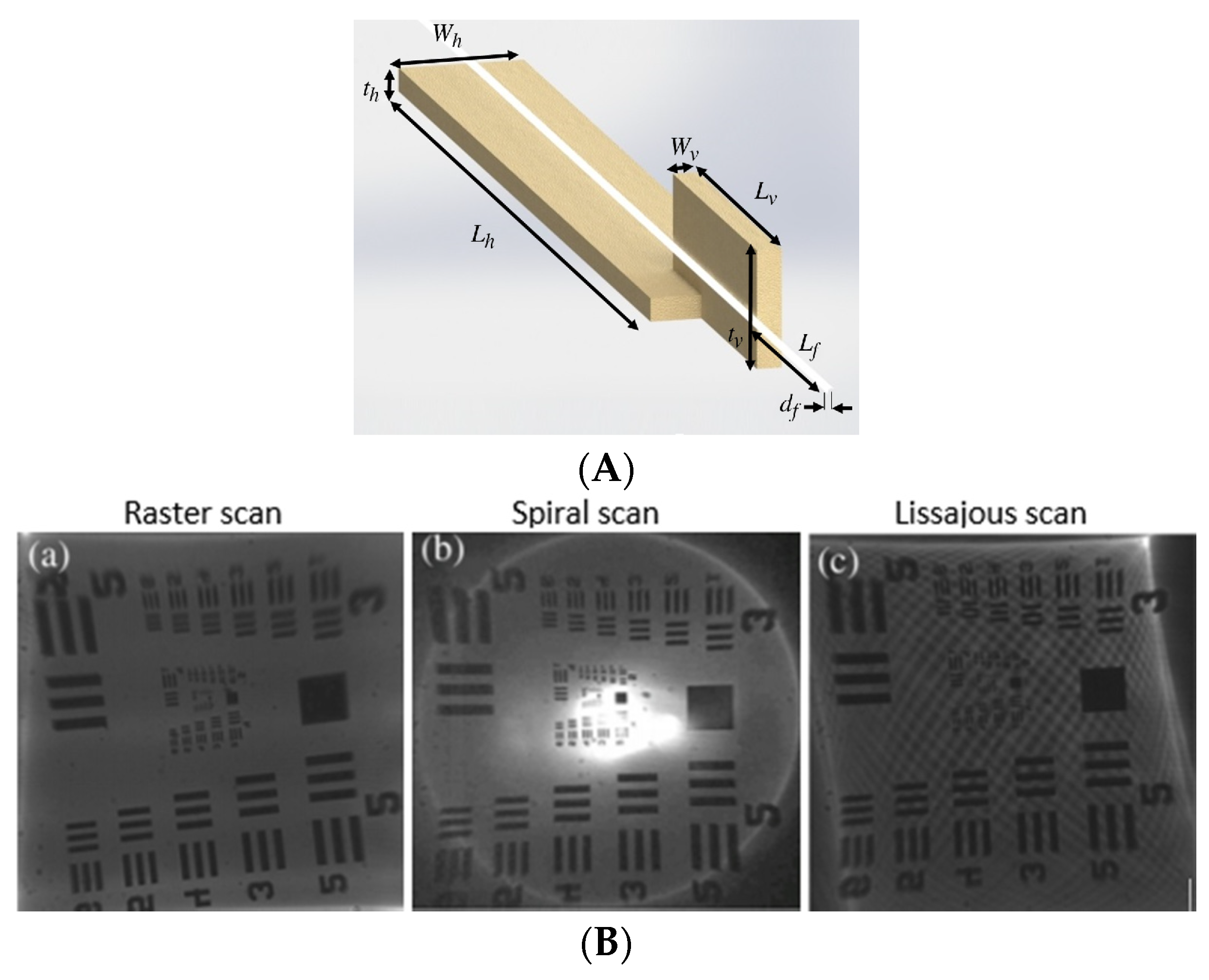
4.2.1. Hot-and-Cold Arm Actuators
4.2.2. Chevron Actuators
4.2.3. Bimorph Actuators
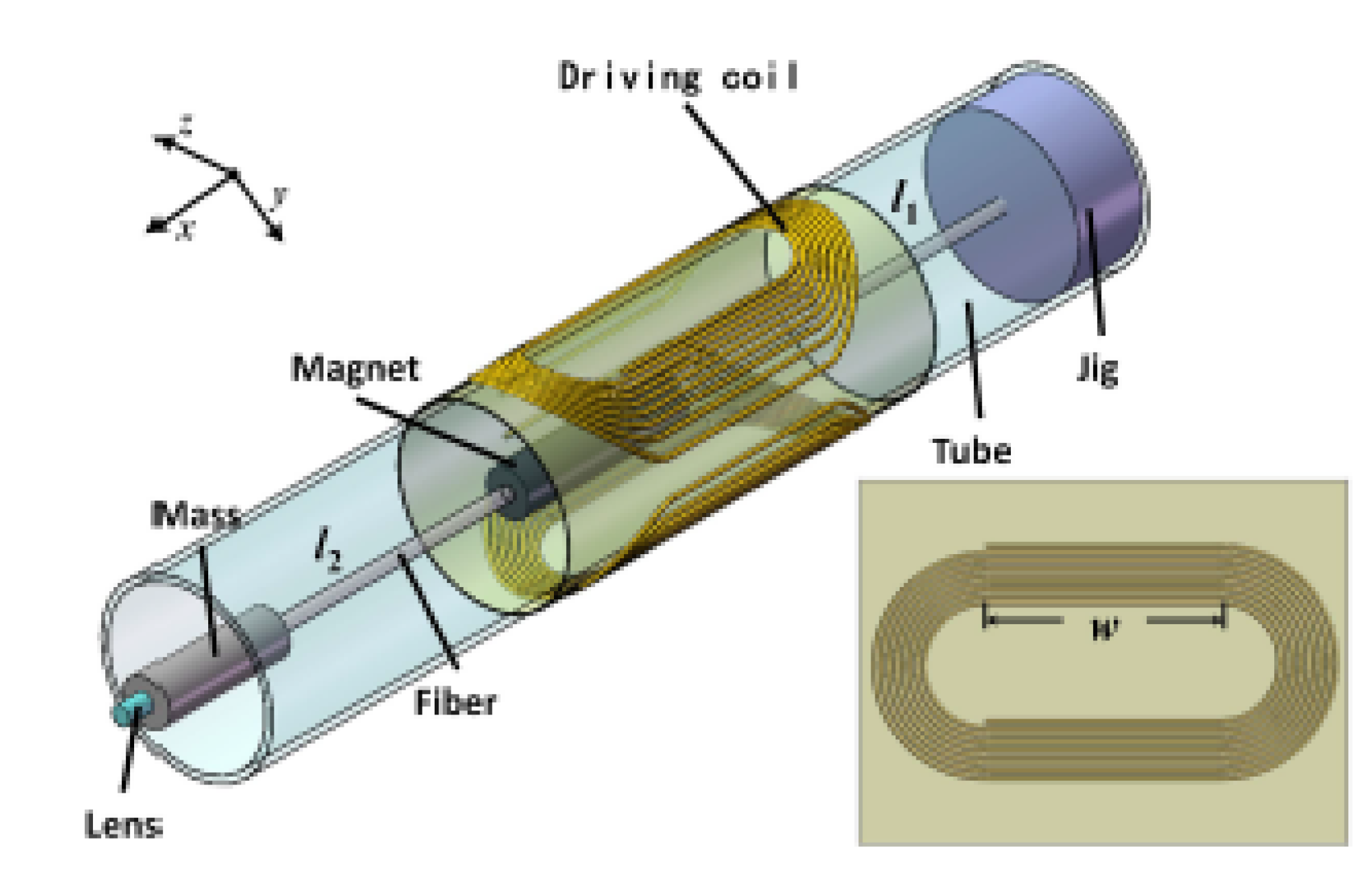
4.3. Electromagnetic Actuators
4.3.1. Moving Magnet Configuration
4.3.2. Moving Coil Configuration
4.3.3. Magnetostrictive Actuation
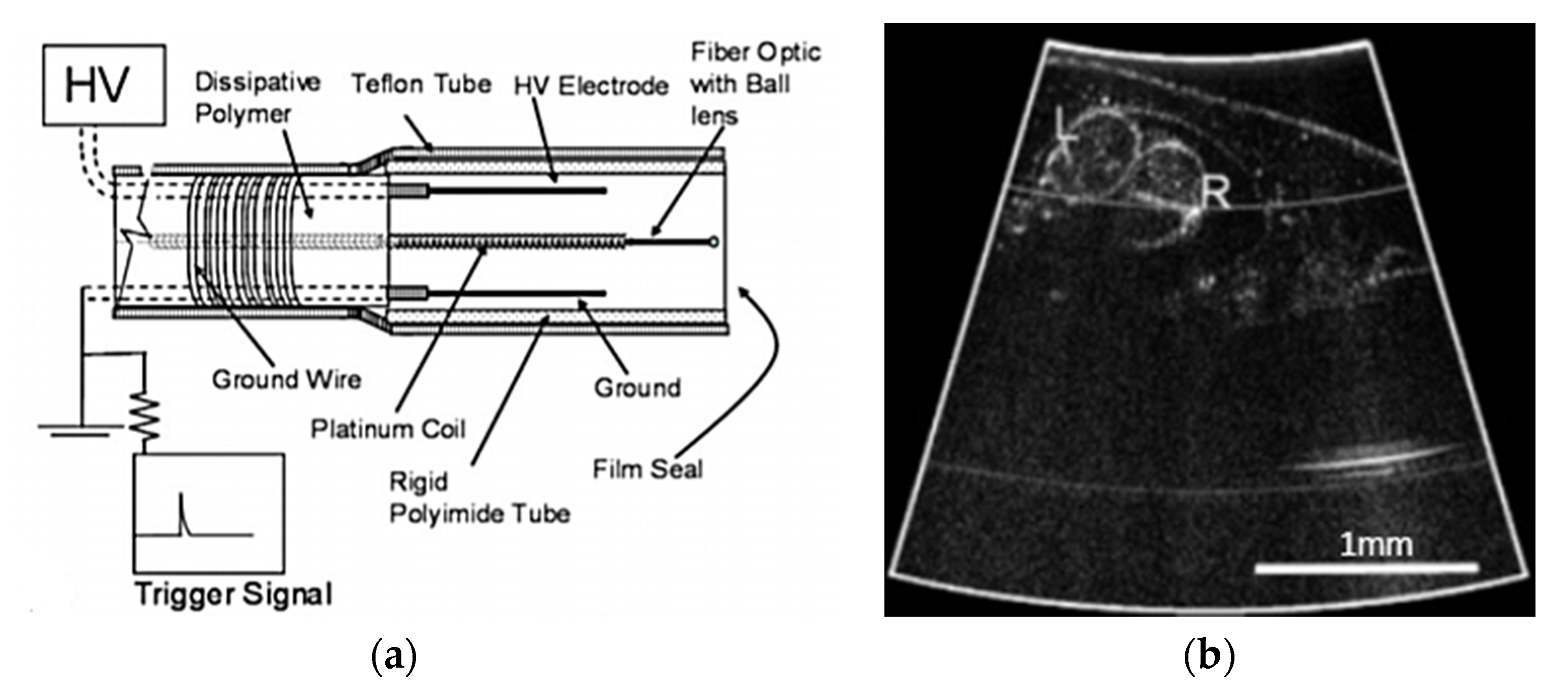
4.4. Electrostatic Actuators
4.4.1. Parallel Plate Actuator
4.4.2. Comb Drive Actuator
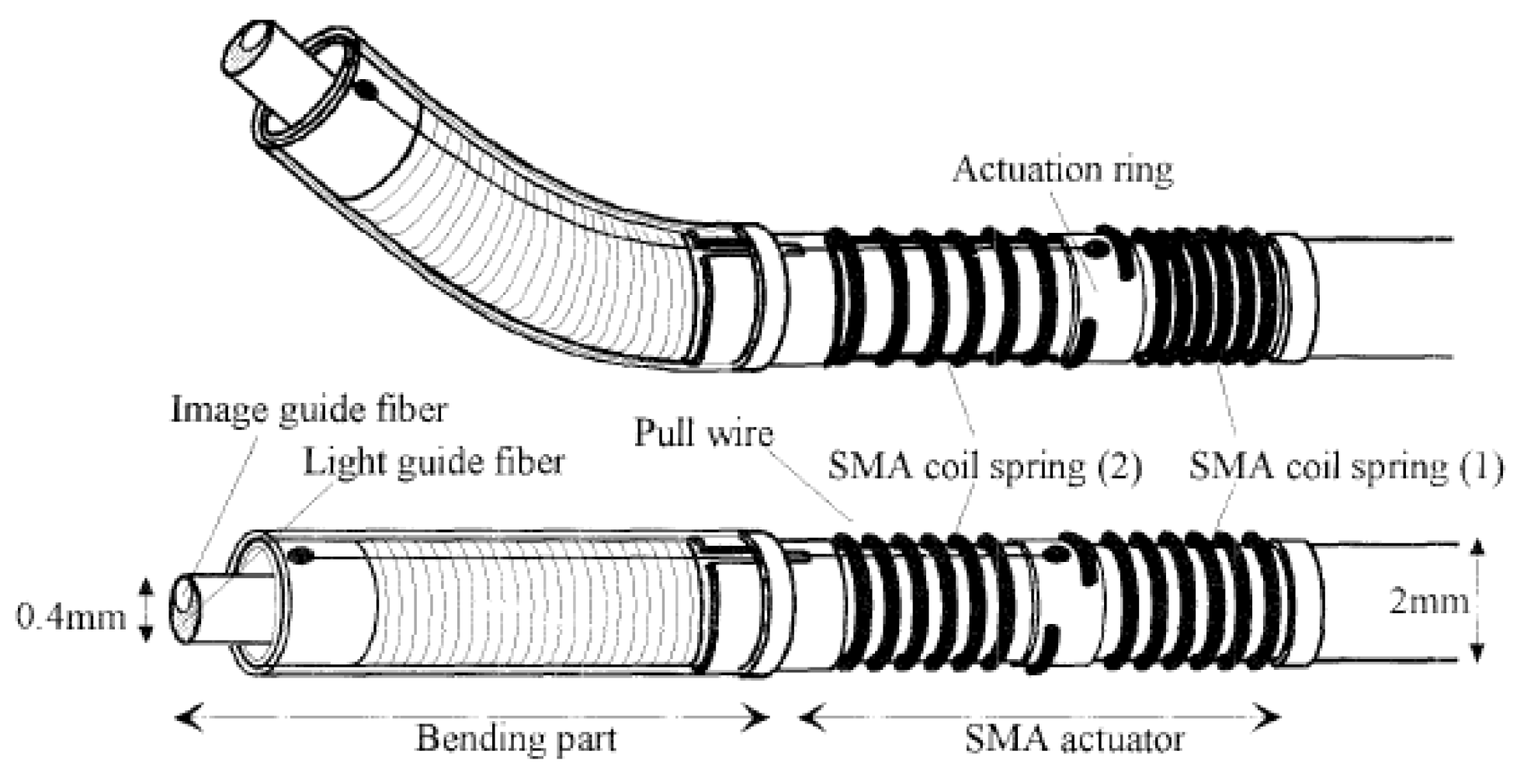
4.5. Shape Memory Alloy Actuators
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coda, S.; Thillainayagam, A.V. State of the art in advanced endoscopic imaging for the detection and evaluation of dysplasia and early cancer of the gastrointestinal tract. Clin. Exp. Gastroenterol. 2014, 7, 133–150. [Google Scholar] [CrossRef]
- Mannath, J.; Banks, M.R. Emerging technologies in endoscopic imaging. F1000 Med. Rep. 2012, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- WHO, Cancer, World Health Organization, 12 09 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 6 October 2020).
- Stewart, B.W.; Wild, C.P. World Cancer Report 2014; IARC: Lyon, France, 2014. [Google Scholar]
- Conigliaro, R.; Pigò, F. New Techniques in Endoscopy: Confocal Laser Endomicroscopy. In New Techniques in Gastrointestinal Endoscopy; IntechOpen: London, UK, 2011; pp. 213–230. [Google Scholar]
- Kaur, M.; Lane, P.M.; Menon, C. Endoscopic Optical Imaging Technologies and Devices for Medical Purposes: State of the Art. Appl. Sci. 2020, 10, 6865. [Google Scholar] [CrossRef]
- Leng, X.; Chapman, W.; Rao, B.; Nandy, S.; Chen, R.; Rais, R.; Gonzalez, I.; Zhou, Q.; Chatterjee, D.; Mutch, M.; et al. Feasibility of co-registered ultrasound and acoustic-resolution photoacoustic imaging of human colorectal cancer. Biomed. Opt. Express 2018, 9, 5159–5172. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jokerst, J.V. Photoacoustic imaging with fiber optic technology: A review. Photoacoustics 2020, 20, 100211. [Google Scholar] [CrossRef] [PubMed]
- Ubbink, R.; Van Dijk, L.J.; Van Noord, D.; Johannes, T.; Specht, P.A.C.; Bruno, M.; Mik, E.G. Evaluation of endoscopic visible light spectroscopy: Comparison with microvascular oxygen tension measurements in a porcine model. J. Transl. Med. 2019, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Benaron, A.D.; Parachikov, H.I.; Cheong, W.-F.; Friedland, S.; Rubinsky, B.E.; Otten, D.M.; Liu, F.W.H.; Levinson, C.J.; Murphy, A.L.; Price, J.W.; et al. Design of a visible-light spectroscopy clinical tissue oximeter. J. Biomed. Opt. 2005, 10, 44005–44009. [Google Scholar] [CrossRef]
- Gorevoy, A.; Machikhin, A.S.; Batshev, V.; Kolyuchkin, V.Y. Optimization of stereoscopic imager performance by computer simulation of geometrical calibration using optical design software. Opt. Express 2019, 27, 17819–17839. [Google Scholar] [CrossRef]
- Geng, J.; Xie, J. Review of 3-D Endoscopic Surface Imaging Techniques. IEEE Sens. J. 2013, 14, 945–960. [Google Scholar] [CrossRef]
- Udovich, J.A.; Kirkpatrick, N.D.; Kano, A.; Tanbakuchi, A.; Utzinger, U.; Gmitro, A.F. Spectral background and transmission characteristics of fiber optic imaging bundles. Appl. Opt. 2008, 47, 4560–4568. [Google Scholar] [CrossRef]
- Scheffer, D. Endoscopes Use CMOS Image Sensors, Vision Systems Design, 30 07 2007. Available online: https://www.vision-systems.com/home/article/16750278/endoscopes-use-cmos-image-sensors (accessed on 28 July 2020).
- Lee, C.M.; Engelbrecht, C.J.; Soper, T.D.; Helmchen, F.; Seibel, E.J. Scanning fiber endoscopy with highly flexible, 1 mm catheterscopes for wide-field, full-color imaging. J. Biophotonics 2010, 3, 385–407. [Google Scholar] [CrossRef] [PubMed]
- Seibel, E.J.; Johnston, R.S.; Melville, C.D. A full-color scanning fiber endoscope. Opt. Fibers Sens. Med. Diagn. Treat. Appl. VI 2006, 6083, 608303. [Google Scholar] [CrossRef]
- Piyawattanametha, W. A review of MEMS scanner based endoscopic optical imaging probe. In Proceedings of the 2013 International Conference on Optical MEMS and Nanophotonics (OMN), Kanazawa, Japan, 18–22 August 2013; pp. 53–54. [Google Scholar]
- Qiu, Z.; Piyawattanamatha, W. New Endoscopic Imaging Technology Based on MEMS Sensors and Actuators. Micromachines 2017, 8, 210. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.; Seo, Y.-H.; Jeong, K.-H. Microscanners for optical endomicroscopic applications. Micro Nano Syst. Lett. 2017, 5, 1–11. [Google Scholar] [CrossRef]
- Powers, J.P. An Introduction to Fiber Optic Systems; Irwin: Chicago, IL, USA, 1997. [Google Scholar]
- Ghatak, A.; Thyagarajan, K. Optical Waveguides and Fibers. In Fundamentals of Photonics; SPIE: Bellingham, WA, USA, 2009; pp. 249–292. [Google Scholar]
- Göbel, W.; Kerr, J.N.D.; Nimmerjahn, A.; Helmchen, F. Miniaturized two-photon microscope based on a flexible coherent fiber bundle and a gradient-index lens objective. Opt. Lett. 2004, 29, 2521–2523. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Oesterberg, U.L. Measurement of cross talk in order-packed image fiber bundles. In Proceedings of the SPIE’s 1995 International Symposium on Optical Science, Engineering, and Instrumentation; SPIE: Bellingham, WA, USA, 1995; pp. 480–488. [Google Scholar]
- Flusberg, A.; Cocker, B.; Piyawattanametha, E.D.; Jung, W.; Cheung, J.C.; Schnitzer, E.L.M. Fiber-optic fluorescence imaging. Nat. Methods 2005, 2, 941–950. [Google Scholar] [CrossRef]
- Shao, J.; Liao, W.-C.; Liang, R.; Barnard, K. Resolution enhancement for fiber bundle imaging using maximum a posteriori estimation. Opt. Lett. 2018, 43, 1906–1909. [Google Scholar] [CrossRef]
- Dumripatanachod, M.; Piyawattananmetha, W. A Fast Depixation Method of Fiber Bundle Image for an Embedded System. In Proceedings of the 8th Biomedical Engineering International Conference, Pattaya, Thailand, 25–27 November 2015. [Google Scholar]
- Shinde, A.; Perinchery, S.M.; Matham, M.V. Fiber pixelated image database. Opt. Eng. 2016, 55, 83105. [Google Scholar] [CrossRef]
- Vyas, K.; Hughes, M.; Gil Rosa, B.; Yang, G.-Z. Fiber bundle shifting endomicroscopy for high-resolution imaging. Biomed. Opt. Express 2018, 9, 4649–4664. [Google Scholar] [CrossRef]
- Sung, K.-B.; Liang, C.; Descour, M.; Collier, T.; Follen, M.; Richards-Kortum, R. Fiber-optic confocal reflectance microscope with miniature objective for in vivo imaging of human tissues. IEEE Trans. Biomed. Eng. 2002, 49, 1168–1172. [Google Scholar] [CrossRef]
- Knittel, J.; Schnieder, L.; Buess, G.; Messerschmidt, B.; Possner, T. Endoscope-compatible confocal microscope using a gradient index-lens system. Opt. Commun. 2001, 188, 267–273. [Google Scholar] [CrossRef]
- Lane, P.M.; Lam, S.; McWilliams, A.; Le Riche, J.C.; Anderson, M.W.; Macaulay, C.E. Confocal fluorescence microendoscopy of bronchial epithelium. J. Biomed. Opt. 2009, 14, 024008. [Google Scholar] [CrossRef] [PubMed]
- Cellvizio, Cellvizio Targeted Biopsies, Mauna Kea Technolgies. Available online: https://www.maunakeatech.com/en/cellvizio/10-cellvizio-targeted-biopsies (accessed on 9 October 2020).
- Osdoit, A.; Lacombe, F.; Cave, C.; Loiseau, S.; Peltier, E. To see the unseeable: Confocal miniprobes for routine microscopic imaging during endoscopy. Biomed. Opt. 2007, 6432, 64320. [Google Scholar] [CrossRef]
- Giniunas, L.; Juskaitis, R.; Shatalin, S. Scanning fiber-optic microscope. Electron. Lett. 1991, 27, 724–726. [Google Scholar] [CrossRef]
- Kimura, S.; Wilson, T. Confocal scanning optical microscope using single-mode fiber for signal detection. Appl. Opt. 1991, 30, 2143–2150. [Google Scholar] [CrossRef]
- Pitris, C.; Bouma, B.E.; Shiskov, M.; Tearney, G.J. A GRISM-based probe for spectrally encoded confocal microscopy. Opt. Express 2003, 11, 120–124. [Google Scholar] [CrossRef]
- Yelin, D.; Rizvi, I.; White, W.M.; Motz, J.T.; Hasan, T.; Bouma, B.E.; Tearney, G.J. Three-dimensional miniature endoscopy. Nat. Cell Biol. 2006, 443, 765. [Google Scholar] [CrossRef]
- Zhao, J.-H.; Zeng, H. Advanced Spectroscopy Technique for Biomedicine. In Biophysics of Skin and Its Treatments; Springer Science and Business Media LLC: Geneva, Swizterland, 2012; pp. 1–54. [Google Scholar]
- Choi, Y.; Yoon, C.; Kim, M.; Yang, T.D.; Fang-Yen, C.; Dasari, R.R.; Lee, K.J.; Choi, W. Scanner-Free and Wide-Field Endoscopic Imaging by Using a Single Multimode Optical Fiber. Phys. Rev. Lett. 2012, 109, 203901. [Google Scholar] [CrossRef]
- Yang, J.-M.; Chen, R.; Favazza, C.; Yao, J.; Li, C.; Hu, Z.; Zhou, Q.; Shung, K.K.; Wang, L.V. A 25-mm diameter probe for photoacoustic and ultrasonic endoscopy. Opt. Express 2012, 20, 23944–23953. [Google Scholar] [CrossRef]
- Wei, W.; Li, X.; Zhou, Q.; Shung, K.K.; Chen, Z. Integrated ultrasound and photoacoustic probe for co-registered intravascular imaging. J. Biomed. Opt. 2011, 16, 106001. [Google Scholar] [CrossRef]
- Papadopoulos, I.N.; Farahi, S.; Moser, C.; Psaltis, D. Focusing and scanning light through a multimode optical fiber using digital phase conjugation. Opt. Express 2012, 20, 10583–10590. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Kanno, T.; Ishihara, S.; Suto, H.; Takahashi, T.; Nishidate, I. Reflectance Imaging by Graded-Index Short Multimode Fiber. Appl. Phys. Express 2013, 6, 052503. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, Y.; Cheng, J.-X. High-resolution photoacoustic endoscope through beam self-cleaning in a graded index fiber. Opt. Lett. 2019, 44, 3841–3844. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Gan, X.; Gu, M. Nonlinear optical microscopy based on double-clad photonic crystal fibers. Opt. Express 2005, 13, 5528–5534. [Google Scholar] [CrossRef]
- Yelin, D.; Bouma, B.E.; Yun, S.H.; Tearney, G.J. Double-clad fiber for endoscopy. Opt. Lett. 2004, 29, 2408–2410. [Google Scholar] [CrossRef]
- Wang, L.; Choi, H.Y.; Jung, Y.; Lee, B.-H.; Kim, K.-T. Optical probe based on double-clad optical fiber for fluorescence spectroscopy. Opt. Express 2007, 15, 17681–17689. [Google Scholar] [CrossRef]
- Mavadia, J.; Xi, J.; Chen, Y.; Li, X. An all-fiber-optic endoscopy platform for simultaneous OCT and fluorescence imaging. Biomed. Opt. Express 2012, 3, 2851–2859. [Google Scholar] [CrossRef][Green Version]
- Xi, J.; Chen, Y.; Zhang, Y.; Murari, K.; Li, M.-J.; Li, X. Integrated multimodal endomicroscopy platform for simultaneous en face optical coherence and two-photon fluorescence imaging. Opt. Lett. 2012, 37, 362–364. [Google Scholar] [CrossRef]
- Buenconsejo, A.L.; Hohert, G.; Manning, M.; Abouei, E.; Tingley, R.; Janzen, I.; McAlpine, J.N.; Miller, D.; Lee, A.; Lane, P.M.; et al. Submillimeter diameter rotary-pullback fiber-optic endoscope for narrowband red-green-blue reflectance, optical coherence tomography, and autofluorescence in vivo imaging. J. Biomed. Opt. 2019, 25, 1–7. [Google Scholar] [CrossRef]
- Tearney, G.J.; Brezinski, M.E.; Fujimoto, J.G.; Weissman, N.J.; Boppart, S.A.; Bouma, B.E.; Southern, J.F. Scanning single-mode fiber optic catheter–endoscope for optical coherence tomography. Opt. Lett. 1996, 21, 543–545. [Google Scholar] [CrossRef]
- Liu, X.; Cobb, M.J.; Chen, Y.; Kimmey, M.B.; Li, X. Rapid-scanning forward-imaging miniature endoscope for real-time optical coherence tomography. Opt. Lett. 2004, 29, 1763–1765. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-C.; Seo, Y.-H.; Hwang, K.; Lim, J.-K.; Yoon, S.Z.; Jeong, K.-H. Micromachined tethered silicon oscillator for an endomicroscopic Lissajous fiber scanner. Opt. Lett. 2014, 39, 6675–6678. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Ding, Z. Rapid two-dimensional transversal scanning fiber probe for optical coherence tomography. In Coherence Domain Optical Methods and Optical Coherence Tomography in Biomedicine XI; SPIE: Bellingham, WA, USA, 2007; p. 64292W. [Google Scholar]
- Liu, Z.; Fu, L.; Gao, F.; Zhang, X. Design and implementation of a 2-D endoscopic optical fiber scanner. In Proceedings of the Seventh International Conference on Photonics and Imaging in Biology and Medicine, Wuhan, China, 24–27 November 2008; Volume 7280, p. 72801D. [Google Scholar]
- Wu, T.; Ding, Z.; Wang, K.; Chen, M.; Wang, C. Two-dimensional scanning realized by an asymmetry fiber cantilever driven by single piezo bender actuator for optical coherence tomography. Opt. Express 2009, 17, 13819–13829. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhou, A.; Gao, H.; Liu, Z. Endoscope two dimensional scanning fiber probe and the driving method. In Proceedings of the International Conference on Optical Instruments and Technology (OIT2011), Beijing, China, 6–9 November 2011; Volume 8199, p. 819913. [Google Scholar]
- Kretschmer, S.; Jäger, J.; Vilches, S.; Ataman, Ç.; Zappe, H. A bimodal endoscopic imager in a glass package. J. Micromech. Microeng. 2018, 28, 105009. [Google Scholar] [CrossRef]
- Rajiv, A.; Zhou, Y.; Ridge, J.; Reinhall, P.G.; Seibel, E.J. Electromechanical Model-Based Design and Testing of Fiber Scanners for Endoscopy. J. Med. Devices 2018, 12, 041003. [Google Scholar] [CrossRef]
- Akhoundi, F.; Qin, Y.; Peyghambarian, N.; Barton, J.K.; Kieu, K. Compact fiber-based multi-photon endoscope working at 1700 nm. Biomed. Opt. Express 2018, 9, 2326–2335. [Google Scholar] [CrossRef]
- Aguirre, A.D.; Hertz, P.R.; Chen, Y.; Fujimoto, J.G.; Piyawattanametha, W.; Fan, L.; Wu, M.C. Two-axis MEMS Scanning Catheter for Ultrahigh Resolution Three-dimensional and En Face Imaging. Opt. Express 2007, 15, 2445–2453. [Google Scholar] [CrossRef]
- Zara, J.M.; Yazdanfar, S.; Rao, K.D.; Izatt, J.A.; Smith, S.W. Electrostatic micromachine scanning mirror for optical coherence tomography. Opt. Lett. 2003, 28, 628–630. [Google Scholar] [CrossRef]
- Kim, K.H.; Park, B.H.; Maguluri, G.N.; Lee, T.W.; Rogomentich, F.J.; Bancu, M.G.; Bouma, B.E.; De Boer, J.F.; Bernstein, J.J. Two-axis magnetically-driven MEMS scanning catheter for endoscopic high-speed optical coherence tomography. Opt. Express 2007, 15, 18130–18140. [Google Scholar] [CrossRef]
- Yao, J.; Yang, Z.; Peng, T.; Sun, B.; Zhang, H.; Zhao, M.; Dai, B.; Liu, H.; Ding, G.; Sawada, R. A Single-Fiber Endoscope Scanner Probe Utilizing Two-Degrees-of-Freedom (2DOF) High-Order Resonance to Realize Larger Scanning Angle. IEEE Trans. Compon. Packag. Manuf. Technol. 2019, 9, 2332–2340. [Google Scholar] [CrossRef]
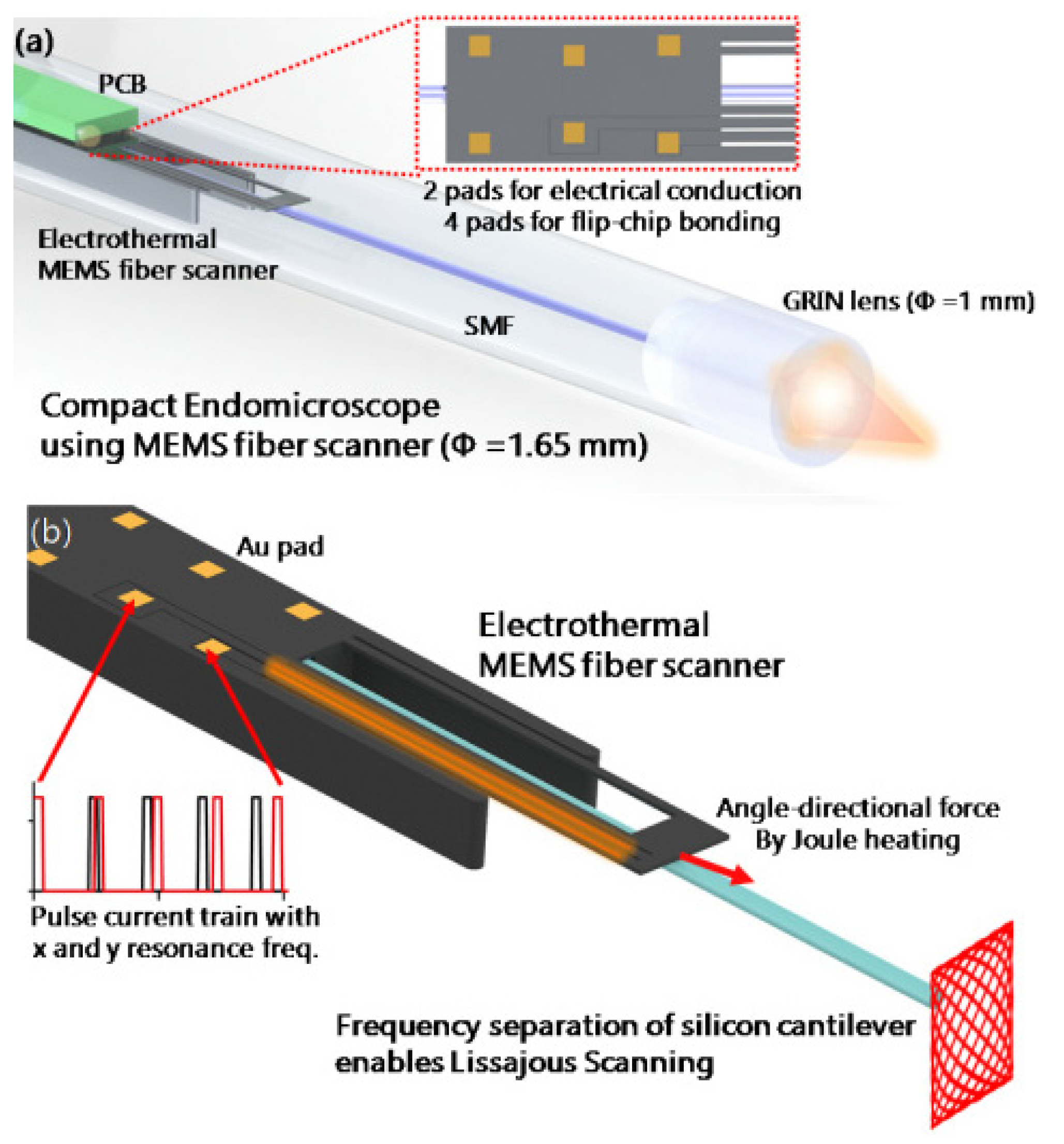
- Seo, H.Y.; Hwang, K.; Jeong, K.-H. 1.65 mm diameter forward-viewing confocal endomicroscopic catheter using a glip-chip bonded electrothermal MEMS fiber scanner. Opt. Express 2018, 26, 4780–4785. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.-H.; Hwang, K.; Park, H.-C.; Jeong, K.-H. Electrothermal MEMS fiber scanner for optical endomicroscopy. Opt. Express 2016, 24, 3903–3909. [Google Scholar] [CrossRef] [PubMed]
- Tanguy, Q.A.; Gaiffe, O.; Passilly, N.; Cote, J.-M.; Cabodevila, G.; Bargiel, S.; Lutz, P.; Xie, H.; Gorecki, C. Real-time Lissajous imaging with a low-voltage 2-axis MEMS scanner based on electro-thermal actuation. Opt. Express 2020, 28, 8512–8527. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Guo, S.; Wu, L.; Liu, L.; Choe, S.-W.; Sorg, B.S.; Xie, H. 3D In Vivo optical coherence tomography based on a low-voltage, large-scan-range 2D MEMS mirror. Opt. Express 2010, 18, 12065–12075. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Tanguy, Q.; Pozzi, A.; Xie, H. Optical coherence tomography endoscopic probe based on a tilted MEMS mirror. Biomed. Opt. Express 2016, 7, 3345–3354. [Google Scholar] [CrossRef]
- Li, H.; Oldham, K.; Wang, T.D. 3 degree-of-freedom resonant scanner with full-circumferential range and large out-of-plane displacement. Opt. Express 2019, 27, 16296–16307. [Google Scholar] [CrossRef]
- Kobayashi, T.; Matsunaga, T.; Haga, Y. Active Bending Electric Endoscope Using Shape Memory Alloy Wires. In Computational and Experimental Simulations in Engineering; Springer Science and Business Media LLC: Geneva, Switzerland, 2015; pp. 131–139. [Google Scholar]
- Pan, Y.; Xie, H.; Fedder, G.K. Endoscopic optical coherence tomography based on a microelectromechanical mirror. Opt. Lett. 2001, 26, 1966–1968. [Google Scholar] [CrossRef]
- Polglase, A.L.; McLaren, W.J.; Skinner, S.A.; Kiesslich, R.; Neurath, M.F.; Delaney, P.M. A fluorescence confocal endomicroscope for in vivo microscopy of the upper- and the lower-GI tract. Gastrointest. Endosc. 2005, 62, 686–695. [Google Scholar] [CrossRef]
- Wu, J.; Conry, M.; Gu, C.; Wang, F.; Yaqoob, Z.; Yang, C. Paired-angle-rotation scanning optical coherence tomography forward-imaging probe. Opt. Lett. 2006, 31, 1265–1267. [Google Scholar] [CrossRef]
- Sarunic, V.M.; Han, S.; Wu, J.; Yaqoob, Z.; Humayun, M.; Yang, C. Endoscopic Optical Coherence Tomography of the Retina at 1310 nm Using Paried-Angle-Rotating Scanning; SPIE: San Jose, CA, USA, 2007. [Google Scholar]
- Kim, K.J.; Choi, J.W.; Yun, S.H. 350-um side-view optical probe for imaging the murine brain in vivo from the cortex to the hypothalamus. J. Biomed. Opt. 2013, 18, 502–511. [Google Scholar] [CrossRef]
- Lee, C.M.; Chandler, J.E.; Seibel, E.J. Wide field fluorescence imaging in narrow passageways using scanning fiber endoscope technology. Endosc. Microsc. V 2010, 7558, 755806. [Google Scholar] [CrossRef]
- Inman, D.J. Engineering Vibrations; CRC Press: Pearson, NJ, USA, 2014. [Google Scholar]
- Qiu, Z.; Piyawattanametha, W. MEMS Actuators for Optical Microendoscopy. Micromachines 2019, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, K.J.; David, I.J.; Delu, S.D.; Ebenezer, D.R.; Jennifer, L.S.; Aaishah, R.L.; Wei, P.L.; Gui-Shuang, Y.L.; Tomas, A.L.; Dunaief, J.L.; et al. Optical coherence tomography identifies outer retina thinning in frontotemporal degeneration. Neurology 2017, 89, 1604–1611. [Google Scholar]
- Lumbroso, B.; Huang, D.; Chen, C.J.; Jia, Y.; Rispoli, M.; Romano, A.; Waheed, N.K. Clinical OCT Angiography Atlas; Jaypee Brothers Medical Publishers: New Delhi, India, 2015. [Google Scholar]
- Boppart, S.A.; Bouma, B.E.; Pitris, C.; Tearney, G.J.; Fujimoto, J.G.; Brezinski, M.E. Forward-imaging instruments for optical coherence tomography. Opt. Lett. 1997, 22, 1618–1620. [Google Scholar] [CrossRef] [PubMed]
- Paddock, S.W.; Fellers, T.J.; Davidson, M.W. Introductory Confocal Concepts, MicroscopyU. Available online: https://www.microscopyu.com/techniques/confocal/introductory-confocal-concepts (accessed on 23 June 2020).
- Liu, L.; Wang, E.; Zhang, X.; Liang, W.; Li, X.; Xie, H. MEMS-based 3D confocal scanning microendoscope using MEMS scanners for both lateral and axial scan. Sens. Actuators A 2014, 215, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Dickensheets, D.L.; Kino, G.S. Micromachined scanning confocal optical microscope. Opt. Lett. 1996, 21, 764–766. [Google Scholar] [CrossRef]
- Maitland, K.C.; Shin, H.J.; Ra, H.; Lee, D.; Solgaard, O.; Richards-Kortum, R. Single fiber confocal microscope with a two-axis gimbaled MEMS scanner for cellular imaging. Opt. Express 2006, 14, 8604–8612. [Google Scholar] [CrossRef]
- Jung, I.W.; Lopez, D.; Qiu, Z.; Piyawattanametha, W. 2-D MEMS Scanner for Handheld Multispectral Dual-Axis Confocal Microscopes. J. Microelectromech. Syst. 2018, 27, 605–612. [Google Scholar] [CrossRef]
- Li, R.; Wang, X.; Zhou, Y.; Zong, H.; Chen, M.; Sun, M. Advances in nonlinear optical microscopy for biophotonics. J. Nanophotonics 2018, 12, 033007. [Google Scholar] [CrossRef]
- Piston, D.W.; Fellers, T.J.; Davidson, M.W. Multiphoton Microscopy, MicroscopyU. Available online: https://www.microscopyu.com/techniques/multi-photon/multiphoton-microscopy (accessed on 23 June 2020).
- Holmstrom, S.T.S.; Baran, U.; Urey, H. MEMS Laser Scanners: A Review. J. Microelectromech. Syst. 2014, 23, 259–275. [Google Scholar] [CrossRef]
- Haight, E.C.; King, W.W. Stability of Nonlinear Oscillations of an Elastic Rod. J. Acoust. Soc. Am. 1972, 52, 899–911. [Google Scholar] [CrossRef]
- Hyer, M.W. Whirling of a base-excited cantilever beam. J. Acoust. Soc. Am. 1979, 65, 931–939. [Google Scholar] [CrossRef]
- Kundrat, M.J.; Reinhall, P.G.; Lee, C.M.; Seibel, E.J. High performance open loop control of scanning with a small cylindrical cantilever beam. J. Sound Vib. 2011, 330, 1762–1771. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ding, Z.; Huang, G. Realization of 2D Scanning Pattern of a Fiber Cantilever by Nonlinear Coupling; SPIE: Wuhan, China, 2007; p. 65340I. [Google Scholar]
- Zhang, X.; Duan, C.; Liu, L.; Li, X.; Xie, H. A non-resonant fiber scanner based on an electrothermally-actuated MEMS stage. Sens. Actuators A Phys. 2015, 233, 239–245. [Google Scholar] [CrossRef]
- Park, H.-C.; Zhang, X.; Yuan, W.; Zhou, L.; Xie, H.; Li, X. Ultralow-voltage electrothermal MEMS based fiber-optic scanning probe for forward-viewing endoscopic OCT. Opt. Lett. 2019, 44, 2232–2235. [Google Scholar] [CrossRef]
- Moon, S.; Lee, S.-W.; Rubinstein, M.; Wong, B.J.F.; Chen, Z. Semi-resonant operation of a fiber-cantilever piezotube scanner for stable optical coherence tomography endoscope imaging. Opt. Express 2010, 18, 21183–21197. [Google Scholar] [CrossRef]
- Yang, S.; Xu, Q. A review on actuation and sensing techniques for MEMS-based microgrippers. J. Micro-Bio Robot. 2017, 13, 1–14. [Google Scholar] [CrossRef]
- Zhang, P. Sensors and actuators. In Advanced Industrial Control Technology; Elsevier BV: Heidelberg, Germany, 2010; pp. 73–116. [Google Scholar]
- Pengwang, E.; Rabenorosoa, K.; Rakotondrabe, M.; Andreff, N. Scanning Micromirror Platform Based on MEMS Technology for Medical Application. Micromachines 2016, 7, 24. [Google Scholar] [CrossRef]
- Emery, J. Piezoelectricity, 04 03 1997. Available online: http://stem2.org/je/piezoelc.pdf (accessed on 12 June 2020).
- Bahadur, I.M.; Mills, J.K. A new model of hysteresis in piezoelectric actuators. In Proceedings of the 2011 IEEE International Conference on Mechatronics and Automation, Beijing, China, 7–10 August 2011; pp. 789–794. [Google Scholar]
- ThorLabs. Piezoelectric Tutorial. Available online: https://www.thorlabs.com/newgrouppage9.cfm?objectgroup_id=5030 (accessed on 15 June 2020).
- Tekpınar, M.; Khayatzadeh, R.; Ferhanoğlu, O. Multiple-pattern generating piezoelectric fiber scanner toward endoscopic applications. Opt. Eng. 2019, 58, 023101. [Google Scholar] [CrossRef]
- Rivera, D.R.; Brown, C.M.; Ouzounov, D.G.; Pavlova, I.; Kobat, D.; Webb, W.W.; Xu, C. Compact and flexible raster scanning multiphoton endoscope capable of imaging unstained tissue. Proc. Natl. Acad. Sci. USA 2011, 108, 17598–17603. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Hall, G.; Messerschmidt, B.; Li, M.-J.; Li, X. Nonlinear optical endomicroscopy for label-free functional histology in vivo. Light Sci. Appl. 2017, 6, e17082. [Google Scholar] [CrossRef] [PubMed]
- Vilches, S.; Kretschmer, S.; Ataman, Ç.; Zappe, H. Miniaturized Fourier-plane fiber scanner for OCT endoscopy. J. Micromech. Microeng. 2017, 27, 105015. [Google Scholar] [CrossRef]
- Mayyas, M. Comprehensive Thermal Modeling of ElectroThermoElastic Microsturctures. In Actuators; Molecular Diversity Preservation International: New York, NY, USA, 2012; Volume 1, pp. 21–35. [Google Scholar]
- Lin, L.; Chiao, M. Electrothermal responses of lineshape microstructures. Sens. Actuators A 1996, 55, 35–41. [Google Scholar] [CrossRef]
- So, H.; Pisano, A.P. Electrothermal modeling, fabrication and analysis of low-power consumption thermal actuator with buckling arm. Microsyst. Technol. 2013, 21, 195–202. [Google Scholar] [CrossRef]
- Buser, R.; De Rooij, N.; Tischhauser, H.; Dommann, A.; Staufert, G. Biaxial scanning mirror activated by bimorph structures for medical applications. Sens. Actuators A Phys. 1992, 31, 29–34. [Google Scholar] [CrossRef]
- Guckel, H.; Klein, J.; Christenson, T.; Skrobis, K.; Laudon, M.; Lovell, E. Thermo-magnetic metal flexure actuators. In Technical Digest IEEE Solid-State Sensor and Actuator Workshop; Institute of Electrical and Electronics Engineers: New York, NY, USA, 2003. [Google Scholar]
- Huang, Q.-A.; Lee, N.K.S. Analysis and design of polysilicon thermal flexure actuator. J. Micromech. Microeng. 1999, 9, 64–70. [Google Scholar] [CrossRef]
- Hickey, R.; Kujath, M.; Hubbard, T. Heat transfer analysis and optimization of two-beam microelectromechanical thermal actuators. J. Vac. Sci. Technol. A 2002, 20, 971–974. [Google Scholar] [CrossRef]
- Huang, Q.-A.; Lee, N.K.S. Analytical modeling and optimization for a laterally-driven polysilicon thermal actuator. Microsyst. Technol. 1999, 5, 133–137. [Google Scholar] [CrossRef]
- Lee, C.-C.; Hsu, W. Optimization of an electro-thermally and laterally driven microactuator. Microsyst. Technol. 2003, 9, 331–334. [Google Scholar] [CrossRef]
- Moulton, T.; Ananthasuresh, G. Micromechanical devices with embedded electro-thermal-compliant actuation. Sens. Actuators A 2001, 90, 38–48. [Google Scholar] [CrossRef]
- Lara-Castro, M.; Herrera-Amaya, A.; Escarola-Rosas, M.A.; Vázquez-Toledo, M.; López-Huerta, F.; Aguilera-Cortés, L.A.; Herrera-May, A.L. Design and Modeling of Polysilicon Electrothermal Actuators for a MEMS Mirror with Low Power Consumption. Micromachines 2017, 8, 203. [Google Scholar] [CrossRef] [PubMed]
- Venditti, R.; Lee, J.S.H.; Sun, Y.; Li, D. An in-plane, bi-directional electrothermal MEMS actuator. J. Micromech. Microeng. 2006, 16, 2067–2070. [Google Scholar] [CrossRef]
- Seo, Y.-H.; Park, H.-C.; Jeong, K.-H. Electrothermal MEMS fiber scanner with lissajous patterns for endomicroscopic applications. In Proceedings of the 2016 IEEE 29th International Conference on Micro Electro Mechanical Systems (MEMS), Shanghai, China, 24–28 January 2016; Volume 24, pp. 367–370. [Google Scholar] [CrossRef]
- Enikov, E.; Kedar, S.; Lazarov, K. Analytical model for analysis and design of V-shaped thermal microactuators. J. Microelectromech. Syst. 2005, 14, 788–798. [Google Scholar] [CrossRef]
- Sinclair, M. A high force low area MEMS thermal actuator. In Proceedings of the ITHERM 2000—The Seventh Intersociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems (Cat. No.00CH37069), Las Vegas, NV, USA, 23–26 May 2002. [Google Scholar]
- Que, L.; Park, J.-S.; Gianchandani, Y. Bent-beam electrothermal actuators-Part I: Single beam and cascaded devices. J. Microelectromechan. Syst. 2001, 10, 247–254. [Google Scholar] [CrossRef]
- Iqbal, S.; Malik, A.A.; Shakoor, R.I. Design and analysis of novel micro displacement amplification mechanism actuated by chevron shaped thermal actuators. Microsyst. Technol. 2018, 25, 861–875. [Google Scholar] [CrossRef]
- Guan, C.; Zhu, Y. An electrothermal microactuator with Z-shaped beams. J. Micromechan. Microeng. 2010, 20, 1–9. [Google Scholar] [CrossRef]
- Rawashdeh, E.; Karam, A.; Foulds, I.G. Characterization of Kink Actuators as Compared to Traditional Chevron Shaped Bent-Beam Electrothermal Actuators. Micromachines 2012, 3, 542–549. [Google Scholar] [CrossRef]
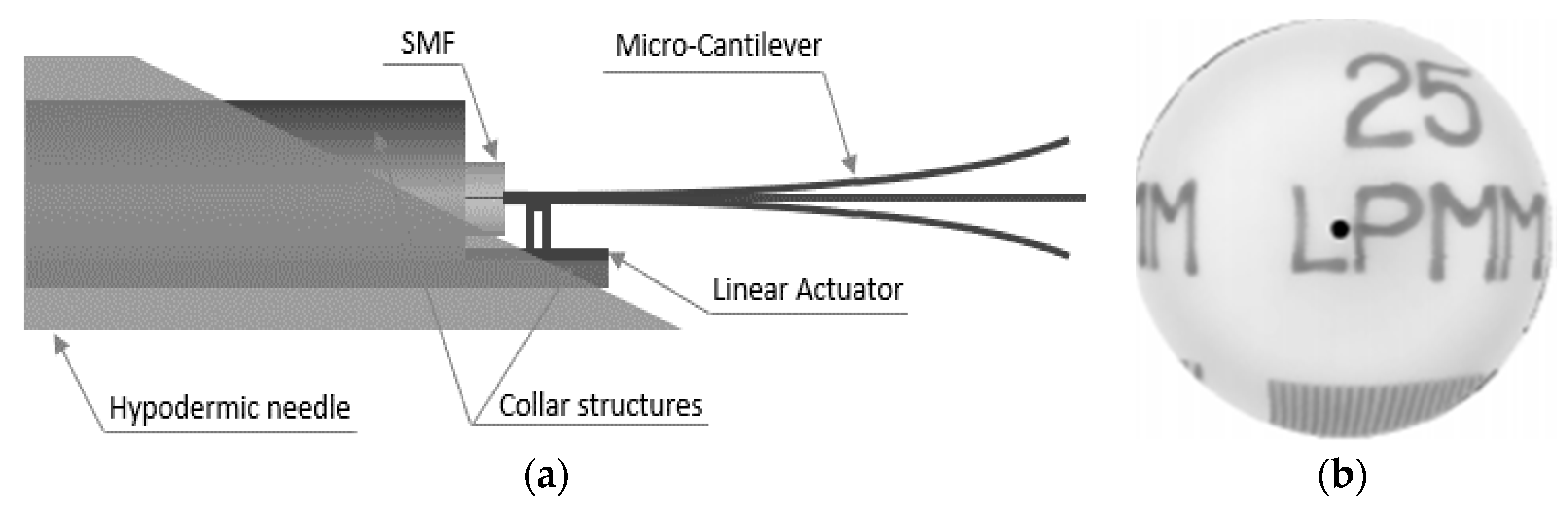
- Kaur, M.; Brown, M.; Lane, P.M.; Menon, C. An Electro-Thermally Actuated Micro-Cantilever-Based Fiber Optic Scanner. IEEE Sens. J. 2020, 20, 9877–9885. [Google Scholar] [CrossRef]
- Chu, W.-H.; Mehregany, M.; Mullen, R.L. Analysis of tip deflection and force of a bimetallic cantilever microactuator. J. Micromechan. Microeng. 1993, 3, 4–7. [Google Scholar] [CrossRef]
- Tanguy, Q.A.A.; Bargiel, S.; Xie, H.; Passilly, N.; Barthès, M.; Gaiffe, O.; Rutkowski, J.; Lutz, P.; Gorecki, C. Design and Fabrication of a 2-Axis Electrothermal MEMS Micro-Scanner for Optical Coherence Tomography. Micromachines 2017, 8, 146. [Google Scholar] [CrossRef]
- Pawinanto, R.E.; Yunas, J.; Majlis, B.; Hamzah, A. Design and Fabrication of Compact MEMS Electromagnetic Micro-Actuator with Planar Micro-Coil Based on PCB. TELKOMNIKA Telecommun. Comput. Electron. Control. 2016, 14, 856–866. [Google Scholar] [CrossRef]
- Judy, J.W.; Muller, R.S. Magnetically actuated, addressable microstructures. J. Microelectromechan. Syst. 1997, 6, 249–256. [Google Scholar] [CrossRef]
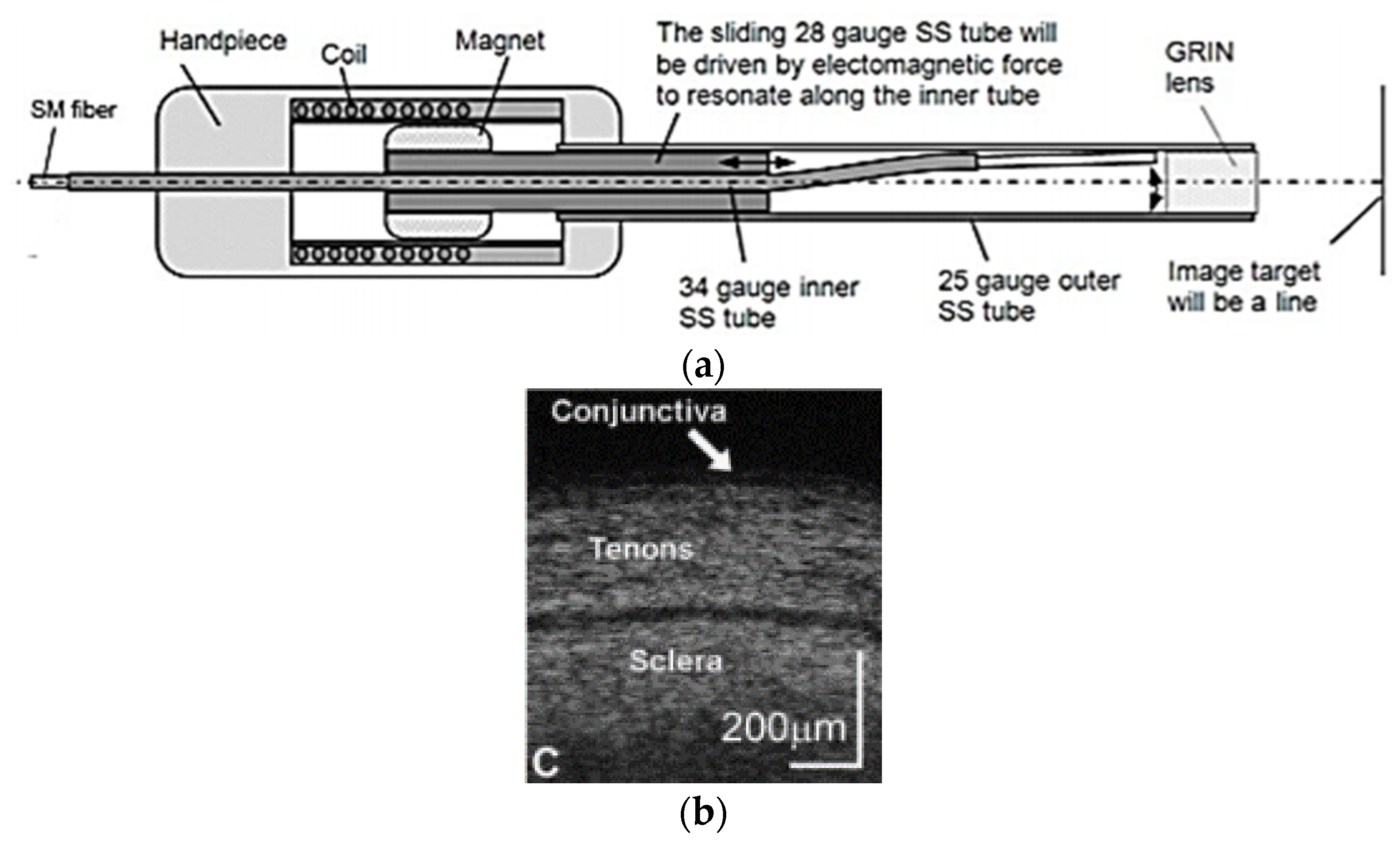
- Joos, K.M.; Shen, J.-H. Miniature real-time intraoperative forward-imaging optical coherence tomography probe. Biomed. Opt. Express 2013, 4, 1342–1350. [Google Scholar] [CrossRef]
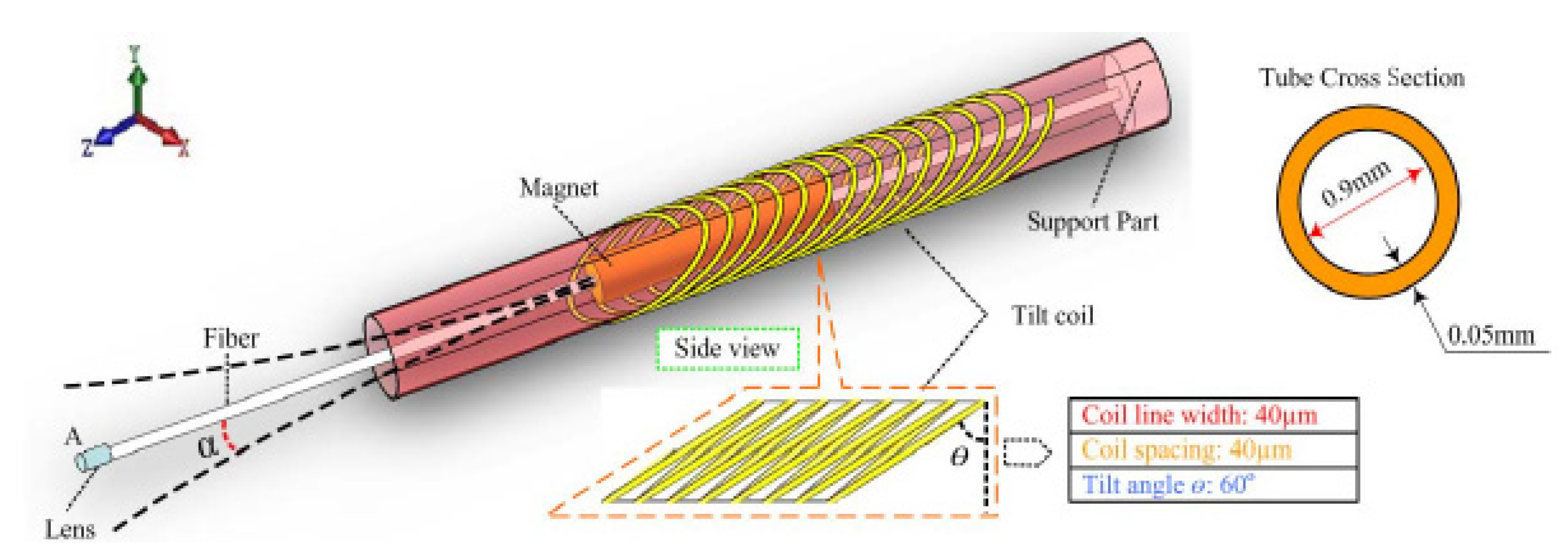
- Sun, B.; Sawada, R.; Yang, Z.; Zhang, Y.; Itoh, T.; Maeda, R. Design and fabrication of driving microcoil with large tilt-angle for medical scanner application. In Proceedings of the 2014 Symposium on Design, Test, Integration and Packaging of MEMS/MOEMS, Cannes, France, 1–4 April 2014; pp. 1–6. [Google Scholar] [CrossRef]
- Sun, B.; Nogami, H.; Pen, Y.; Sawada, R. Microelectromagnetic actuator based on a 3D printing process for fiber scanner application. J. Micromechan. Microeng. 2015, 25, 075014. [Google Scholar] [CrossRef]
- Lv, X.; Wei, W.; Mao, X.; Chen, Y.; Yang, J.; Yang, F. A novel MEMS electromagnetic actuator with large displacement. Sens. Actuators A 2015, 221, 22–28. [Google Scholar] [CrossRef]
- Barbaroto, P.R.; Ferreira, L.O.S.; Doi, I. Micromachined scanner actuated by electromagnetic induction. In Optomechatronic Systems III; International Society for Optics and Photonics: New York, NY, USA, 2002; pp. 691–698. [Google Scholar]
- Tang, T.-L.; Hsu, C.-P.; Chen, W.-C.; Fang, W. Design and implementation of a torque-enhancement 2-axis magnetostatic SOI optical scanner. J. Micromechan. Microeng. 2010, 20, 025020. [Google Scholar] [CrossRef][Green Version]
- Cullity, B.D.; Graham, C.D. Magnetostriction and the Effects of Stress. In Introduction to Magnetic Materials; Wiley: Hoboken, NJ, USA, 2009; pp. 241–273. [Google Scholar]
- Bourouina, T.; Lebrasseur, E.; Reyne, G.; Debray, A.; Fujita, H.; Ludwig, A.; Quandt, E.; Muro, H.; Oki, T.; Asaoka, A. Integration of two degree-of-freedom magnetostrictive actuation and piezoresistive detection: Application to a two-dimensional optical scanner. J. Microelectromechan. Syst. 2002, 11, 355–361. [Google Scholar] [CrossRef]
- Garnier, A.; Bourouina, T.; Fujita, H.; Orsier, E.; Masuzawa, T.; Hiramoto, T.; Peuzin, J.-C. A fast, robust and simple 2-D micro-opticalscanner based on contactless magnetostrictive actuation. In Proceedings of the IEEE Thirteenth Annual International Conference on Micro Electro Mechanical Systems, Miyazaki, Japan, 23–27 January 2000. [Google Scholar]
- Pandojirao-Sunkojirao, P.; Rao, S.; Phuyal, P.C.; Dhaubanjar, N.; Chiao, J.-C. A Magnetic Actuator for Fiber-Optic Applications. Int. J. Optomechatron. 2009, 3, 215–232. [Google Scholar] [CrossRef]
- Collard, D.; Fujita, H.; Toshiyoshi, B.; Legrand, B.; Buchaillot, L. Electrostatic Micro-actuators. In Microsystems Technology: Fabrication, Test & Reliability; Kogan Page Science: London, UK, 2003; pp. 75–115. [Google Scholar]
- Bourouina, T.; Fujita, H.; Reyne, G.; Motamedi, M.E. Optical Scanning. In MOEMS: Micro-Opto-Electro-Mechanical Systems; SPIE: Bellingham, WA, USA, 2005; pp. 323–367. [Google Scholar]
- Henri, C.; Franck, L. Fabrication, Simulation and experiment of a rotating electrostatic silicon mirror with large angular deflection. In Proceedings of the IEEE Thirteen Annual International Conference on Micro Electro Mechanical Systems, Miyazaki, Japan, 23–27 January 2000. [Google Scholar]
- Patterson, P.R.; Hah, D.; Fujino, M.; Piyawattanametha, W.; Wu, M.C. Scanning micromirrors: An overview. In Optomechatronic Micro/Nano Components, Devices, and Systems; SPIE: Bellingham, WA, USA, 2004; Volume 5604, pp. 195–208. [Google Scholar]
- Piyawattanametha, W.; Barretto, R.P.J.; Ko, T.H.; Flusberg, B.A.; Cocker, E.D.; Ra, H.; Lee, D.; Solgaard, O.; Schnitzer, M.J. Fast-scanning two-photon fluorescence imaging based on a microelectromechanical systems two- dimensional scanning mirror. Opt. Lett. 2006, 31, 2018–2020. [Google Scholar] [CrossRef]
- Hofmann, U.; Janes, J.; Quenzer, H.-J. High-Q MEMS Resonators for Laser Beam Scanning Displays. Micromachines 2012, 3, 509–528. [Google Scholar] [CrossRef]
- Izawa, T.; Sasaki, T.; Hane, K. Scanning Micro-Mirror with an Electrostatic Spring for Compensation of Hard-Spring Nonlinearity. Micromachines 2017, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Schenk, H.; Dürr, P.; Kunze, D.; Lakner, H.; Kück, H. A resonantly excited 2D-micro-scanning-mirror with large deflection. Sens. Actuators A 2001, 89, 104–111. [Google Scholar] [CrossRef]
- Munce, N.R.; Mariampillai, A.; Standish, B.A.; Pop, M.; Anderson, K.J.; Liu, G.Y.; Luk, T.; Courtney, B.K.; Wright, G.A.; Vitkin, I.A.; et al. Electrostatic forward-viewing scanning probe for Doppler optical coherence tomography using a dissipative polymer catheter. Opt. Lett. 2008, 33, 657–659. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huebsch, W.; Hamburg, S.; Guiler, R. Aircraft morphing technologies. In Innovation in Aeronautics; Elsevier BV: Amsterdam, The Netherlands, 2012; pp. 37–55. [Google Scholar]
- Ishii, T. Design of shape memory alloy (SMA) coil springs for actuator applications. In Shape Memory and Superelastic Alloys; Elsevier BV: Heidelberg, Germany, 2011; pp. 63–76. [Google Scholar]
- Maeda, S.; Abe, K.; Yamamoto, K.; Tohyama, O.; Ito, H. Active endoscope with SMA (Shape Memory Alloy) coil springs. In Proceedings of the Ninth International Workshop on Micro Electromechanical Systems, San Diego, CA, USA, 24 January 2002. [Google Scholar]
- Haga, Y.; Esashi, M. Small Diameter Active Catheter Using Shape Memory Alloy Coils. IEEJ Trans. Sens. Micromach. 2000, 120, 509–514. [Google Scholar] [CrossRef]
- Makishi, W.; Matsunaga, T.; Esashi, M.; Haga, Y. Active Bending Electric Endoscope Using Shape Memory Alloy Coil Actuators. IEEJ Trans. Sens. Micromach. 2007, 127, 75–81. [Google Scholar] [CrossRef][Green Version]
- Liang, W.; Park, H.-C.; Li, K.; Li, A.; Chen, D.; Guan, H.; Yue, Y.; Gau, Y.-T.A.; Bergles, D.E.; Li, M.-J.; et al. Throughput-Speed Product Augmentation for Scanning Fiber-Optic Two-Photon Endomicroscopy. IEEE Trans. Med. Imaging 2020, 39, 3779–3787. [Google Scholar] [CrossRef]
- Naono, T.; Fujii, T.; Esashi, M.; Tanaka, S. Non-resonant 2-D piezoelectric MEMS optical scanner actuated by Nb doped PZT thin film. Sens. Actuators A 2015, 233, 147–157. [Google Scholar] [CrossRef]
- Thong, P.S.-P.; Olivo, M.; Kho, K.-W.; Zheng, W.; Mancer, K.; Harris, M.R.; Soo, K.-C. Laser confocal endomicroscopy as a novel technique for fluorescence diagnostic imaging of the oral cavity. J. Biomed. Opt. 2007, 12, 014007. [Google Scholar] [CrossRef]
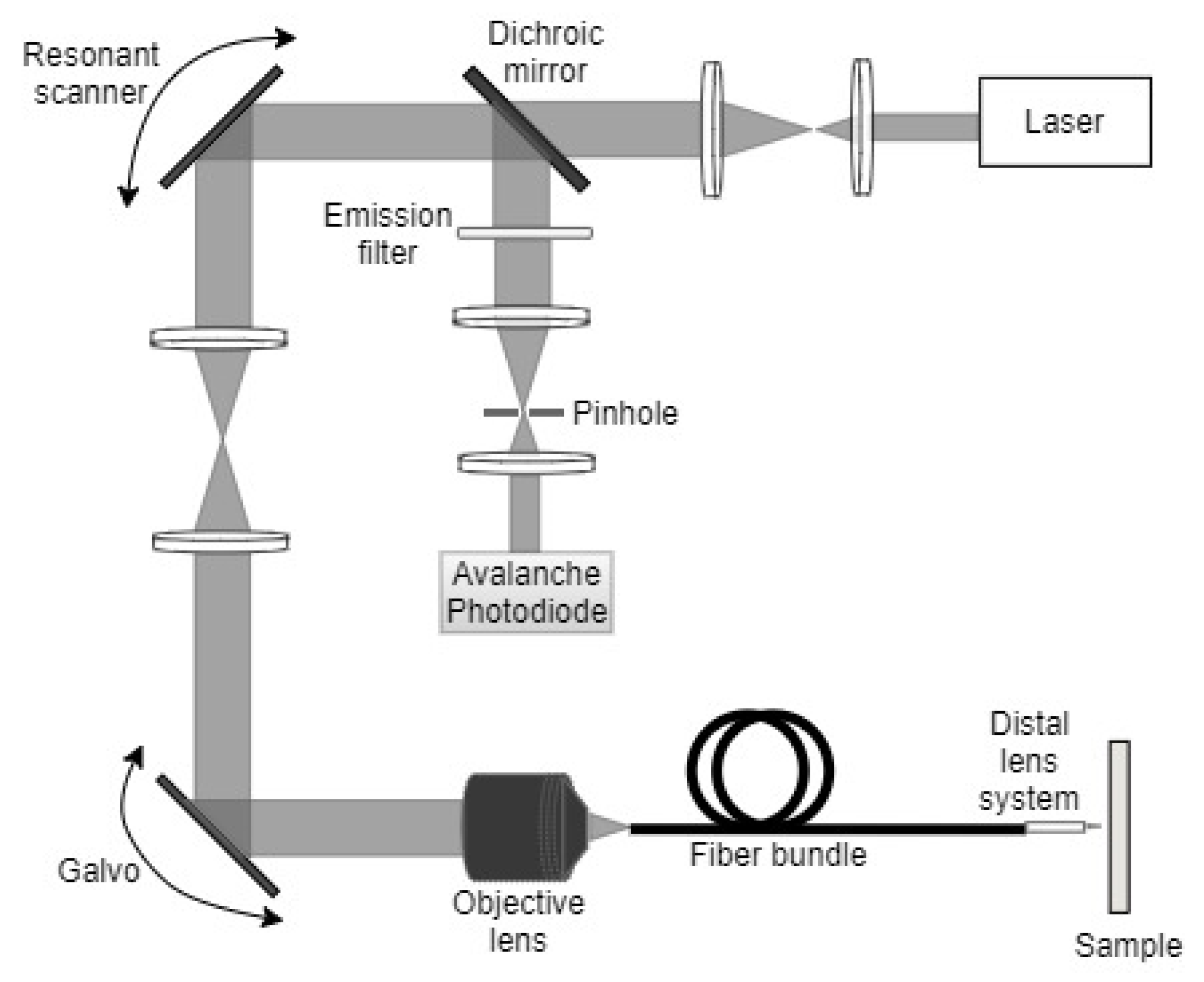
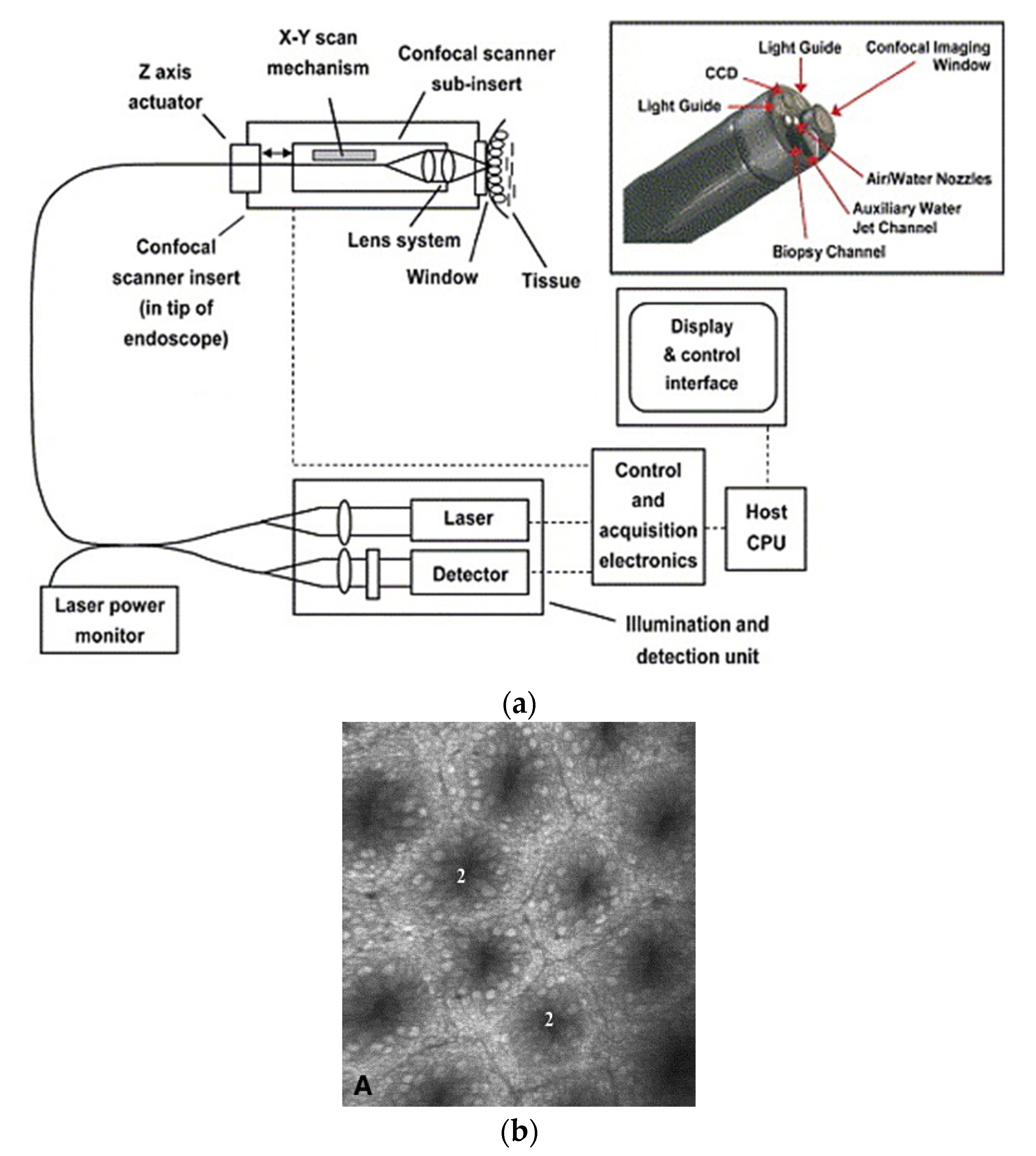
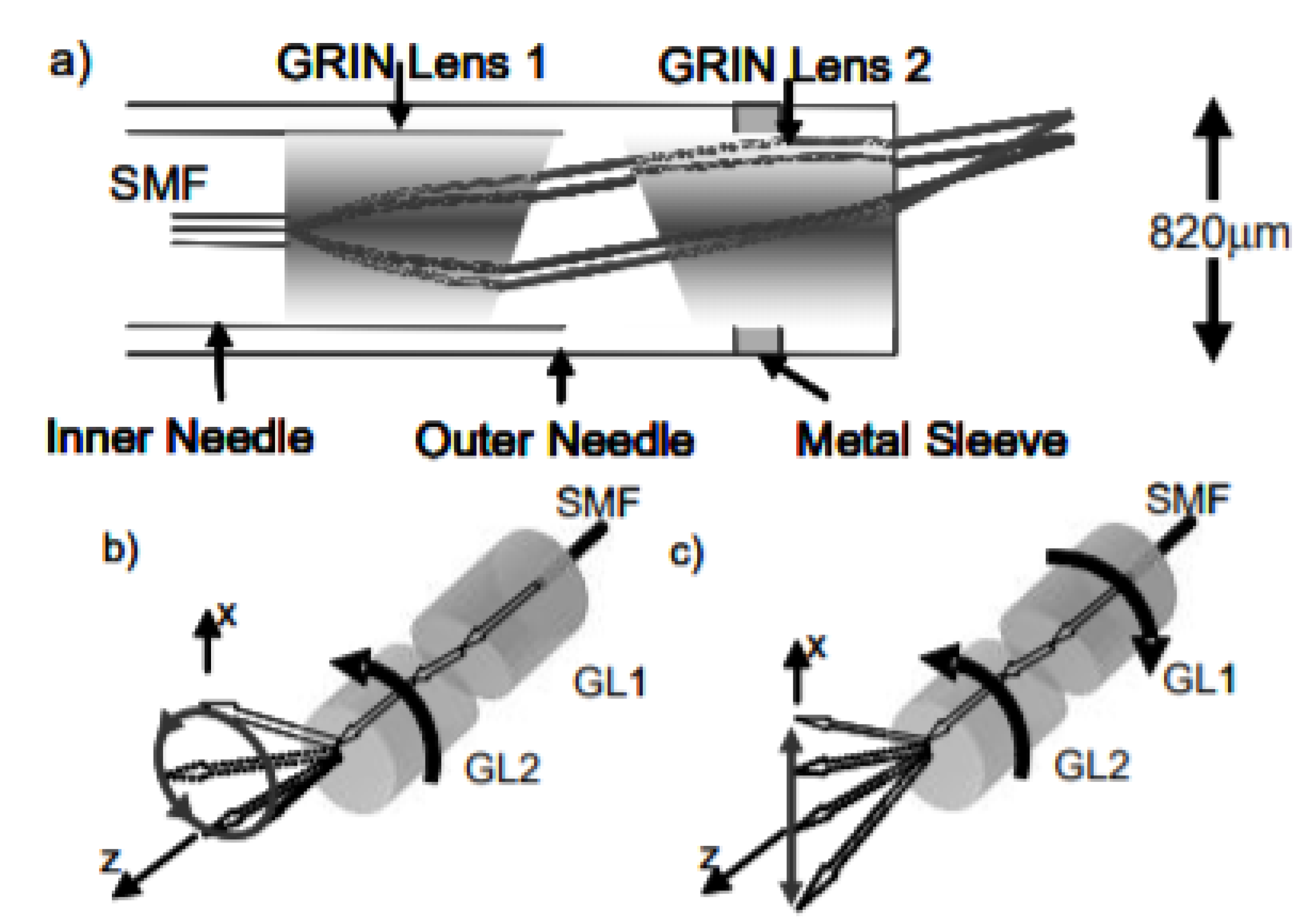
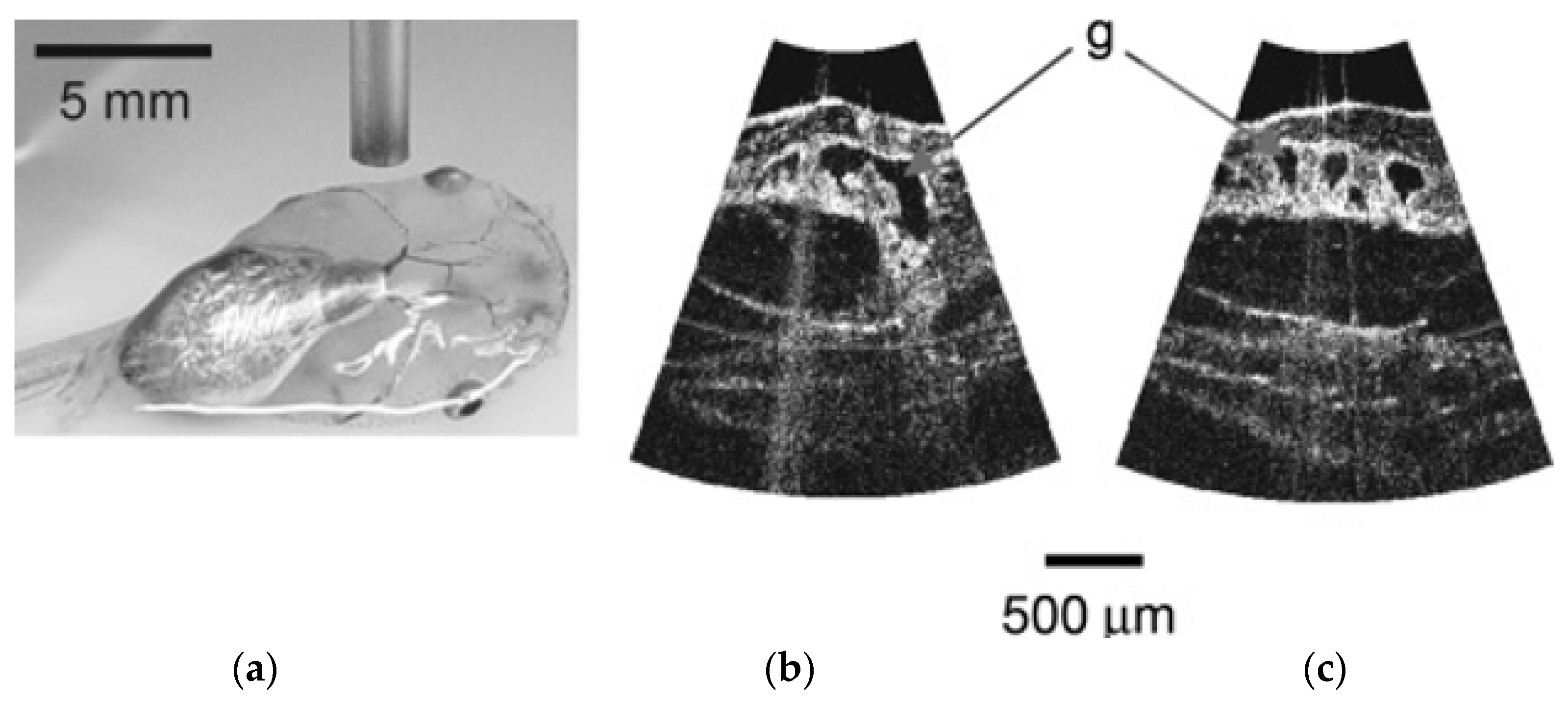

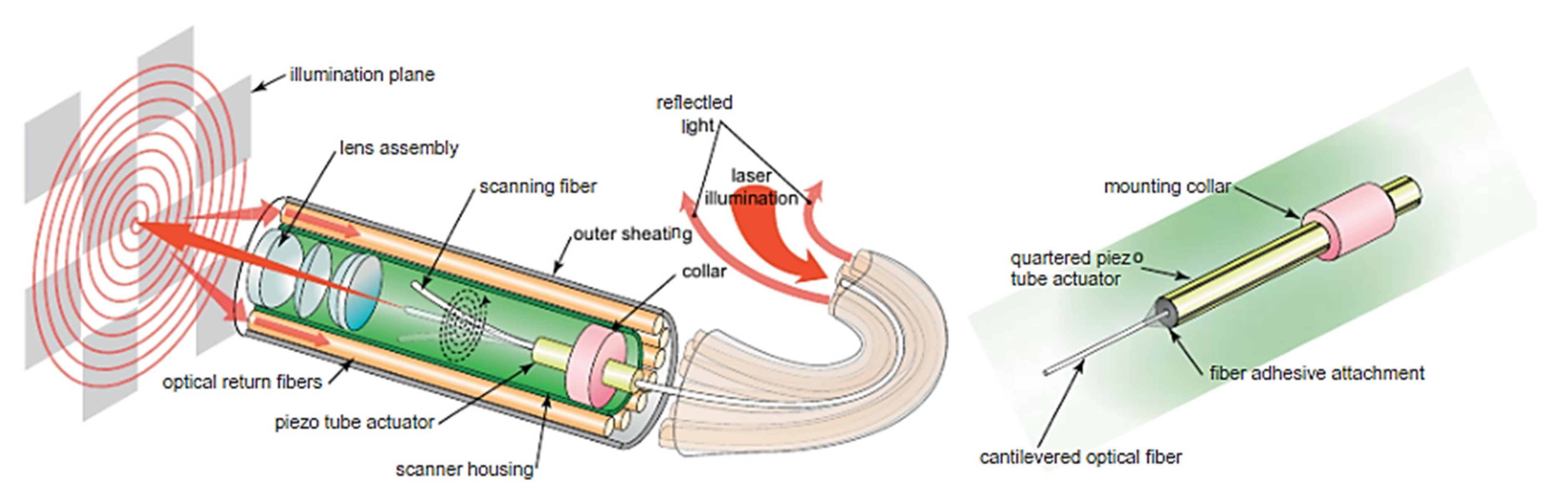
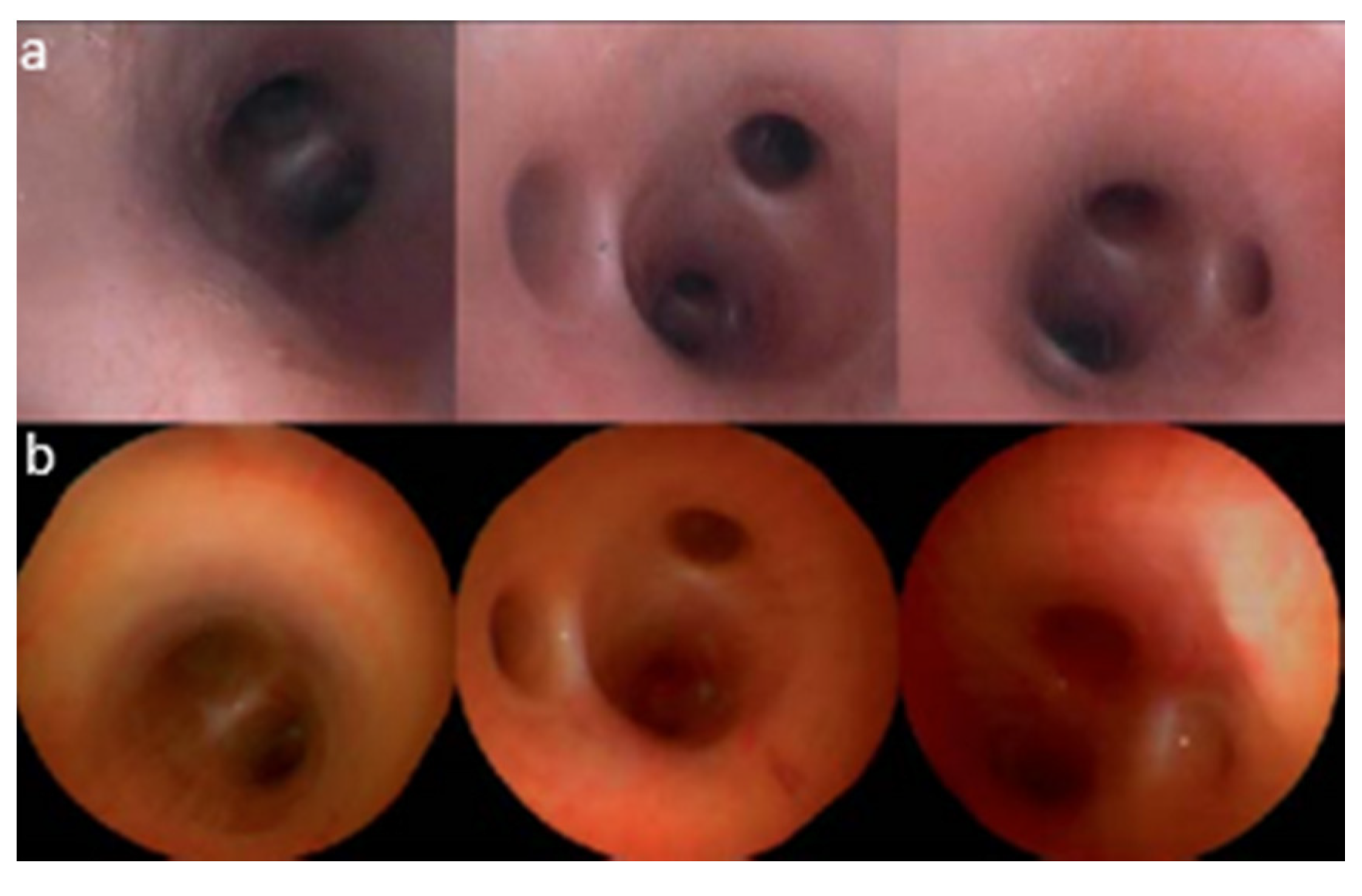
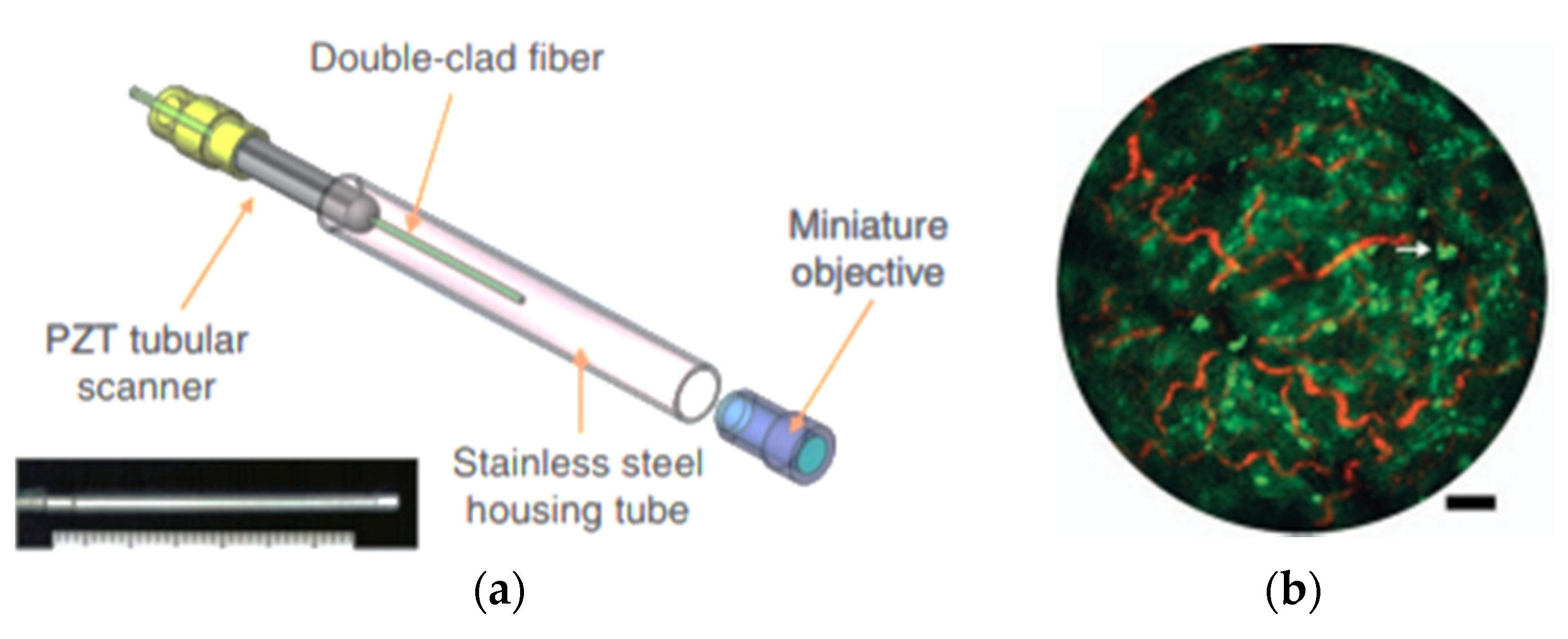
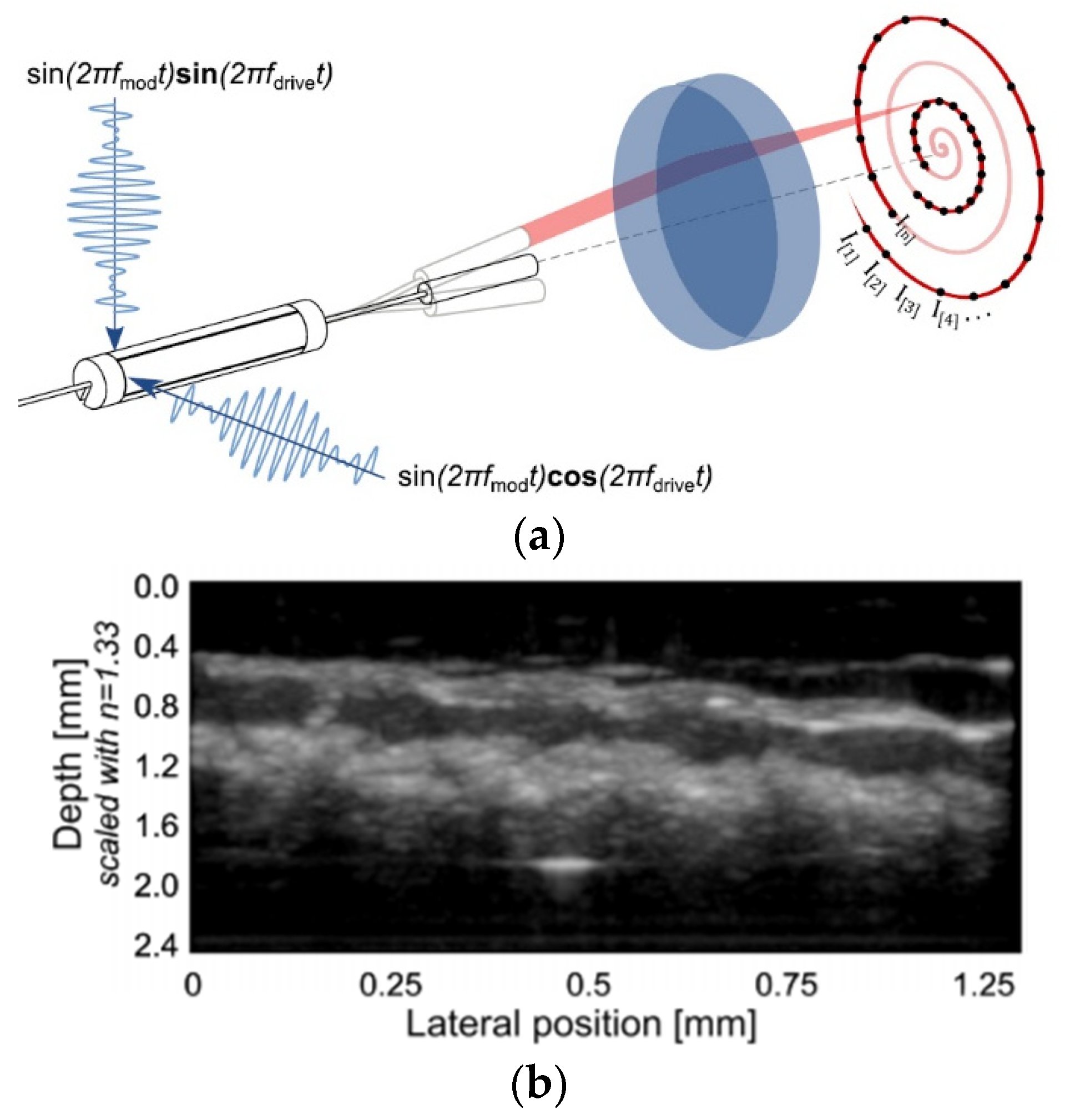


| Pixel Density (Pixels/mm2) | Image Resolution (Pixels) | Pixel Size | Advantages | Disadvantages | |
|---|---|---|---|---|---|
| CFB | 113 k | 30.0 k/64.0 k | 2 µm Ø | Small form factor, low cost | Cross-coupling and honeycomb effect degrade image resolution Aging effect results in non-working pixels due to fractured fibers within the bundle |
| CCD | 238 k | 95.0 k | 0.5 µm × 0.5 µm | Small pixel size, low cost, no aging effect | Rectangular geometry limits the usable area, low dynamic range, poor light collection in low illumination area |
| CMOS | 476 k | 190 k | 1.45 µm × 1.45 µm | Higher image resolution, low cost | Rectangular geometry limits the usable area, poor resolution in devices with diameter < 1 mm |
| SFE | 345 k | 282 k | Dependent on scanning pattern and sampling rate | Higher sampling rate and resolution in sub-millimeter-sized devices | Performance dependent on actuation method and sampling rate. Spatial point spread dependent on objective lens at the tip and illumination properties. |
| Electrostatic | Electro-Thermal | Piezoelectric | Electromagnetic | Shape Memory Alloy | |
|---|---|---|---|---|---|
| Force | ✓ | ✓ | ✓✓✓ | ✓✓ | ✓✓✓ |
| Displacement amplitude | ✓✓ | ✓✓✓ | ✓ | ✓✓✓ | ✓✓ |
| Compactness | ✓✓✓ | ✓✓✓ | ✓✓ | ✓ | ✓✓ |
| Power consumption | ✓✓ | ✓ | ✓ | ✓✓✓ | ✓✓✓ |
| Working principle | Electrostatic force | Thermal expansion | Piezoelectric effect | Magnetization effect | Material deformation |
| Motion range | 1D/2D | 1D/2D | 2D | 1D | 1D |
| Scanning pattern | Spiral | Lissajous | Spiral | Linear | Linear |
| Advantages | Fast response, low voltage required, easy fabrication, and no hysteresis | Large displacement, low operating voltage, small dimensions | Large force generated, wide operating frequency range, low power consumption | Large displacement obtained, quick and linear response, easy to control | Flexibility, large frequency response |
| Disadvantages | Large device dimensions, pull-in problem, complicated circuit | High working temperature, not operable at very high frequencies | Limited displacement | Large device dimensions, difficult to manufacture | Low displacement |
| Actuation Principle | Imaging Modality | Scanning Direction | Resolution | FOV | Frequency | Driving Voltage | Frame Rate | Scanner Dimensions | Scanning Pattern | Cantilever Fiber | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Piezoelectric bimorph | Multiphoton | Forward | 0.8 µm (lateral), 10 µm (axial) | 110 µm × 110 µm | 1.05 kHz (fast axis), 4.1 Hz | 50 Vpp (resonant bimorph) 200 Vpp (non-resonant) | 4.1 fps (512 × 512 pixels) | 3 mm (diameter) 40 mm (rigid length) | Raster scan | DCF | [105] |
| Piezoelectric tube | Two-photon | Forward | 0.61 µm ÷ 1.10 µm (from center of FOV to peripheric zone) | 160 µm | 3.1 kHz | 48 Vpp | 6 fps | ~2 mm (diameter) | Spiral | DCF with GRIN lens | [156] |
| Piezoelectric tube | Two-Photon | Forward | 10 µm | 70° | 5 kHz | 25 V | 15 fps | 1.6 mm (diameter) | Spiral | SMF | [16] |
| Piezoelectric thin film | E-OCT | Side-view | 5 µm (axial) | 152° | 394 Hz | 1.3 Vpp | - | 3.4 mm × 2.5 mm | radial | - | [157] |
| Electrothermal | Confocal endomicroscope | Forward | ~1.7 µm | 378 µm × 439 µm | 239 Hz (x-axis) 207 Hz (y-axis) | 16 Vpp | 1 fps | 1.65 mm (diameter) 28 mm (rigid length) | Lissajous | SMF | [65] |
| Electrothermal | OCT (A-scan) | Forward | ~17 µm (lateral) ~9 µm (axial) | ~3 mm (beam scanning) | ~100 Hz | 3 Vac_pp, 1.5 V DC offset | 200 fps | 5.5 mm (diameter) 55 mm (rigid length) | raster | - | [96] |
| Electromagnetic | OCT (B-scan) | Forward | 4–6 µm (axial) 25–35 µm (lateral) | 2 mm | 5 Hz | ±10 V (triangle wave) | - | 0.51 mm (diameter) | Linear | SMF | [132] |
| Micromotor | PA and US endoscopy | Side-view | ~58 µm (PA radial) ~30 µm (US radial) ~100 µm (PA transvers) ~120 µm (US transverse) | ~310° | ~4 Hz | ~3.2V DC | 4 fps | 2.5 mm (diameter) ~35 mm (Rigid length) | Radial | MMF | [40] |
| Electromagnetic | Confocal | Forward | 0.8 µm (lateral) | 390 µm × 390 µm | 700 Hz (fast scan) 1–2 Hz (slow scan) | - | 1 fps | 8 mm (diameter) | Raster | SMF | [158] |
| Cellvizio | Confocal | Forward | 5–15 µm (axial) 2–5 µm (lateral) | 600 µm × 500 µm | - | - | 12 fps | 2.5 mm | - | - | [33] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, M.; Lane, P.M.; Menon, C. Scanning and Actuation Techniques for Cantilever-Based Fiber Optic Endoscopic Scanners—A Review. Sensors 2021, 21, 251. https://doi.org/10.3390/s21010251
Kaur M, Lane PM, Menon C. Scanning and Actuation Techniques for Cantilever-Based Fiber Optic Endoscopic Scanners—A Review. Sensors. 2021; 21(1):251. https://doi.org/10.3390/s21010251
Chicago/Turabian StyleKaur, Mandeep, Pierre M. Lane, and Carlo Menon. 2021. "Scanning and Actuation Techniques for Cantilever-Based Fiber Optic Endoscopic Scanners—A Review" Sensors 21, no. 1: 251. https://doi.org/10.3390/s21010251
APA StyleKaur, M., Lane, P. M., & Menon, C. (2021). Scanning and Actuation Techniques for Cantilever-Based Fiber Optic Endoscopic Scanners—A Review. Sensors, 21(1), 251. https://doi.org/10.3390/s21010251







