A FRET Based Two-Photon Fluorescent Probe for Visualizing Mitochondrial Thiols of Living Cells and Tissues
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
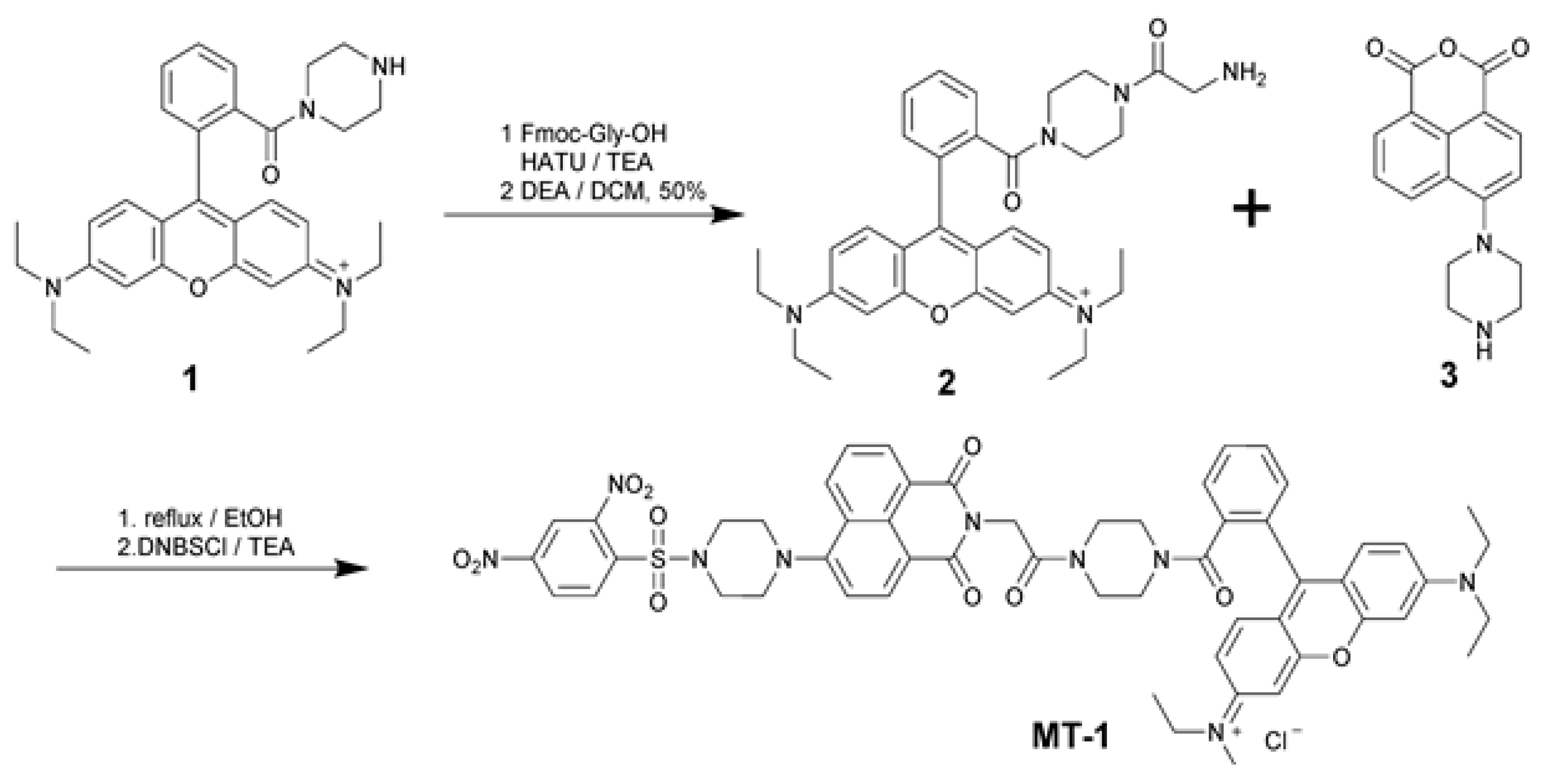
2.2. Synthesis and Characterization
2.3. UV-vis and Fluorescence Measurements
2.4. Determination of the Detection Limit
2.5. Cell Culture and MTT Assay
2.6. Fluorescence Imaging in Cells
2.7. Two-Photon Tissue Imaging Experiments
3. Results
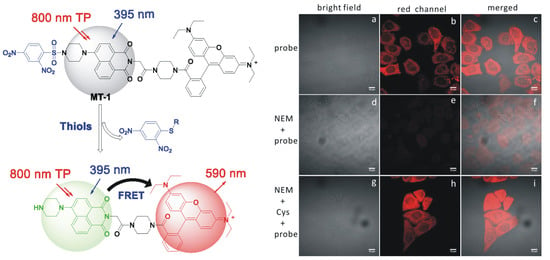
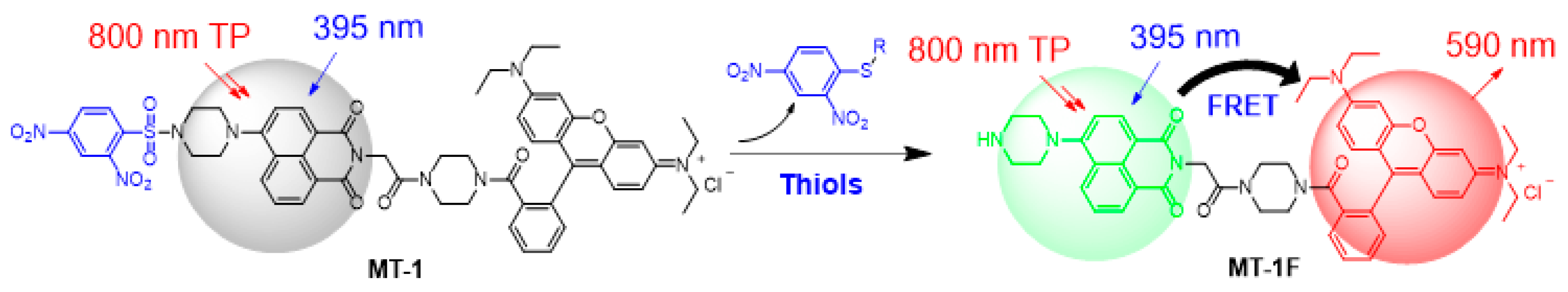
3.1. Design and Synthesis of MT-1
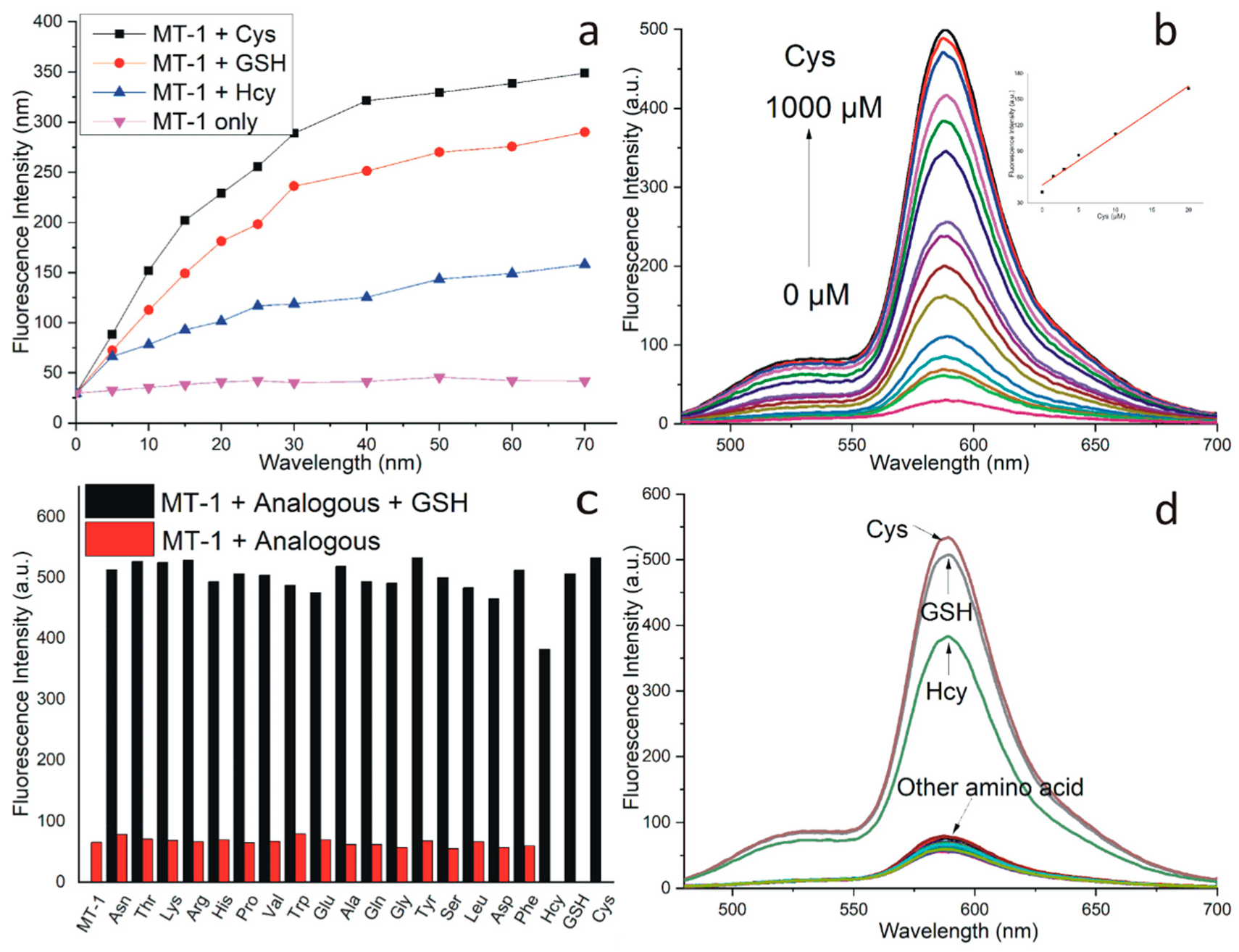
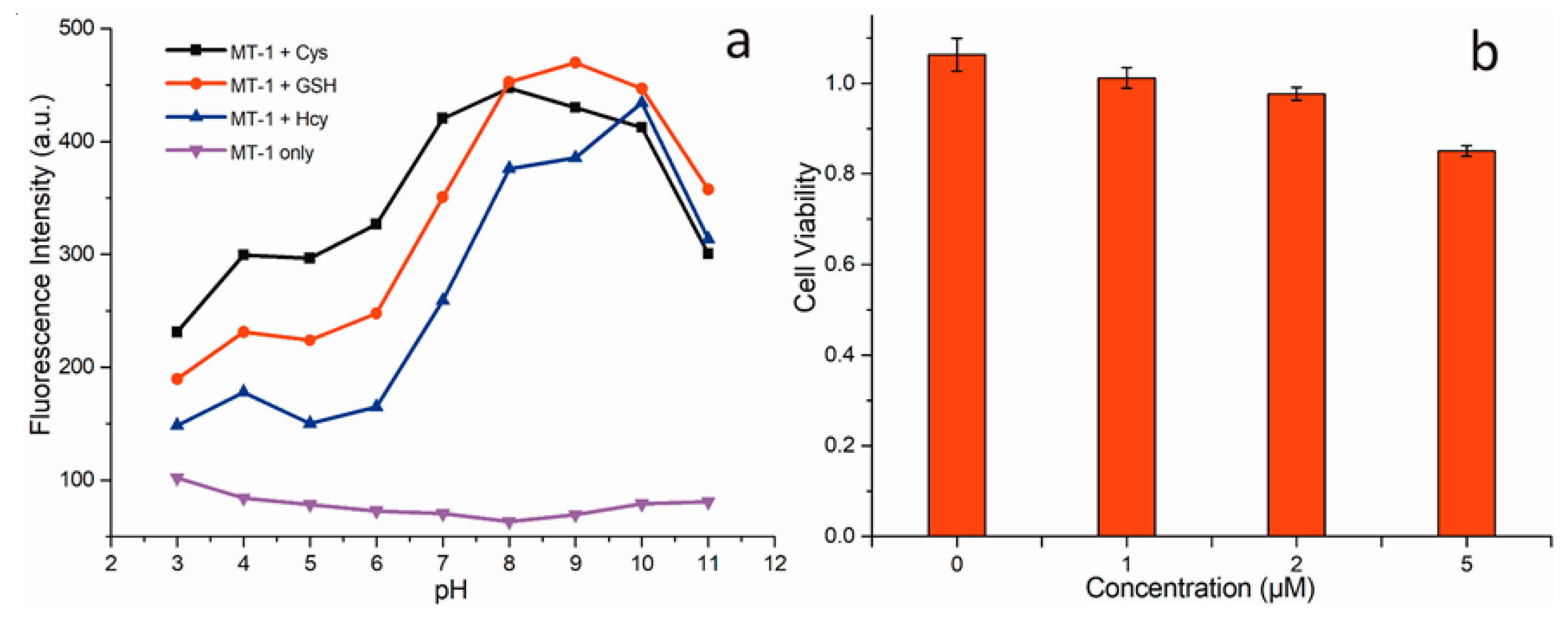
3.2. Response of MT-1 to the Biothiols
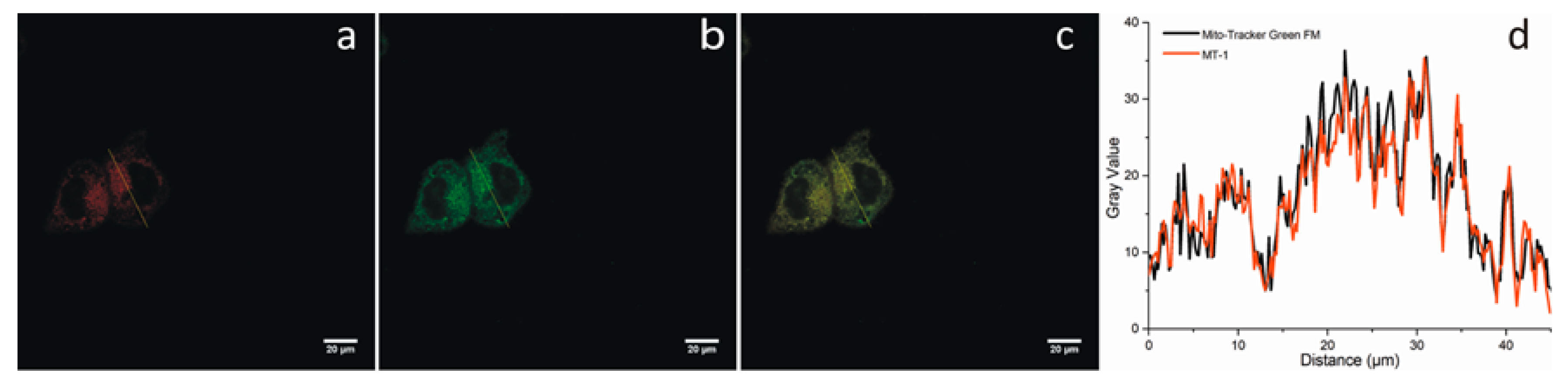
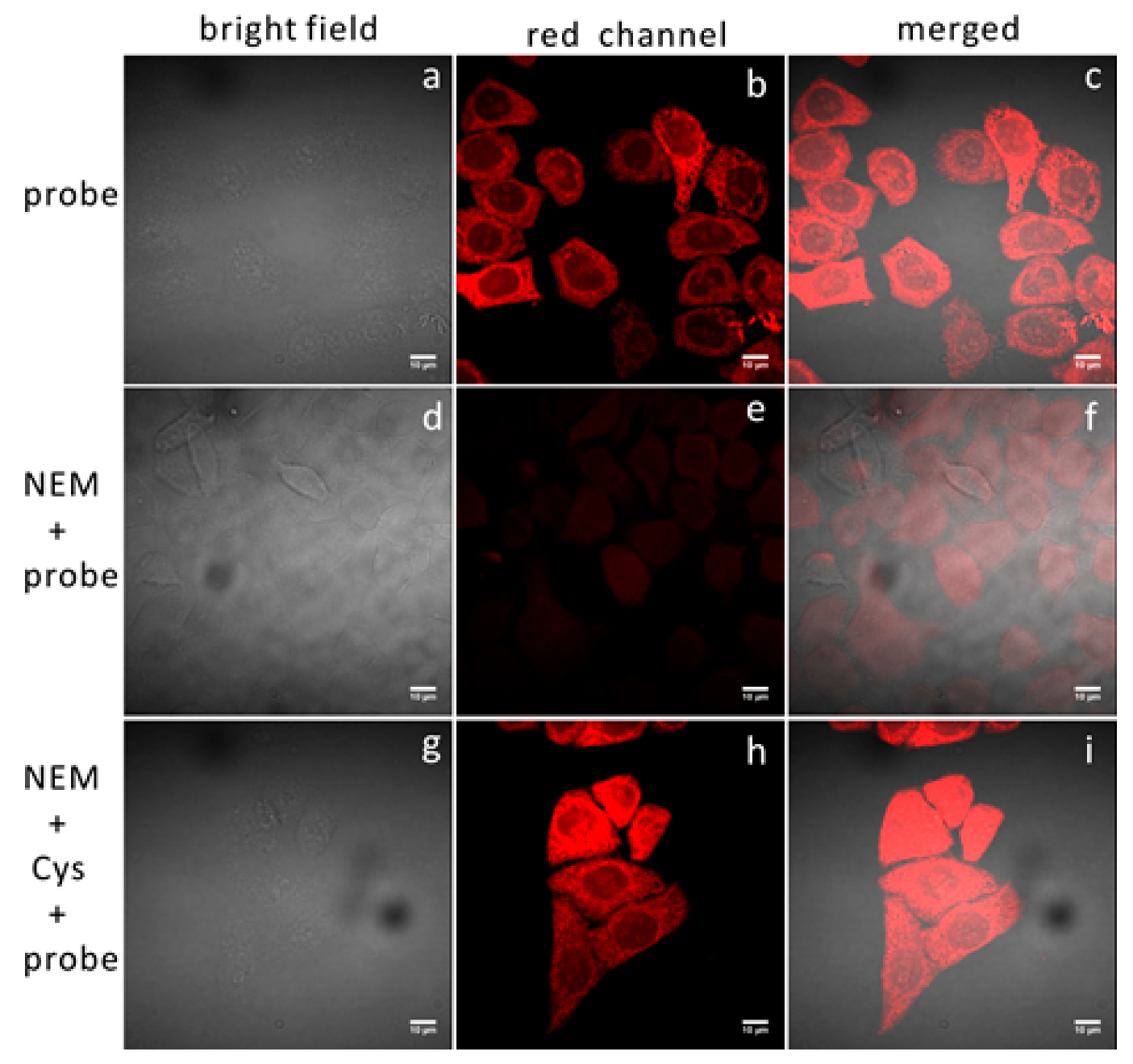
3.3. Two-Photon Imaging of Thiols in Cell Lines
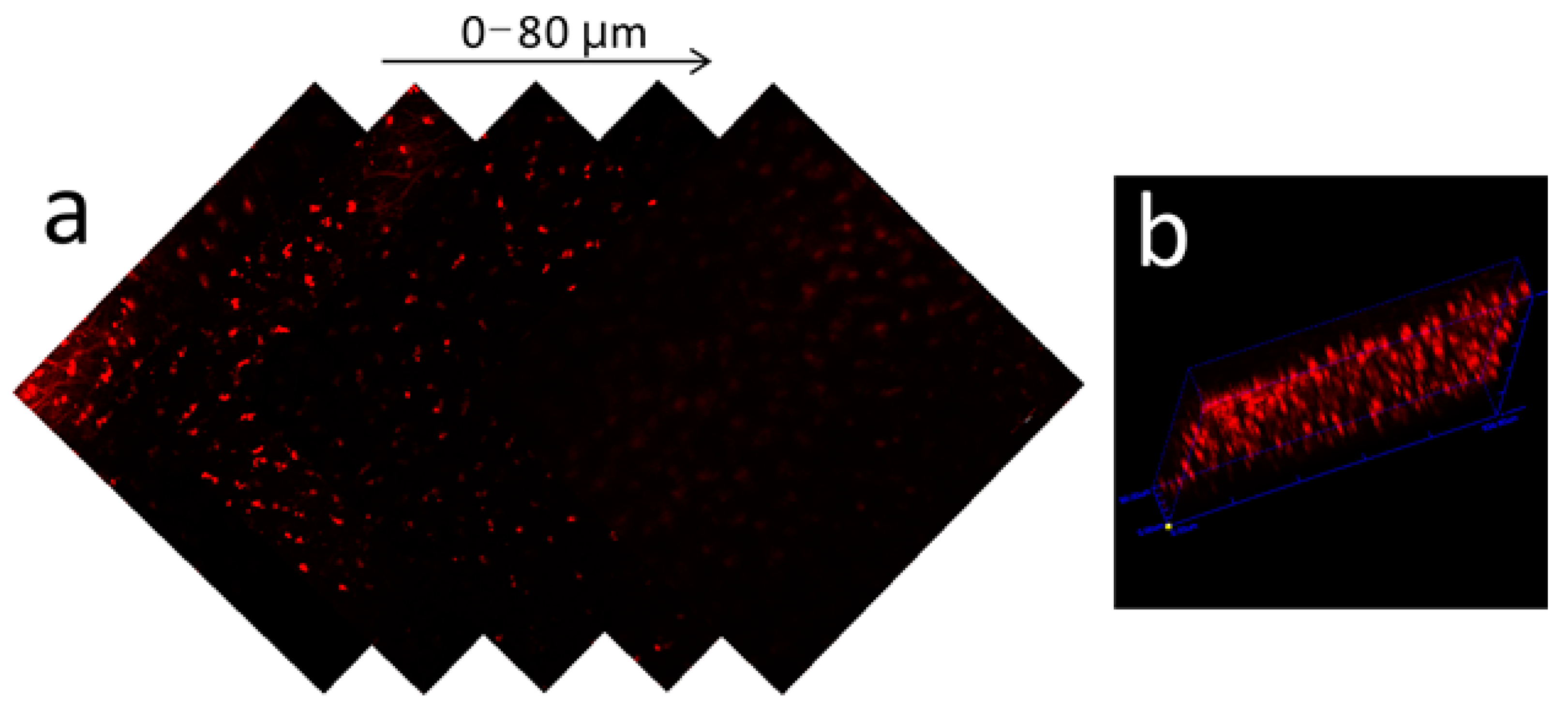
3.4. Two-Photon Tissue Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mari, M.; Morales, A.; Colell, A.; Garcia-Ruiz, C.; Fernandez-Checa, J.C. Mitochondrial glutathione, a key survival antioxidant. Antioxid. Redox Signal. 2009, 11, 2685–2700. [Google Scholar] [CrossRef] [PubMed]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Finkel, T.; Serrano, M.; Blasco, M.A. The common biology of cancer and ageing. Nature 2007, 448, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.W.; Shao, J.; Lin, J.; Zhang, N.; Lu, B.J.; Lin, S.C.; Dong, M.Q.; Han, J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 2009, 325, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Desagher, S.; Martinou, J.C. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000, 10, 369–377. [Google Scholar] [CrossRef]
- Gourlay, C.W.; Ayscough, K.R. The actin cytoskeleton: A key regulator of apoptosis and ageing? Nat. Rev. Mol. Cell Biol. 2005, 6, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Ribas, V.; Garcia-Ruiz, C.; Fernandez-Checa, J.C. Glutathione and mitochondria. Front. Pharmacol. 2014, 5, 151. [Google Scholar] [CrossRef]
- Han, Y.H.; Kim, S.Z.; Kim, S.H.; Park, W.H. Apoptosis in pyrogallol-treated Calu-6 cells is correlated with the changes of intracellular GSH levels rather than ROS levels. Lung Cancer 2008, 59, 301–314. [Google Scholar] [CrossRef]
- Han, Y.H.; Kim, S.H.; Kim, S.Z.; Park, W.H. Apoptosis in arsenic trioxide-treated Calu-6 lung cells is correlated with the depletion of GSH levels rather than the changes of ROS levels. J. Cell. Biochem. 2008, 104, 862–878. [Google Scholar] [CrossRef]
- Bak, D.W.; Weerapana, E. Cysteine-mediated redox signalling in the mitochondria. Mol. Biosyst. 2015, 11, 678–697. [Google Scholar] [CrossRef]
- Quintana-Cabrera, R.; Bolaños, J.P. Glutathione and γ-Glutamylcysteine in the Antioxidant and Survival Functions of Mitochondria. Biochem. Soc. Trans 2013, 41, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Yang, H.; Chen, M.; Su, Q.; Feng, W.; Li, F. Mitochondria-targeted near-infrared fluorescent off–on probe for selective detection of cysteine in living cells and in vivo. ACS Appl. Mater. Interfaces 2015, 7, 27968–27975. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Yu, F.; Zhang, W.; Chen, L. A near-infrared ratiometric fluorescent probe for cysteine detection over glutathione indicating mitochondrial oxidative stress in vivo. Biosens. Bioelectron. 2015, 74, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W.; Meister, A. Glutathione: Interorgan translocation, turnover, and metabolism. Proc. Natl. Acad. Sci. USA 1979, 76, 5606–5610. [Google Scholar] [CrossRef] [PubMed]
- Jahoor, F.; Jackson, A.; Gazzard, B.; Philips, G.; Sharpstone, D.; Frazer, M.E.; Heird, W. Erythrocyte glutathione deficiency in symptom-free HIV infection is associated with decreased synthesis rate. Am. J. Physiol. Endocrinol. Metab. 1999, 276, E205–E211. [Google Scholar] [CrossRef]
- Stipanuk, M.H.; Coloso, R.M.; Garcia, R.A.; Banks, M.F. Cysteine concentration regulates cysteine metabolism to glutathione, sulfate and taurine in rat hepatocytes. J. Nutr. 1992, 122, 420–427. [Google Scholar] [CrossRef]
- Hidalgo, J.; Garvey, J.S.; Armario, A. On the metallothionein, glutathione and cysteine relationship in rat liver. J. Pharmacol. Exp. Ther. 1990, 255, 554–564. [Google Scholar]
- Guan, X.M.; Hoffman, B.; Dwivedi, C.; Matthees, D.P. A simultaneous liquid chromatography/mass spectrometric assay of glutathione, cysteine, homocysteine and their disulfides in biological samples. J. Pharm. Biomed. Anal. 2003, 31, 251–261. [Google Scholar] [CrossRef]
- Kubalczyk, P.; Bald, E. Analysis of orange juice for total cysteine and glutathione content by CZE with UV-absorption detection. Electrophoresis 2009, 30, 2280–2283. [Google Scholar] [CrossRef]
- Newton, G.L.; Dorian, R.; Fahey, R.C. Analysis of biological thiols: Derivatization with monobromobimane and separation by reverse-phase high-performance liquid chromatography. Anal. Biochem. 1981, 114, 383–387. [Google Scholar] [CrossRef]
- Shabani-Nooshabadi, M.; Tahernejad-Javazmi, F. Rapid and fast strategy for the determination of glutathione in the presence of vitamin B-6 in biological and pharmaceutical samples using a nanostructure based electrochemical sensor. RSC Adv. 2015, 5, 56255–56261. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Peng, X.; Yoon, J. Fluorescent and colorimetric probes for detection of thiols. Chem. Soc. Rev. 2010, 39, 2120–2135. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Sun, X.; Zu, F.; Bai, Z.; Jiang, Y.; Fan, K.; Wang, J. Fluorescent probes for detecting cysteine. Methods Appl. Fluoresc. 2018, 6, 42001. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Li, J.; Zhou, X.; Yin, J.; Yoon, J. Recent progress on the development of glutathione (GSH) selective fluorescent and colorimetric probes. Coord. Chem. Rev. 2018, 366, 29–68. [Google Scholar] [CrossRef]
- Peng, H.; Chen, W.; Cheng, Y.; Hakuna, L.; Strongin, R.; Wang, B. Thiol reactive probes and chemosensors. Sensors 2012, 12, 15907–15946. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, X.; Miao, Y.; Hu, Y.; Kwon, N.; Wu, X.; Yoon, J. A Reversible Fluorescent Probe for Real-Time Quantitative Monitoring of Cellular Glutathione. Angew. Chem. Int. Ed. 2017, 56, 5812–5816. [Google Scholar] [CrossRef]
- Xu, Y.; Li, R.; Zhou, X.; Li, W.; Ernest, U.; Wan, H.; Li, L.; Chen, H.; Yuan, Z. A visible and near-infrared, dual emission fluorescent probe based on thiol reactivity for selectively tracking mitochondrial glutathione in vitro. Talanta 2019, 205, 120125. [Google Scholar] [CrossRef]
- Lim, S.-Y.; Hong, K.-H.; Kim, D.I.; Kwon, H.; Kim, H.-J. Tunable Heptamethine–Azo Dye Conjugate as an NIR Fluorescent Probe for the Selective Detection of Mitochondrial Glutathione over Cysteine and Homocysteine. J. Am. Chem. Soc. 2014, 136, 7018–7025. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, X.; Zhang, C.; Mackenzie, K.R.; Stossi, F.; Palzkill, T.; Wang, M.C.; Wang, J. Reversible Reaction-Based Fluorescent Probe for Real-Time Imaging of Glutathione Dynamics in Mitochondria. ACS Sens. 2017, 2, 1257–1261. [Google Scholar] [CrossRef]
- Zhang, J.; Bao, X.; Zhou, J.; Peng, F.; Ren, H.; Dong, X.; Zhao, W. A mitochondria-targeted turn-on fluorescent probe for the detection of glutathione in living cells. Biosens. Bioelectron. 2016, 85, 164–170. [Google Scholar] [CrossRef]
- Liu, X.; Niu, L.; Chen, Y.; Zheng, M.; Yang, Y.; Yang, Q. A mitochondria-targeting fluorescent probe for the selective detection of glutathione in living cells. Org. Biomol. Chem. 2017, 15, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Guo, L.; Li, Y.; Shuang, S.; Dong, C.; Wong, M.S. Highly selective two-photon fluorescent probe for ratiometric sensing and imaging cysteine in mitochondria. Anal. Chem. 2016, 88, 1908–1914. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.; Yang, X.; Ning, P.; Xi, X.; Yu, H.; Feng, Y.; Shao, R.; Meng, X. A mitochondria-targeted ratiometric two-photon fluorescent probe for detecting intracellular cysteine and homocysteine. Talanta 2018, 178, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.Y.; Kang, H.; Chung, S.J.; Kim, H.; Na, S.; Kim, H. Mitochondria-Targeting Chromogenic and Fluorescence Turn-On Probe for the Selective Detection of Cysteine by Caged Oxazolidinoindocyanine. Anal. Chem. 2016, 88, 7178–7182. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.-B.; Zhang, Q.-L.; Su, D.; Zhang, X.-X.; Yuan, L.; Zhang, X.-B. Detection of analytes in mitochondria without interference from other sites based on an innovative ratiometric fluorophore. Chem. Sci. 2018, 9, 5461–5466. [Google Scholar] [CrossRef]
- Zhang, X.; He, N.; Huang, Y.; Yu, F.; Li, B.; Lv, C.; Chen, L. Mitochondria-targeting near-infrared ratiometric fluorescent probe for selective imaging of cysteine in orthotopic lung cancer mice. Sens. Actuators B Chem. 2019, 282, 69–77. [Google Scholar] [CrossRef]
- Lee, M.H.; Han, J.H.; Lee, J.-H.; Choi, H.G.; Kang, C.; Kim, J.S. Mitochondrial thioredoxin-responding off–on fluorescent probe. J. Am. Chem. Soc. 2012, 134, 17314–17319. [Google Scholar] [CrossRef]
- Su, L.C.; Goutam, M.; Hyung Joong, K.; Hee, H.J.; Hwan Myung, K.; Bong Rae, C. Ratiometric detection of mitochondrial thiols with a two-photon fluorescent probe. J. Am. Chem. Soc. 2011, 133, 11132–11135. [Google Scholar]
- Singha, S.; Kim, D.; Rao, A.S.; Wang, T.; Kim, K.H.; Lee, K.H.; Kim, K.T.; Ahn, K.H. Two-photon probes based on arylsulfonyl azides: Fluorescence detection and imaging of biothiols. Dye. Pigment. 2013, 99, 308–315. [Google Scholar] [CrossRef]
- Li, Y.; Wang, K.N.; Liu, B.; Lu, X.R.; Li, M.F.; Ji, L.N.; Mao, Z.W.; Li, Y.; Wang, K.N.; Liu, B. Mitochondria-targeted two-photon fluorescent probe for the detection of biothiols in living cells. Sens. Actuators B Chem. 2017, 255, S0925400517314636. [Google Scholar] [CrossRef]
- Wang, F.-F.; Liu, Y.-J.; Wang, B.-B.; Gao, L.-X.; Jiang, F.-L.; Liu, Y. A BODIPY-based mitochondria-targeted turn-on fluorescent probe with dual response units for the rapid detection of intracellular biothiols. Dye. Pigment. 2018, 152, 29–35. [Google Scholar] [CrossRef]
- Wang, S.; Yin, H.; Huang, Y.; Guan, X. Thiol Specific and Mitochondria Selective Fluorogenic Benzofurazan Sulfide for Live Cell Nonprotein Thiol Imaging and Quantification in Mitochondria. Anal. Chem. 2018, 90, 8170–8177. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Han, Z.; Kang, Y.; Peng, X. A Two-Photon Fluorescent Probe for Lysosomal Thiols in Live Cells and Tissues. Sci. Rep. 2016, 6, 19562. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Shi, X.; Xiao, H.; Ding, Q.; Bai, X.; Wu, C.; Zhang, W.; Tang, B. Two-photon imaging of the endoplasmic reticulum thiol flux in the brains of mice with depression phenotypes. Analyst 2019, 144, 191–196. [Google Scholar] [CrossRef]
- Zielonka, J.; Joseph, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chem. Rev. 2017, 117, 10043–10120. [Google Scholar] [CrossRef]
- Jares-Erijman, E.A.; Jovin, T.M. FRET imaging. Nat. Biotechnol. 2003, 21, 1387–1395. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, X.; Mei, B.; Zhang, D.; Tang, A.; An, L.; He, X.; Jiang, J.; Liang, G. Labeling Thiols on Proteins, Living Cells and Tissues with Enhanced Emission Induced by FRET. Sci. Rep. 2013, 3, 3523. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Y.; Zan, W.; Zhang, J.; Chen, Z.; Liu, X.; Qi, F.; Yao, X.; Zhang, X.; Zhang, H. A two-photon off-on fluorescence probe for imaging thiols in live cells and tissues. Photochem. Photobiol. Sci. 2016, 15, 412–419. [Google Scholar] [CrossRef]
- Choi, S.-A.; Park, C.S.; Kwon, O.S.; Giong, H.-K.; Lee, J.-S.; Ha, T.H.; Lee, C.-S. Structural effects of naphthalimide-based fluorescent sensor for hydrogen sulfide and imaging in live zebrafish. Sci. Rep. 2016, 6, 26203. [Google Scholar] [CrossRef]
- Zhang, W.; Huo, F.; Liu, T.; Wen, Y.; Yin, C. A rapid and highly sensitive fluorescent imaging materials for thiophenols. Dye. Pigment. 2016, 133, 248–254. [Google Scholar] [CrossRef]
- Nguyen, T.; Francis, M.B. Practical synthetic route to functionalized rhodamine dyes. Org. Lett. 2003, 5, 3245–3248. [Google Scholar] [CrossRef] [PubMed]
- Valeria, M.; Claudia, C.; Falchi, A.M.; Hyde, S.T.; Vito, L.; Maura, M.; Marc, O.R.; Antonella, R.; Judith, S.; Yeshayahu, T. Docetaxel-Loaded Fluorescent Liquid-Crystalline Nanoparticles for Cancer Theranostics. Langmuir ACS J. Surf. Colloids 2015, 31, 9566–9575. [Google Scholar]
- Zhou, L.Y.; Liu, Y.C.; Hu, S.Q.; Wang, H.F.; Sun, H.Y.; Zhang, X.B. A new ratiometric two-photon fluorescent probe for imaging of lysosomes in living cells and tissues. Tetrahedron 2016, 72, 4637–4642. [Google Scholar] [CrossRef]
- Qi, C.; Tao, Y.; Weiping, Z.; Yufang, X.; Xuhong, Q. A turn-on fluorescent probe for tumor hypoxia imaging in living cells. Chem. Commun. 2015, 51, 14739. [Google Scholar]
- Yao, S.-k.; Qian, Y. A naphthalimide–rhodamine two-photon fluorescent turn-on probe for hypochlorous acid by desulfurization-cyclization and fluorescence resonance energy transfer. Sens. Actuators B Chem. 2017, 252, 877–885. [Google Scholar] [CrossRef]
- Maeda, H.; Matsuno, H.; Ushida, M.; Katayama, K.; Saeki, K.; Itoh, N. 2, 4-Dinitrobenzenesulfonyl fluoresceins as fluorescent alternatives to Ellman’s reagent in thiol-quantification enzyme assays. Angew. Chem. Int. Ed. 2005, 44, 2922–2925. [Google Scholar] [CrossRef] [PubMed]
- Van Meerloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. In Cancer Cell Culture; Humana Press: Totowa, NJ, USA, 2011; pp. 237–245. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Wang, Q.; Wang, H.; Su, W.; Dong, S. A FRET Based Two-Photon Fluorescent Probe for Visualizing Mitochondrial Thiols of Living Cells and Tissues. Sensors 2020, 20, 1746. https://doi.org/10.3390/s20061746
Liu Z, Wang Q, Wang H, Su W, Dong S. A FRET Based Two-Photon Fluorescent Probe for Visualizing Mitochondrial Thiols of Living Cells and Tissues. Sensors. 2020; 20(6):1746. https://doi.org/10.3390/s20061746
Chicago/Turabian StyleLiu, Zhengkun, Qianqian Wang, Hao Wang, Wenting Su, and Shouliang Dong. 2020. "A FRET Based Two-Photon Fluorescent Probe for Visualizing Mitochondrial Thiols of Living Cells and Tissues" Sensors 20, no. 6: 1746. https://doi.org/10.3390/s20061746
APA StyleLiu, Z., Wang, Q., Wang, H., Su, W., & Dong, S. (2020). A FRET Based Two-Photon Fluorescent Probe for Visualizing Mitochondrial Thiols of Living Cells and Tissues. Sensors, 20(6), 1746. https://doi.org/10.3390/s20061746