Noise-Resistant CECG Using Novel Capacitive Electrodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Common Mode Noise
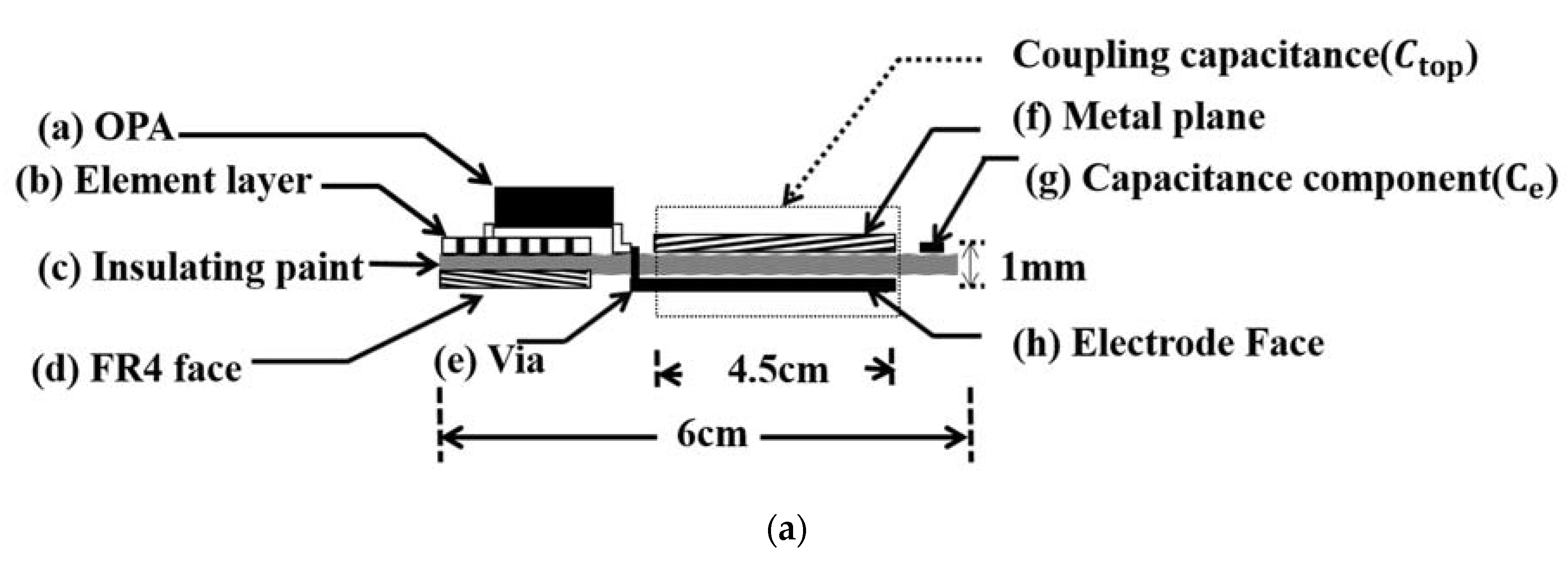
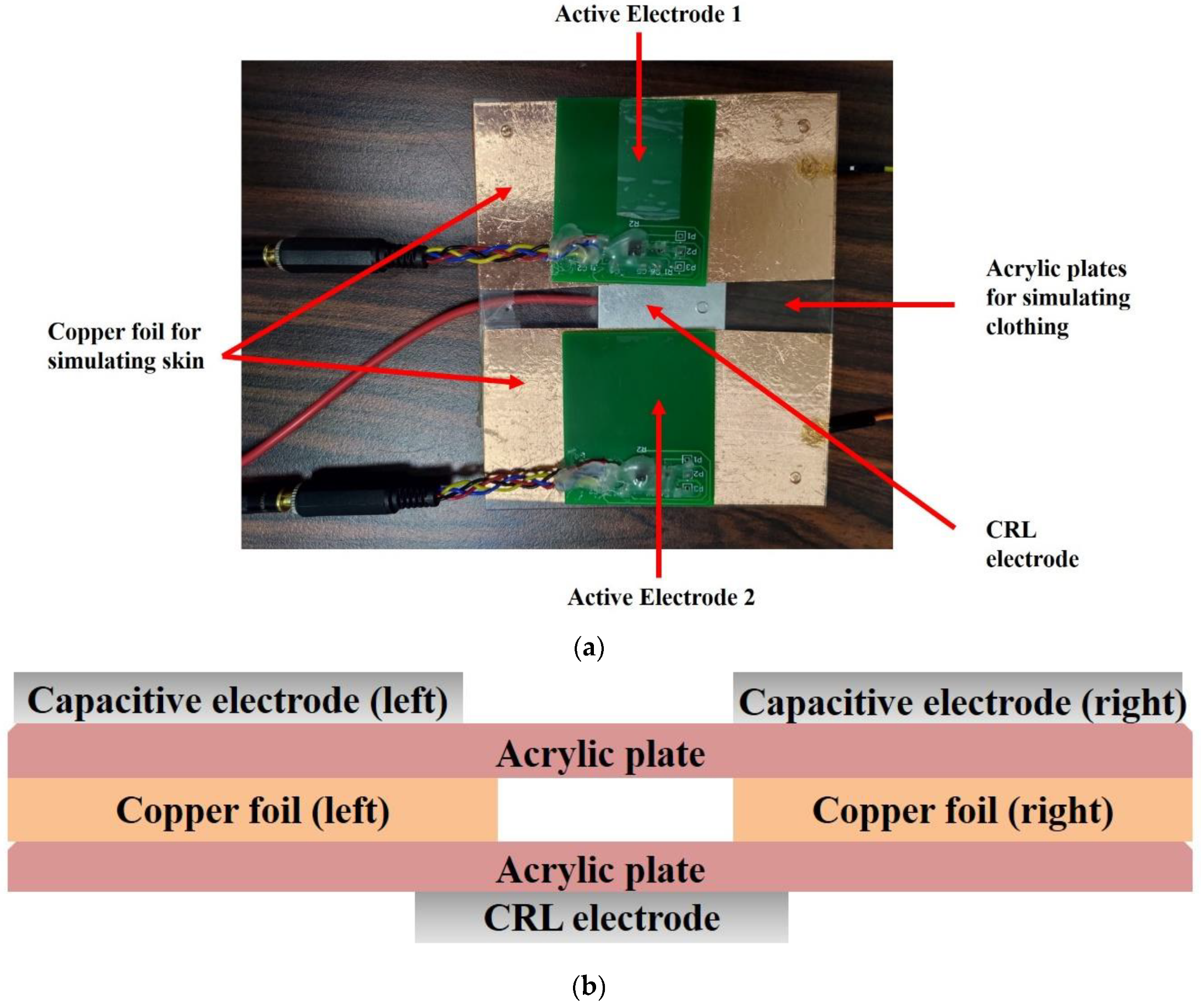
2.2. Electrode Design
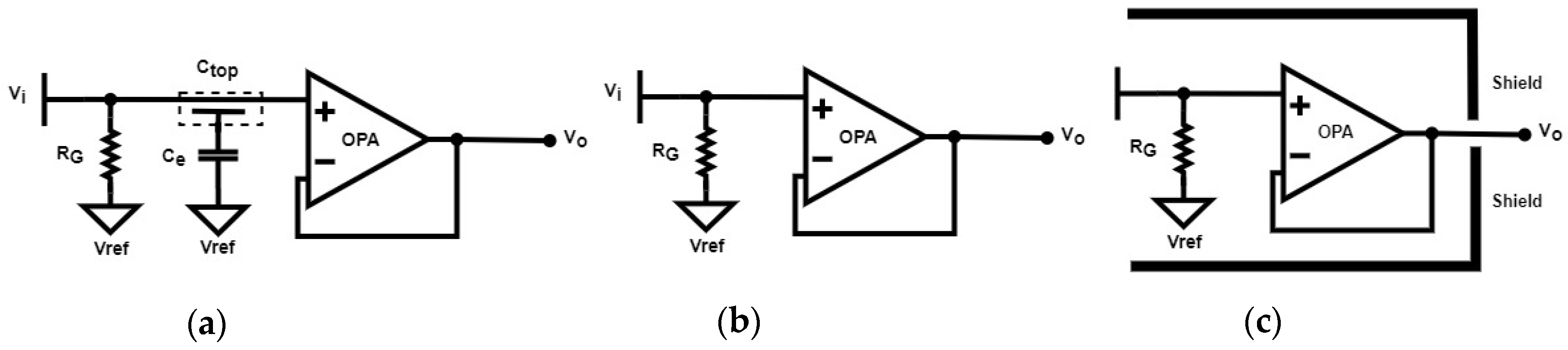
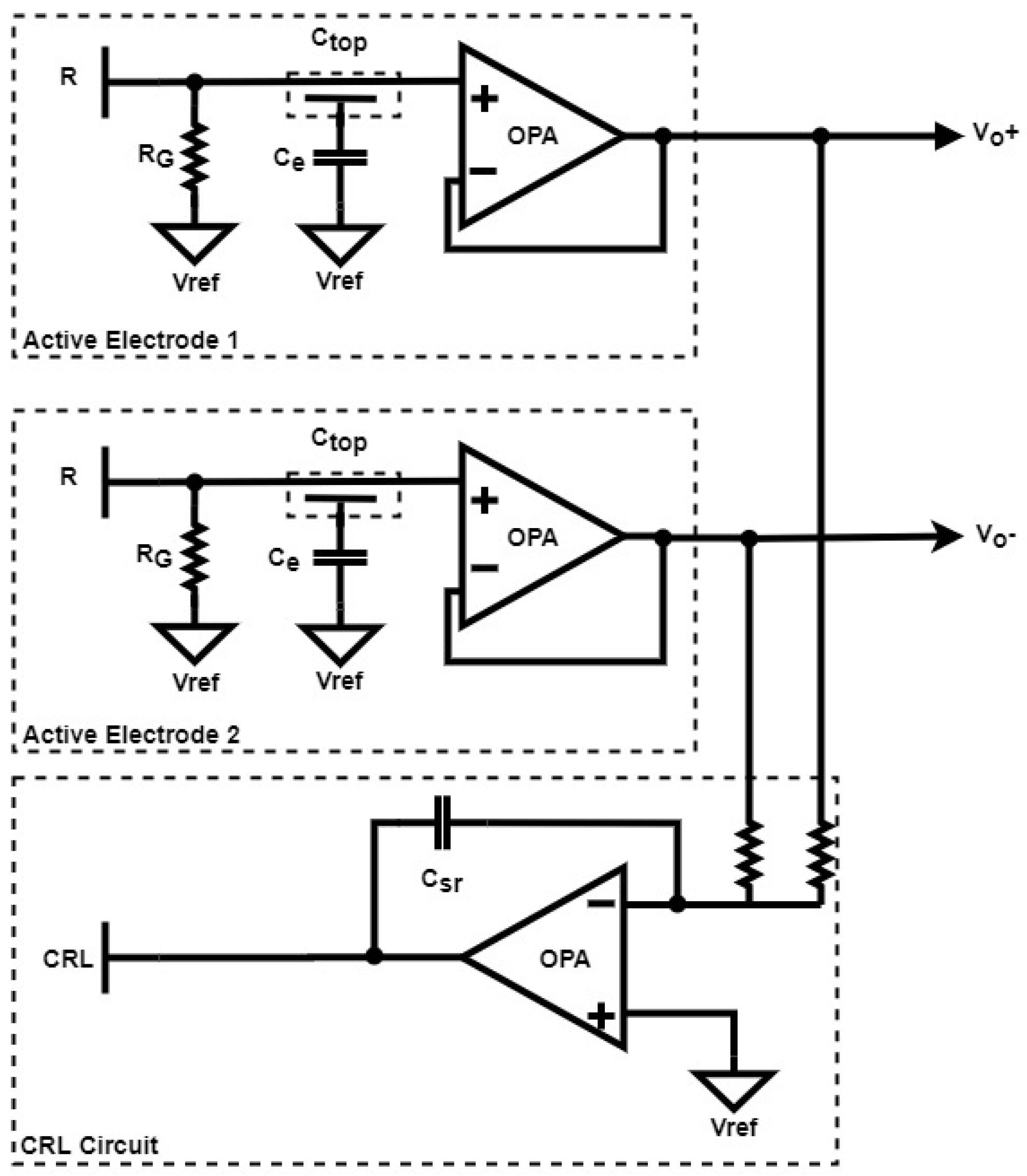
2.2.1. Electrode Equivalent Circuit
2.2.2. CRL Circuit
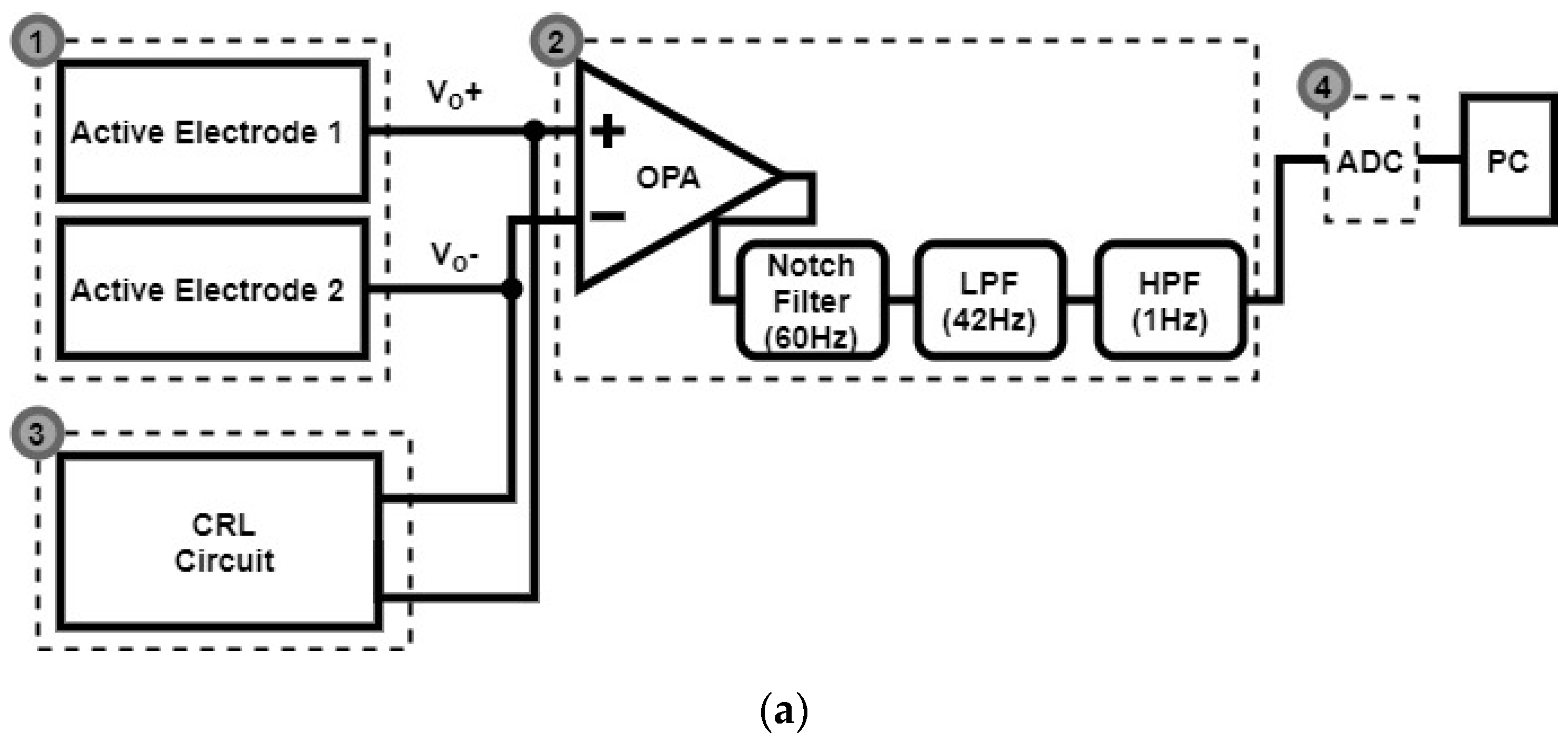
2.3. Signal Acquisition
2.4. Performance Validation
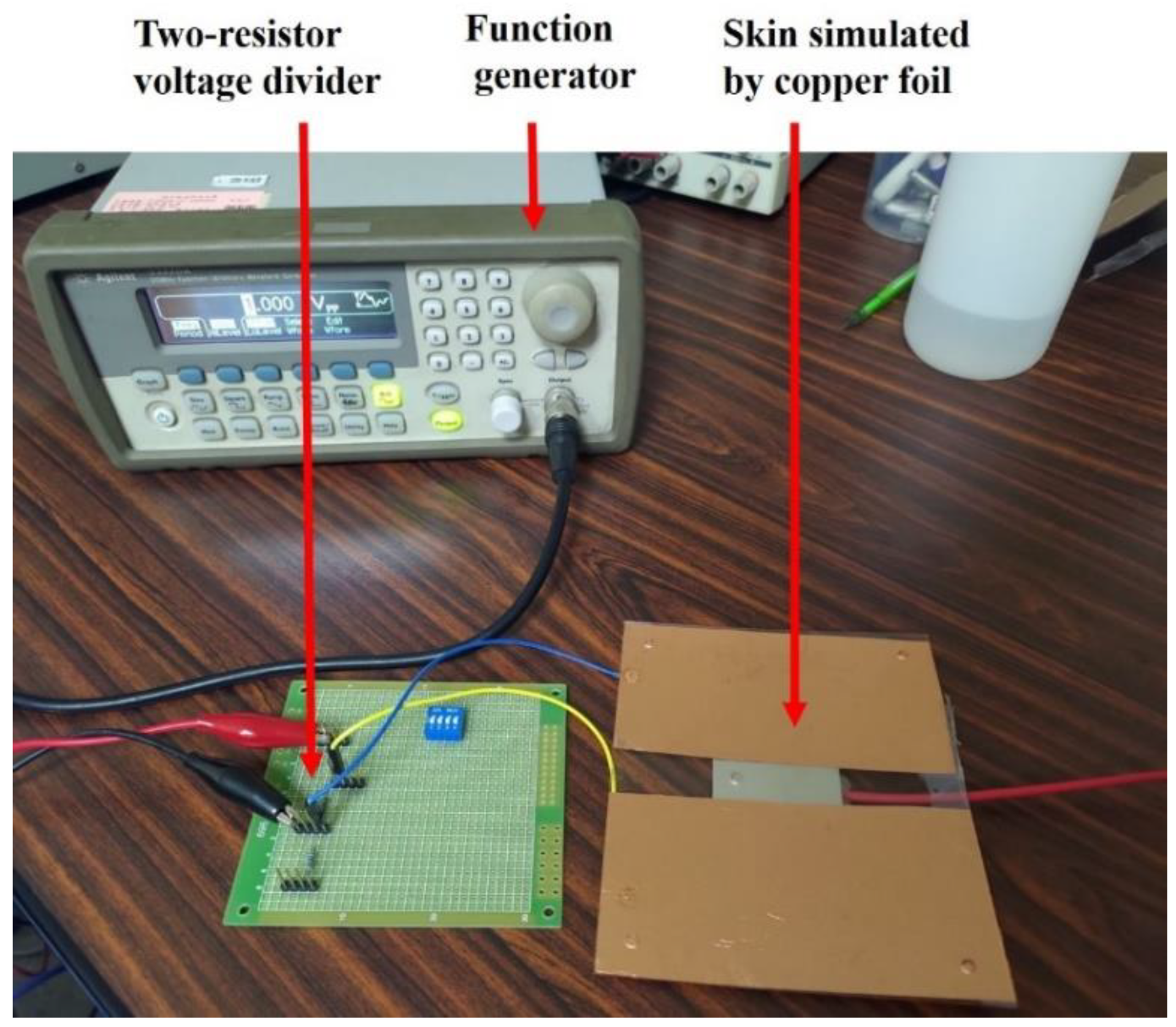
2.4.1. Noise-resistant Performance Testbed
Simulated ECG Signal Source
Signal Capturing and Noise Filtering
AD Converter
Simulated Noise Source
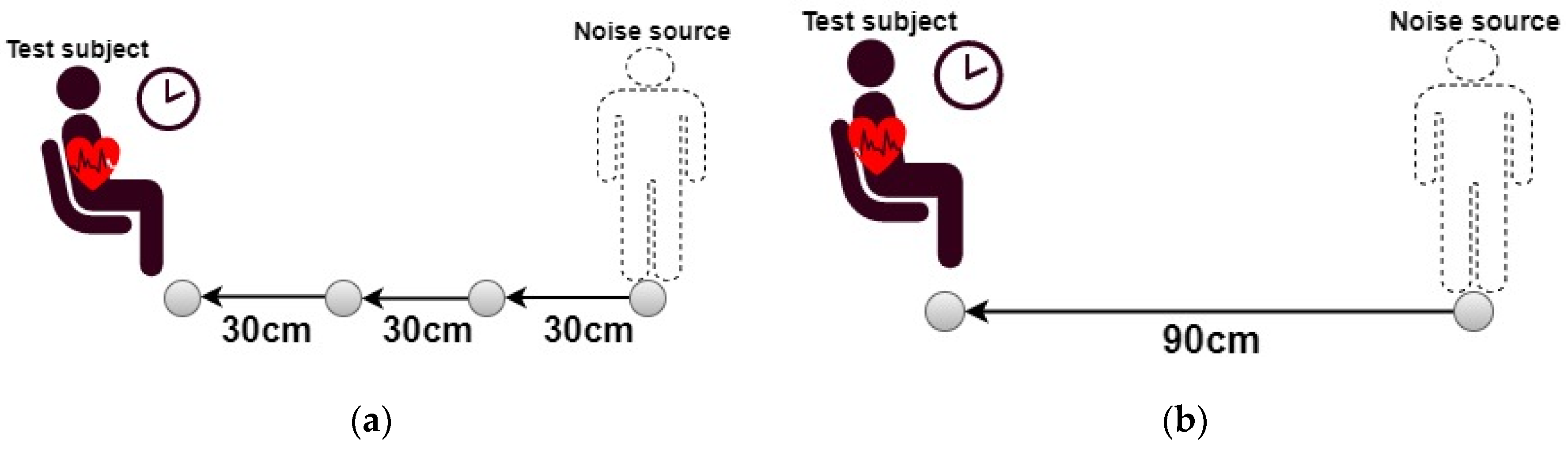
2.4.2. Experimental Protocol
3. Results
3.1. Simulated Noise Cases
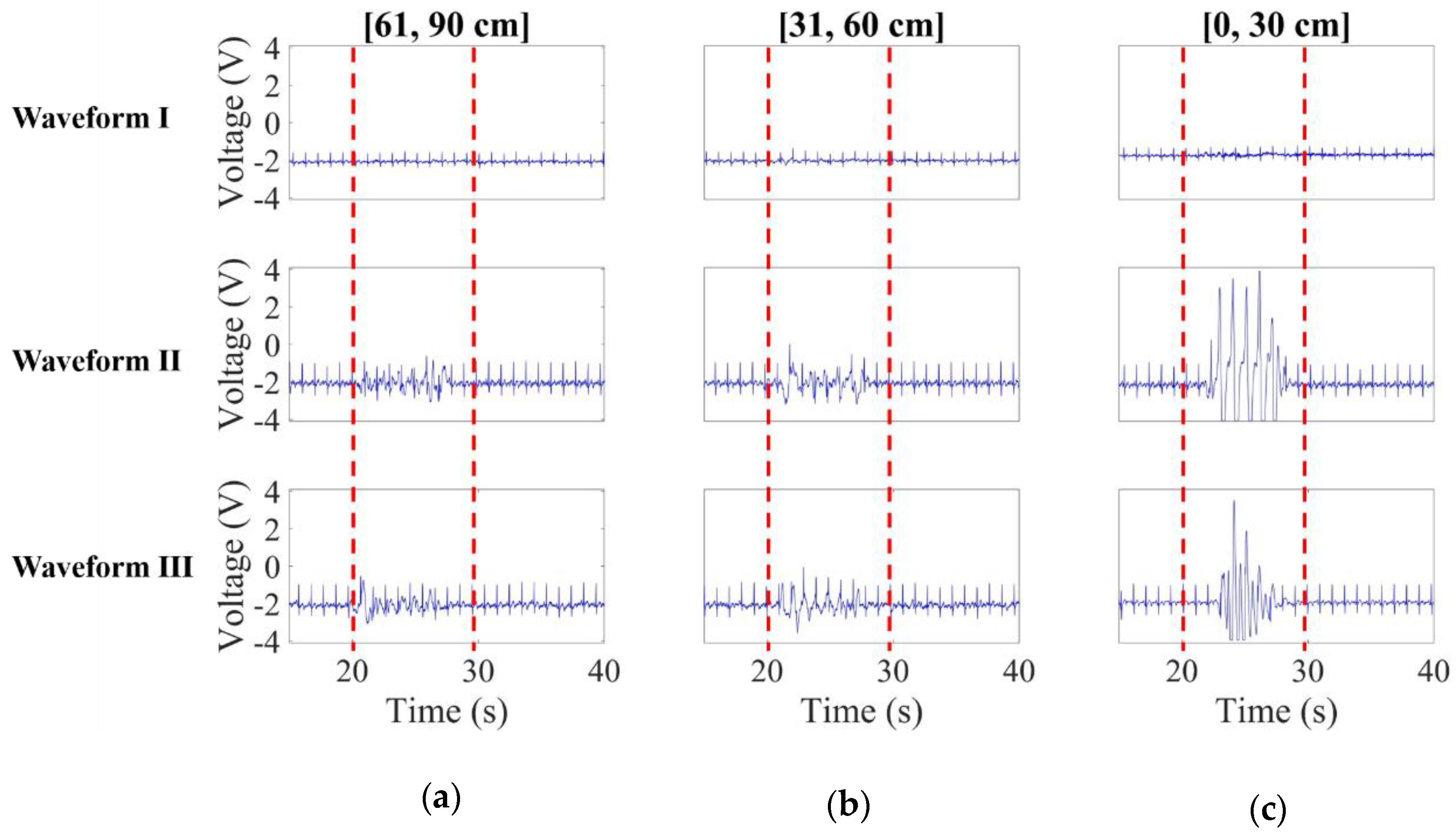
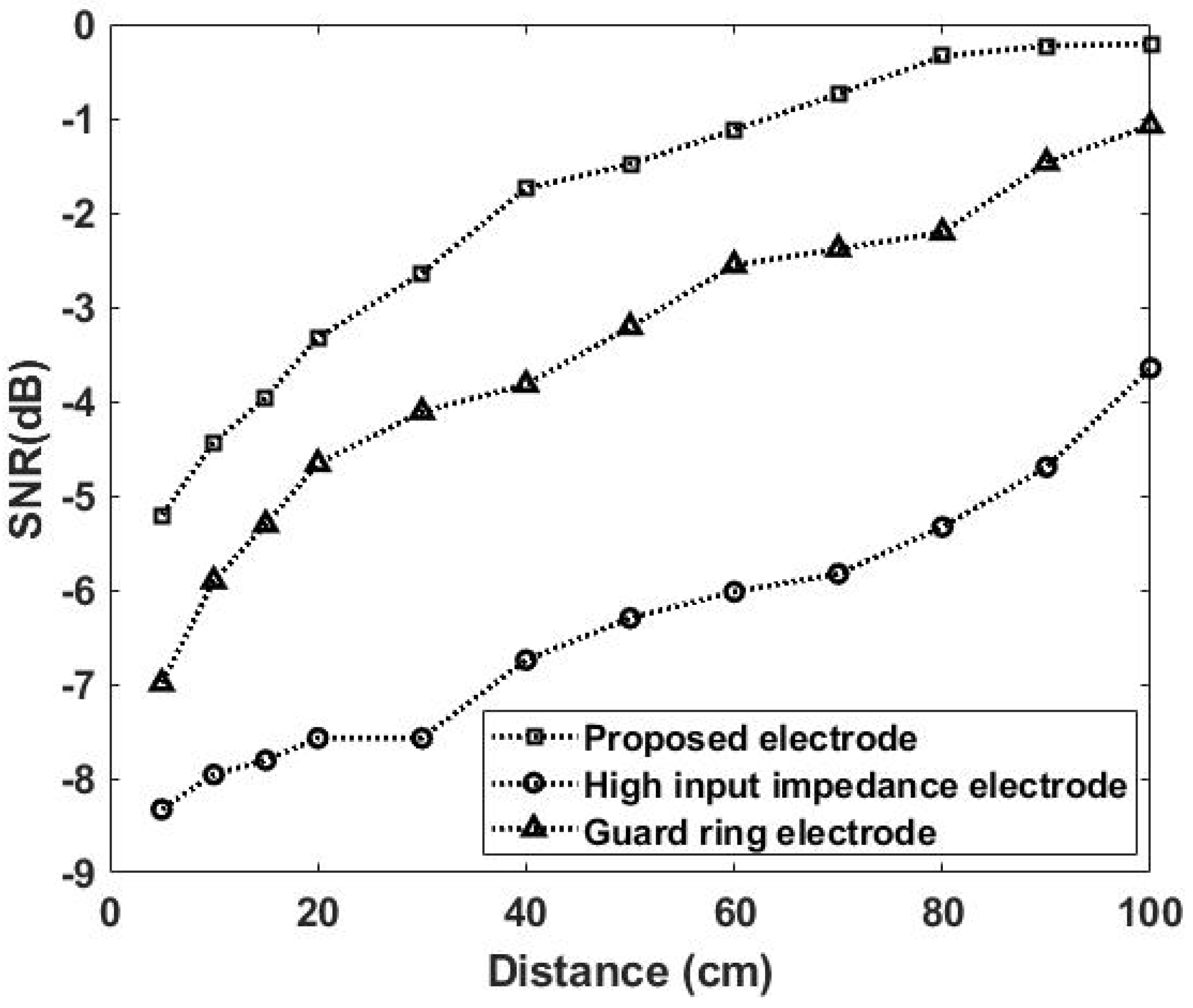
3.1.1. Noise Effect Versus Distance
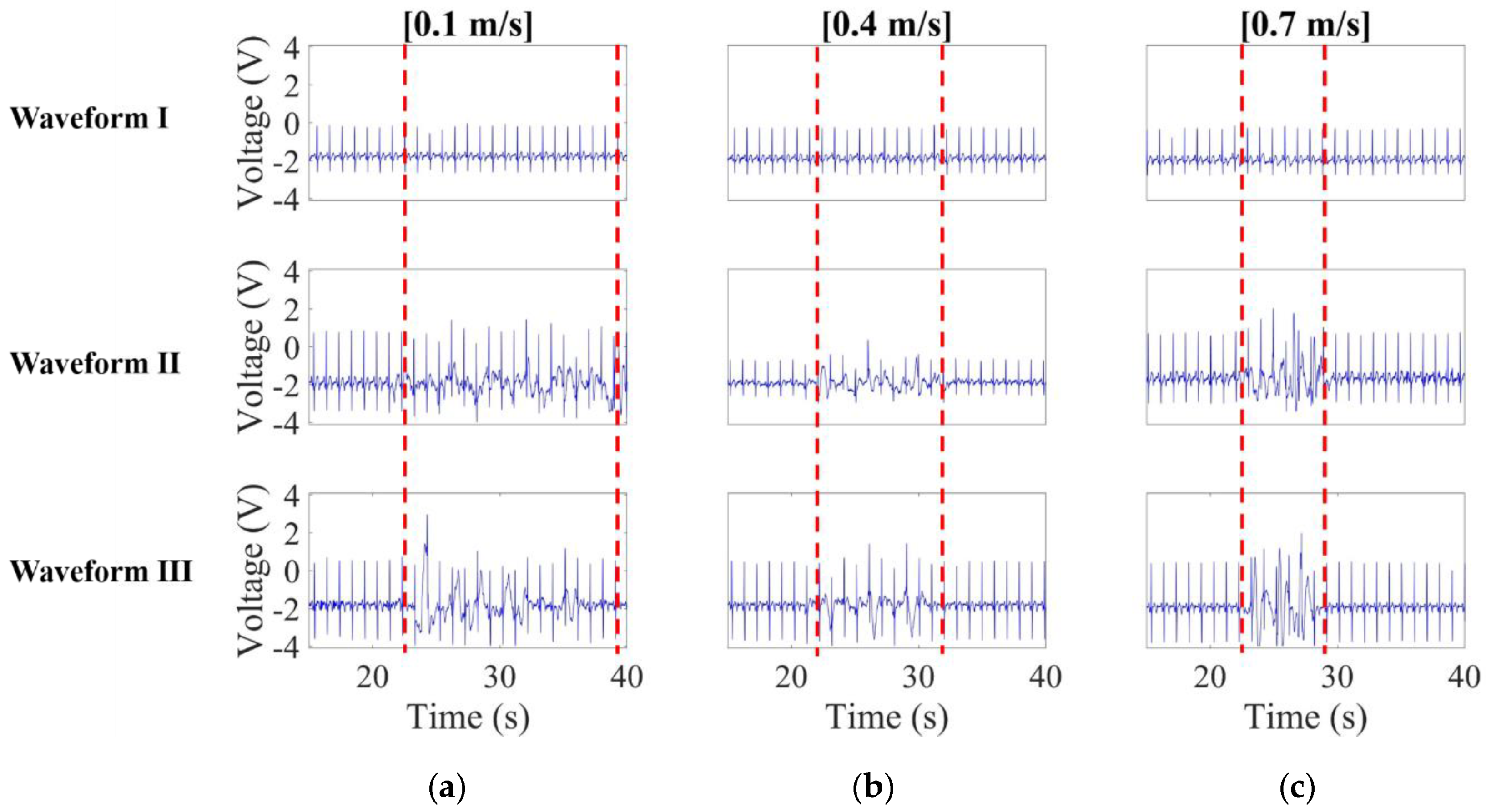
3.1.2. Noise Effect Versus Speed
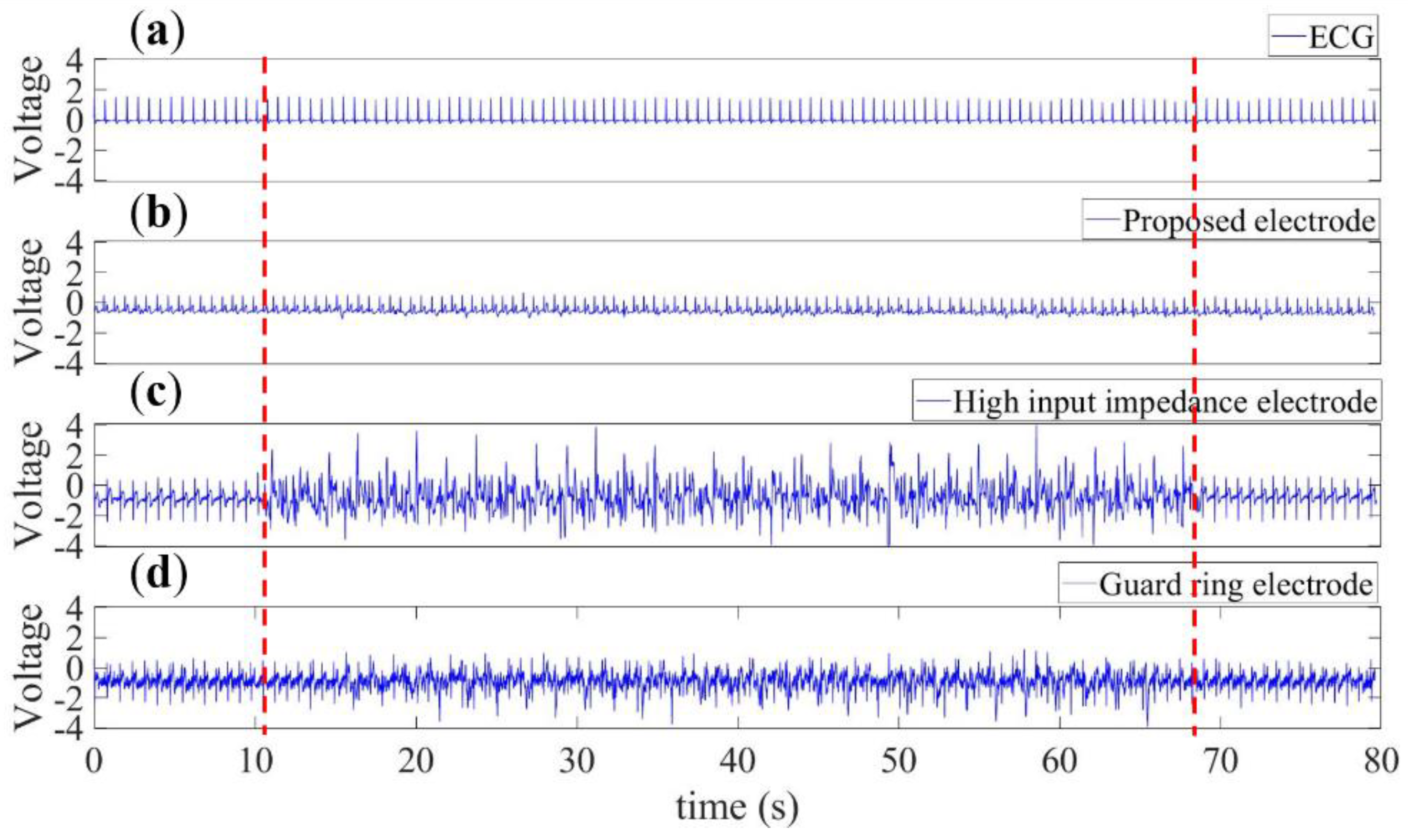
3.2. Human Noise Cases
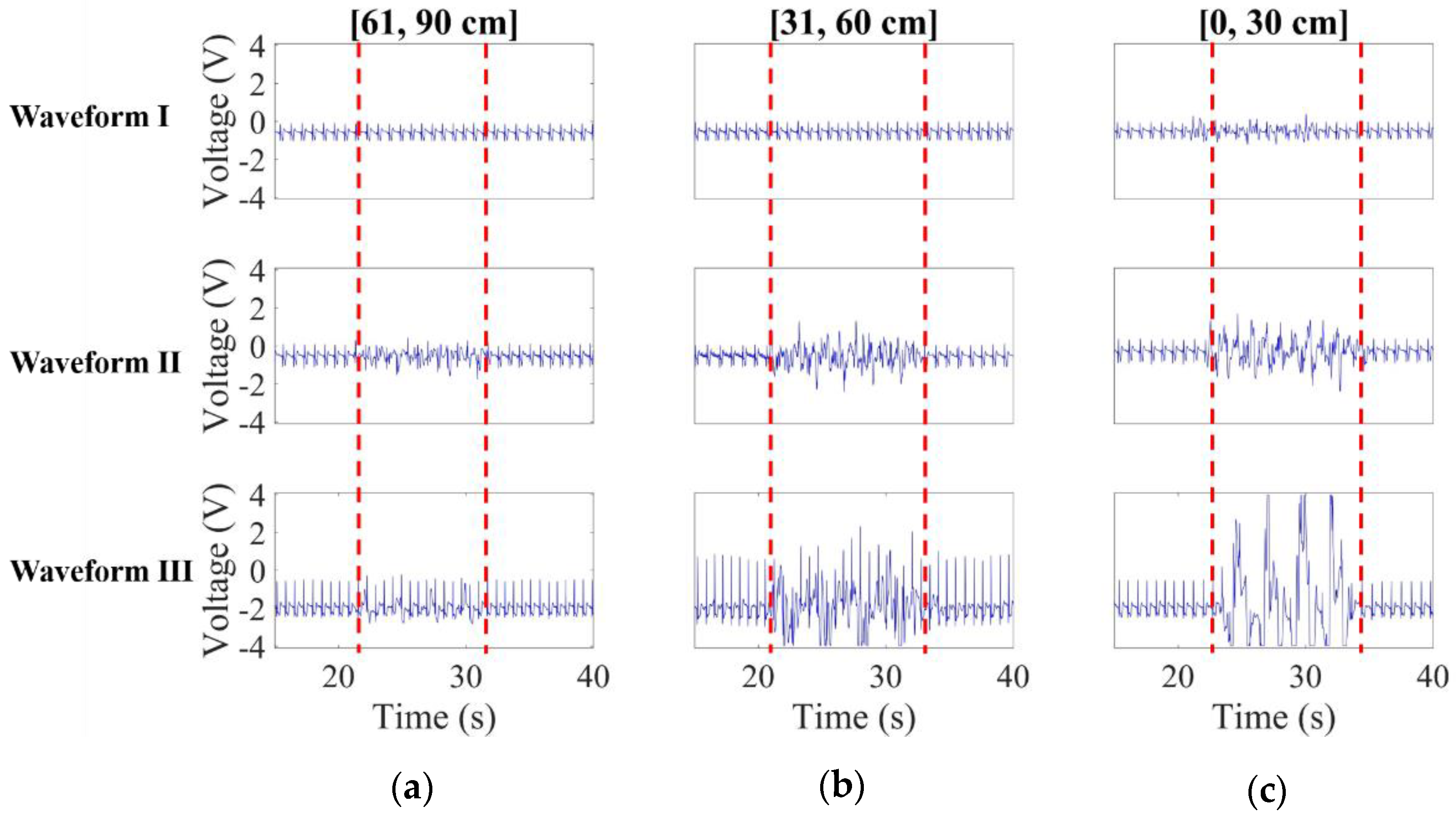
3.2.1. Noise Effect Versus Distance
3.2.2. Noise Effect Versus Speed
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kora, P.; Krishna, K.S.R. ECG Based Heart Arrhythmia Detection Using Wavelet Coherence and Bat Algorithm. Sens. Imaging Int. J. 2016, 17, 17. [Google Scholar] [CrossRef]
- Staszewsky, L.; Pasina, L.; Musazzi, U.M.; Latini, R.; Collaborative Grp Pharmacists, H. Screening for unknown atrial fibrillation in older people: A feasibility study in community pharmacies. Eur. Geriatr. Med. 2018, 9, 113–115. [Google Scholar] [CrossRef]
- Cardinale, M.; Varley, M. Wearable Training-Monitoring Technology: Applications, Challenges, and Opportunities. Int. J. Sports Physiol. Perform. 2017, 12, S255–S262. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, A.; Mastella, G.; Cipriani, A.; Berton, G.; Del Monte, A.; Gusella, B.; Nese, A.; Portolan, L.; Sciacca, F.; Tikvina, S.; et al. Burden of ventricular arrhythmias at 12-lead 24-hour ambulatory ECG monitoring in middle-aged endurance athletes versus sedentary controls. Eur. J. Prev. Cardiol. 2018, 25, 2003–2011. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Wang, Y. Modeling and Recognition of Driving Fatigue State Based on R-R Intervals of ECG Data. IEEE Access 2019, 7, 175584–175593. [Google Scholar] [CrossRef]
- Ahn, J.W.; Ku, Y.; Kim, H.C. A Novel Wearable EEG and ECG Recording System for Stress Assessment. Sensors 2019, 19, 1991. [Google Scholar] [CrossRef]
- Griffith, M.E.; Portnoy, W.M.; Stotts, L.J.; Day, J.L. Improved capacitive electrocardiogram electrodes for burn applications. Med Boil. Eng. 1979, 17, 641–646. [Google Scholar] [CrossRef]
- Searle, A.; Kirkup, L. A direct comparison of wet, dry and insulating bioelectric recording electrodes. Physiol. Meas. 2000, 21, 271–283. [Google Scholar] [CrossRef]
- Torfs, T.; Chen, Y.-H.; Kim, H.; Yazicioglu, R.F. Noncontact ECG Recording System with Real Time Capacitance Measurement for Motion Artifact Reduction. IEEE Trans. Biomed. Circuits Syst. 2014, 8, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.G.; Lee, J.S.; Lee, S.-M.; Lee, H.J.; Park, K.S. Capacitive Measurement of ECG for Ubiquitous Healthcare. Ann. Biomed. Eng. 2014, 42, 2218–2227. [Google Scholar] [CrossRef] [PubMed]
- Weil, M.B.; Oehler, M.; Schilling, M.; Maier, L. First clinical evaluation of a novel capacitive ECG system in patients with acute myocardial infarction. Clin. Res. Cardiol. 2011, 101, 165–174. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leonhardt, S.; Leicht, L.; Teichmann, D. Unobtrusive Vital Sign Monitoring in Automotive Environments—A Review. Sensors 2018, 18, 3080. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.; Eilebrecht, B.; Wartzek, T.; Leonhardt, S. The smart car seat: Personalized monitoring of vital signs in automotive applications. Pers. Ubiquitous Comput. 2011, 15, 707–715. [Google Scholar] [CrossRef]
- Haberman, M.A.; Spinelli, E.; García, P.A.; Guerrero, F.N. Capacitive driven-right-leg circuit design. Int. J. Biomed. Eng. Technol. 2015, 17, 115. [Google Scholar] [CrossRef]
- Liu, S.-H.; Hsieh, C.-H.; Chen, W.; Tan, T.-H. ECG Noise Cancellation Based on Grey Spectral Noise Estimation. Sensors 2019, 19, 798. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, J.; Anzai, D.; Wang, J. Performance of human body communication-based wearable ECG with capacitive coupling electrodes. Heal. Technol. Lett. 2016, 3, 222–225. [Google Scholar] [CrossRef]
- Lee, J.S.; Heo, J.; Lee, W.K.; Lim, Y.G.; Kim, Y.H.; Park, K.S. Flexible Capacitive Electrodes for Minimizing Motion Artifacts in Ambulatory Electrocardiograms. Sensors 2014, 14, 14732–14743. [Google Scholar] [CrossRef]
- Xu, X.; Liang, Y.; He, P.; Yang, J. Adaptive Motion Artifact Reduction Based on Empirical Wavelet Transform and Wavelet Thresholding for the Non-Contact ECG Monitoring Systems. Sensors 2019, 19, 2916. [Google Scholar] [CrossRef]
- Atallah, L.; Serteyn, A.; Meftah, M.; Schellekens, M.; Vullings, R.; Bergmans, J.W.M.; Osagiator, A.; Oetomo, S.B. Unobtrusive ECG monitoring in the NICU using a capacitive sensing array. Physiol. Meas. 2014, 35, 895–913. [Google Scholar] [CrossRef]
- Seo, M.; Choi, M.; Lee, J.S.; Kim, S.W. Adaptive Noise Reduction Algorithm to Improve R Peak Detection in ECG Measured by Capacitive ECG Sensors. Sensors 2018, 18, 2086. [Google Scholar] [CrossRef]
- Rachim, V.P.; Chung, W.-Y. Wearable Noncontact Armband for Mobile ECG Monitoring System. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Asl, S.N.; Oehler, M.; Schilling, M. Noise Model of Capacitive and Textile Capacitive Noncontact Electrodes for Bioelectric Applications. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Oehler, M.; Ling, V.; Melhorn, K.; Schilling, M. A multichannel portable ECG system with capacitive sensors. Physiol. Meas. 2008, 29, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Winter, B.B.; Webster, J.G. Driven-right-leg circuit design. IEEE Trans. Biomed. Eng. 1983, 30, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chang, R.; Zhang, L.; Yan, A.F. An Interference Suppression Method for Non-Contact Bioelectric Acquisition. Electronics 2020, 9, 293. [Google Scholar] [CrossRef]
- Chen, C.; Chang, W.; Xie, T.Y. In Shielded capacitive electrode with high noise immunity. In Proceedings of the 2017 IEEE International Conference on Consumer Electronics—(ICCE-TW), Taipei, Taiwan, 12–14 June 2017; pp. 157–158. [Google Scholar]
- World Health Organization. WHO, Research Agenda for Extremely Low Frequency Fields; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- IEC 60601-2-47: Medical Electrical Equipment—Part 2-47: Particular Requirements for the Basic Safety and Essential Performance of Ambulatory Electrocardiographic Systems, 2nd ed.; International Electrotechnical Commission (IEC): Geneva, Switzerland, 2012; pp. 1–140.







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-C.; Chen, C.-W.; Hsieh, C.-W. Noise-Resistant CECG Using Novel Capacitive Electrodes. Sensors 2020, 20, 2577. https://doi.org/10.3390/s20092577
Chen C-C, Chen C-W, Hsieh C-W. Noise-Resistant CECG Using Novel Capacitive Electrodes. Sensors. 2020; 20(9):2577. https://doi.org/10.3390/s20092577
Chicago/Turabian StyleChen, Chi-Chun, Cheng-Wei Chen, and Chang-Wei Hsieh. 2020. "Noise-Resistant CECG Using Novel Capacitive Electrodes" Sensors 20, no. 9: 2577. https://doi.org/10.3390/s20092577
APA StyleChen, C.-C., Chen, C.-W., & Hsieh, C.-W. (2020). Noise-Resistant CECG Using Novel Capacitive Electrodes. Sensors, 20(9), 2577. https://doi.org/10.3390/s20092577




