A Comparison of the Conventional PiG Marker Method Versus a Cluster-Based Model when recording Gait Kinematics in Trans-Tibial Prosthesis Users and the Implications for Future IMU Gait Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
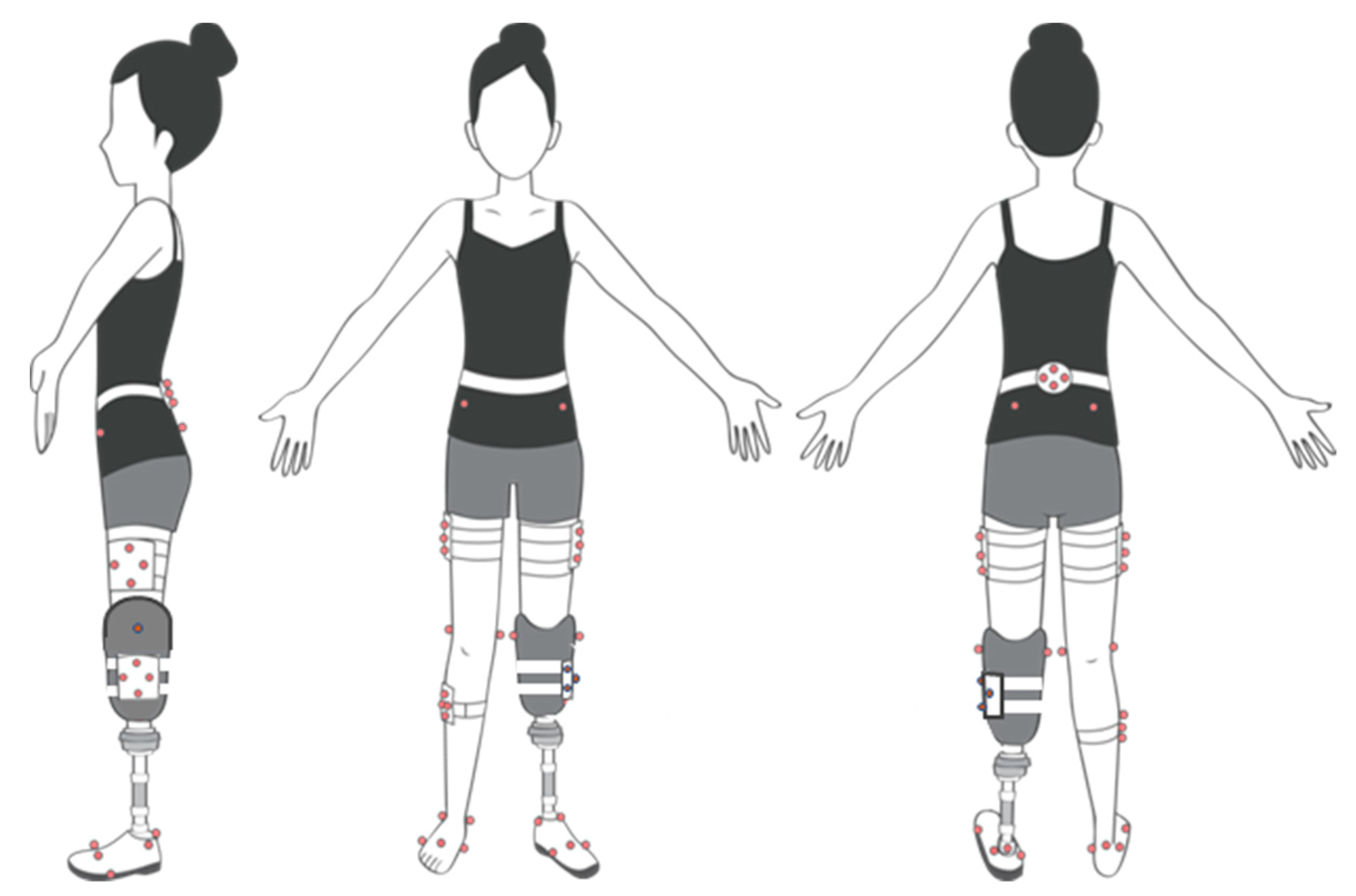
2.2. Protocol
2.3. Data Processing
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schwartz, M.H.; Rozumalski, A.; Trost, J.P. The effect of walking speed on the gait of typically developing children. J. Biomech. 2008, 41, 1639–1650. [Google Scholar] [CrossRef]
- Yeates, K.H.; Segal, A.D.; Neptune, R.R.; Klute, G.K. Balance and recovery on coronally-uneven and unpredictable terrain. J. Biomech. 2016, 49, 2734–2740. [Google Scholar] [CrossRef] [PubMed]
- Voloshina, A.S.; Kuo, A.D.; Daley, M.A.; Ferris, D.P. Biomechanics and energetics of walking on uneven terrain. J. Exp. Biol. 2013, 216, 3963–3970. [Google Scholar] [CrossRef] [PubMed]
- Cappozzo, A.; Catani, F.; Della Croce, U.; Leardini, A. Position and orientation in space of bones during movement: Anatomical frame definition and determination. Clin. Biomech. 1995, 10, 171–178. [Google Scholar] [CrossRef]
- Petropoulos, A.; Sikeridis, D.; Antonakopoulos, T. SPoMo: IMU-based real-time sitting posture monitoring. In Proceedings of the IEEE 7th International Conference on Consumer Electronics—Berlin (ICCE-Berlin), Berlin, Germany, 3–6 September 2017. [Google Scholar]
- Simpson, L.; Maharaj, M.M.; Mobbs, R.J. The role of wearables in spinal posture analysis: A systematic review. BMC Musculoskelet. Disord. 2019, 20, 55. [Google Scholar] [CrossRef]
- Hansson, E.E.; Tornberg, Å. Coherence and reliability of a wearable inertial measurement unit for measuring postural sway. BMC Res. Notes 2019, 12, 201. [Google Scholar]
- Lim, H.; Kim, B.; Park, S. Prediction of Lower Limb Kinetics and Kinematics during Walking by a Single IMU on the Lower Back Using Machine Learning. Sensors 2019, 20, 130. [Google Scholar] [CrossRef]
- Al-Amri, M.; Nicholas, K.; Button, K.; Sparkes, V.; Sheeran, L.; Davies, J.L. Inertial Measurement Units for Clinical Movement Analysis: Reliability and Concurrent Validity. Sensors 2018, 18, 719. [Google Scholar] [CrossRef]
- Lipperts, M.; Heyligers, I.C.; van Laarhoven, S.N.; Grimm, B.; Bolink, S.A.A.N. Inertial sensor motion analysis of gait, sit–stand transfers and step-up transfers: differentiating knee patients from healthy controls. Physiol. Meas. 2012, 33, 1947–1958. [Google Scholar]
- Orendurff, M.S.; Segal, A.D.; Klute, G.K.; Berge, J.S.; Rohr, E.S.; Kadel, N.J. The effect of walking speed on center of mass displacement. J. Rehabil. Res. Dev. 2004, 41, 829–834. [Google Scholar] [CrossRef]
- Krätschmer, R.; Böhm, H.; Döderlein, L. Kinematic adaptation and changes in gait classification in running compared to walking in children with unilateral spastic cerebral palsy. Gait Posture 2019, 67, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Kobayashi, T.; Gao, F.; Kataoka, M.; Orendurff, M.S.; Okuda, K. The effect of transverse prosthetic alignment changes on socket reaction moments during gait in individuals with transtibial amputation. Gait Posture 2018, 65, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Greenland, K.; Bloswick, D.; Zhao, J.; Merryweather, A. Vacuum level effects on knee contact force for unilateral transtibial amputees with elevated vacuum suspension. J. Biomech. 2017, 57, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Rigney, S.M.; Simmons, A.; Kark, L. A prosthesis-specific multi-link segment model of lower-limb amputee sprinting. J. Biomech. 2016, 49, 3185–3193. [Google Scholar] [CrossRef] [PubMed]
- Manal, K.; McClay, I.; Stanhope, S.; Richards, J.; Galinat, B. Comparison of surface mounted markers and attachment methods in estimating tibial rotations during walking: An in vivo study. Gait Posture 2000, 11, 38–45. [Google Scholar] [CrossRef]
- Nair, S.P.; Gibbs, S.; Arnold, G.; Abboud, R.; Wang, W. A method to calculate the centre of the ankle joint: A comparison with the Vicon® Plug-in-Gait model. Clin. Biomech. 2010, 25, 582–587. [Google Scholar] [CrossRef]
- Meng, L.; Childs, C.; Buis, A. A novel Strathclyde cluster model with functional method for joint centre location. In Strathclyde Researcher Conference; University of Strathclyde: Glasgow, UK, 2017. [Google Scholar]
- Mentiplay, B.F.; Clark, R.A. Modified conventional gait model versus cluster tracking: Test-retest reliability, agreement and impact of inverse kinematics with joint constraints on kinematic and kinetic data. Gait Posture 2018, 64, 75–83. [Google Scholar] [CrossRef]
- Meldrum, D.; Shouldice, C.; Conroy, R.; Jones, K.; Forward, M. Test–retest reliability of three dimensional gait analysis: Including a novel approach to visualising agreement of gait cycle waveforms with Bland and Altman plots. Gait Posture 2014, 39, 265–271. [Google Scholar] [CrossRef]
- Kainz, H.; Graham, D.; Edwards, J.; Walsh, H.; Maine, S.; Boyd, R.; Modenese, L.; Carty, C. Reliability of four models for clinical gait analysis. Gait Posture 2017, 54, 325–331. [Google Scholar] [CrossRef]
- Schwartz, M.H.; Rozumalski, A. A new method for estimating joint parameters from motion data. J. Biomech. 2005, 38, 107–116. [Google Scholar] [CrossRef]
- Della Croce, U.; Leardini, A.; Chiari, L.; Cappozzo, A. Human movement analysis using stereophotogrammetry: Part 4: Assessment of anatomical landmark misplacement and its effects on joint kinematics. Gait Posture 2005, 21, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, L.; D’Amico, C.; Turkina, A.Y.; Fabiana, N.; Amoroso, G.; Risitano, G. Endo and Exoskeleton: New Technologies on Composite Materials. Prosthesis 2020, 2, 1–9. [Google Scholar] [CrossRef]
- Cicciù, M. Prosthesis: New Technological Opportunities and Innovative Biomedical Devices. Prosthesis 2019, 1, 1–2. [Google Scholar] [CrossRef]
- Tawy, G.F.; Rowe, P.; Biant, L. Gait variability and motor control in patients with knee osteoarthritis as measured by the uncontrolled manifold technique. Gait Posture 2018, 59, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Zeni, J.A.; Richards, J.G.; Higginson, J.S. Two simple methods for determining gait events during treadmill and overground walking using kinematic data. Gait Posture 2008, 27, 710–714. [Google Scholar] [CrossRef]
- Zuk, M.; Pexowicz, C. Kinematic Analysis of a Six-Degrees-of-Freedom Model Based on ISB Recommendation: A Repeatability Analysis and Comparison with Conventional Gait Model. Appl. Bionics Biomech. 2015, 2015, 503713. [Google Scholar]
- McGinley, J.L.; Baker, R.; Wolfe, R.; Morris, M.E. The reliability of three-dimensional kinematic gait measurements: A systematic review. Gait Posture 2009, 29, 360–369. [Google Scholar] [CrossRef]
- Papi, E.; Ugbolue, U.C.; Solomonidis, S.; Rowe, P.J. Comparative study of a newly cluster based method for gait analysis and plug-in gait protocol. Gait Posture 2014, 39, S9–S10. [Google Scholar] [CrossRef]
- Cappozzo, A.; Della Croce, U.; Leardini, A.; Chiari, L. Human movement analysis using stereophotogrammetry. Part 1: Theoretical background. Gait Posture 2005, 21, 186–196. [Google Scholar] [CrossRef]
- Rusaw, D. Motion Analysis and Postural Stability of Transtibial Prosthesis Users. Ph.D. Thesis, University of Gothenburg, Goteborg, Sweden, 2011. [Google Scholar]
- Cappozzo, A.; Catani, F.; Leardini, A.; Benedetti, M.G.; Della Croce, U. Position and orientation in space of bones during movement: Experimental artefacts. Clin. Biomech. 1996, 11, 90–100. [Google Scholar] [CrossRef]
- Leardini, A.; Chiari, L.; Della Croce, U.; Cappozzo, A. Human movement analysis using stereophotogrammetry. Part 3. Soft tissue artifact assessment and compensation. Gait Posture 2005, 21, 212–225. [Google Scholar] [CrossRef] [PubMed]
| Participant | Hip Joint Rotation | |||||
|---|---|---|---|---|---|---|
| Flex/Extension | Ab/Adduction | Int/External | ||||
| PiG | SCM | PiG | SCM | PiG | SCM | |
| Subject 1 | 1.6 | 2.4 | 1.1 | 1.0 | 2.0 | 1.5 |
| Subject 2 | 1.3 | 1.0 | 0.8 | 1.6 | 1.8 | 1.8 |
| Subject 3 | 1.0 | 1.8 | 1.3 | 2.7 | 3.6 | 2.7 |
| Subject 4 | 1.9 | 2.6 | 1.3 | 2.6 | 5.6 | 2.2 |
| Subject 5 | 2.0 | 1.8 | 1.2 | 2.0 | 1.7 | 2.5 |
| Subject 6 | 2.5 | 5.6 | 1.6 | 2.4 | 3.0 | 2.9 |
| Subject 7 | 3.1 | 3.0 | 1.2 | 3.1 | 4.4 | 4.6 |
| Mean (°) | 1.9 | 2.6 | 1.2 | 2.2 | 3.2 | 2.6 |
| Participant | Hip Joint Rotation | |||||
|---|---|---|---|---|---|---|
| Flex/Extension | Ab/Adduction | Int/External | ||||
| PiG | SCM | PiG | SCM | PiG | SCM | |
| Subject 1 | 1.0 | 1.9 | 0.4 | 0.6 | 1.6 | 1.6 |
| Subject 2 | 1.3 | 1.3 | 0.6 | 1.1 | 1.3 | 1.8 |
| Subject 3 | 3.1 | 4.3 | 0.7 | 2.8 | 2.5 | 1.3 |
| Subject 4 | 2.0 | 4.1 | 1.1 | 2.7 | 2.9 | 3.9 |
| Subject 5 | 3.3 | 3.4 | 1.1 | 1.8 | 2.7 | 2.7 |
| Subject 6 | 2.5 | 3.9 | 0.8 | 1.8 | 2.7 | 4.3 |
| Subject 7 | 2.2 | 2.5 | 1.0 | 1.8 | 4.7 | 1.5 |
| Mean (°) | 2.2 | 3.0 | 0.8 | 1.8 | 2.6 | 2.4 |
| Participant | Knee Joint Rotation | |||||
|---|---|---|---|---|---|---|
| Flex/Extension | Ab/Adduction | Int/External | ||||
| PiG | SCM | PiG | SCM | PiG | SCM | |
| Subject 1 | 2.0 | 4.5 | 1.8 | 2.7 | 1.0 | 2.2 |
| Subject 2 | 2.1 | 1.6 | 1.1 | 1.4 | 0.7 | 1.6 |
| Subject 3 | 1.1 | 1.8 | 1.6 | 1.4 | 0.3 | 2.3 |
| Subject 4 | 2.2 | 3.9 | 3.2 | 2.4 | 1.4 | 2.2 |
| Subject 5 | 2.4 | 3.4 | 2.0 | 1.4 | 2.9 | 2.0 |
| Subject 6 | 4.9 | 6.5 | 3.4 | 1.7 | 0.7 | 2.6 |
| Subject 7 | 5.3 | 3.9 | 3.2 | 2.9 | 9.8 | 4.4 |
| Mean (°) | 2.9 | 3.7 | 2.3 | 2.0 | 2.4 | 2.5 |
| Participant | Knee Joint Rotation | |||||
|---|---|---|---|---|---|---|
| Flex/Extension | Ab/Adduction | Int/External | ||||
| PiG | SCM | PiG | SCM | PiG | SCM | |
| Subject 1 | 0.8 | 2.8 | 0.5 | 0.8 | 1.0 | 1.8 |
| Subject 2 | 1.3 | 2.1 | 1.5 | 0.8 | 1.6 | 1.5 |
| Subject 3 | 4.1 | 6.1 | 2.5 | 1.9 | 2.4 | 3.9 |
| Subject 4 | 2.3 | 3.0 | 1.4 | 2.6 | 2.0 | 2.5 |
| Subject 5 | 3.2 | 7.3 | 1.9 | 1.9 | 2.8 | 2.3 |
| Subject 6 | 2.0 | 2.3 | 1.7 | 1.3 | 3.9 | 1.8 |
| Subject 7 | 2.4 | 4.7 | 3.3 | 2.0 | 2.8 | 2.3 |
| Mean (°) | 2.3 | 4.1 | 1.8 | 1.6 | 2.4 | 2.3 |
| Participant | Ankle Joint Rotation SD | |||||
|---|---|---|---|---|---|---|
| Dorsi/Plantar | Ab/Adduction | Inv/Eversion | ||||
| PiG | SCM | PiG | SCM | PiG | SCM | |
| Subject 1 | 1.1 | 0.9 | 1.2 | 2.9 | 5.5 | 1.4 |
| Subject 2 | 0.3 | 0.5 | 0.2 | 0.8 | 1.5 | 0.7 |
| Subject 3 | 0.5 | 0.5 | 0.9 | 0.8 | 3.6 | 0.8 |
| Subject 4 | 1.0 | 0.8 | 3.7 | 1.1 | 2.2 | 0.8 |
| Subject 5 | 6.4 | 0.6 | 6.9 | 0.7 | 1.7 | 0.6 |
| Subject 6 | 1.1 | 1.5 | 0.4 | 1.0 | 2.4 | 1.3 |
| Subject 7 | 11.1 | 1.6 | 3.3 | 1.2 | 15.7 | 1.4 |
| Mean (°) | 3.1 | 0.9 | 2.3 | 1.2 | 4.6 | 1.0 |
| Participant | Ankle Joint Rotation SD | |||||
|---|---|---|---|---|---|---|
| Dorsi/Plantar | Ab/Adduction | Inv/Eversion | ||||
| PiG | SCM | PiG | SCM | PiG | SCM | |
| Subject 1 | 1.7 | 2.4 | 0.9 | 1.9 | 1.3 | 1.1 |
| Subject 2 | 6.4 | 1.5 | 3.6 | 1.7 | 1.5 | 1.0 |
| Subject 3 | 8.2 | 2.8 | 8.0 | 5.6 | 1.1 | 1.4 |
| Subject 4 | 1.8 | 2.0 | 0.8 | 2.5 | 3.4 | 1.4 |
| Subject 5 | 4.1 | 6.0 | 1.5 | 4.1 | 4.5 | 2.5 |
| Subject 6 | 1.8 | 14.2 | 1.8 | 12.2 | 4.2 | 1.5 |
| Subject 7 | 1.8 | 1.7 | 1.1 | 2.5 | 5.0 | 1.3 |
| Mean (°) | 3.7 | 4.4 | 2.5 | 4.4 | 3.0 | 1.5 |
| Parameters | PiG SD | SCM SD | p-Value | p < 0.05 | p < 0.005 |
|---|---|---|---|---|---|
| Sound side | |||||
| Hip flex/extension ROM | 38.4(4.7) | 42.1(7.0) | 0.12 | ||
| Peak Stance Extension | 15.3(10.5) | −8.9(10.0) | 0.06 | * | |
| Peak Swing Flexion | 17.9(12.4) | 27.2(13.5) | 0.02 | ||
| Hip Ab/Ad ROM | 9.1(3.2) | 12.3(3.3) | 0.11 | * | |
| Hip Int/Ext Rotation ROM | 24.6(5.3) | 12.1(4.0) | 0.01 | ||
| Amputated side | |||||
| Hip flex/extension ROM | 42.1(7.6) | 49.0(5.6) | 0.00 | * | ** |
| Peak Stance Extension | −11.4(9.6) | −7.6(9.2) | 0.22 | ||
| Peak Swing Flexion | 27.5(8.8) | 38.5(9.7) | 0.01 | * | |
| Hip Ab/Ad ROM | 8.9(3.8) | 11.6(3.3) | 0.32 | ||
| Hip Int/Ext Rotation ROM | 37.6(32.3) | 12.3(3.0) | 0.09 |
| Parameters | PiG SD | SCM SD | p-Value | p < 0.05 | p < 0.005 |
|---|---|---|---|---|---|
| Sound side | |||||
| Knee flex/extension ROM | 48.8(10.0) | 63.4(7.7) | 0.01 | * | |
| Peak Stance Extension | 3.2(11.4) | 8.6(8.4) | 0.18 | ||
| Peak Swing Flexion | 43.6(14.3) | 60.8(7.5) | 0.02 | * | |
| Knee Ab/Ad ROM | 34.1(12.6) | 21.3(9.4) | 0.08 | ||
| Knee Int/Ext Rotation ROM | 20.7(8.1) | 19.5(5.2) | 0.71 | ||
| Amputated side | |||||
| Knee flex/extension ROM | 45.3(12.8) | 70.6(8.9) | 0.01 | * | |
| Peak Stance Extension | 6.8(7.2) | 12.2(4.4) | 0.03 | * | |
| Peak Swing Flexion | 41.9(14.6) | 69.3(5.4) | 0.01 | * | |
| Knee Ab/Ad ROM | 38.7(15.1) | 22.5(10.0) | 0.10 | ||
| Knee Int/Ext Rotation ROM | 9.8(6.9) | 21.2(5.6) | 0.01 | * |
| Parameters | PiG SD | SCM SD | p-Value | p < 0.05 | p < 0.005 |
|---|---|---|---|---|---|
| Sound side | |||||
| Ankle Plantar/dorsiflexion | 37.3(16.1) | 25.7(3.5) | 0.12 | ||
| Peak Stance dorsiflexion | 32.8(21.8) | 2.8(6.6) | 0.02 | * | |
| Peak Swing plantarflexion | −2.3(8.5) | −21.1(6.6) | 0.01 | * | |
| Ankle Ab/Adduction | 13.5(13.7) | 11.6(3.9) | 0.76 | ||
| Ankle Inv/Eversion ROM | 11.5(5.0) | 10.6(3.6) | 0.66 | ||
| Amputated side | |||||
| Ankle Plantar/dorsiflexion | 10.5(6.0) | 8.2(2.8) | 0.43 | ||
| Peak Stance dorsiflexion | 15.2(11.8) | −3.0(2.6) | 0.01 | * | |
| Peak Swing plantarflexion | 9.0(13.3) | −8.2(4.0) | 0.02 | * | |
| Ankle Ab/Adduction | 14.4(11.5) | 3.2(0.8) | 0.04 | * | |
| Ankle Inv/Eversion ROM | 34.7(30.5) | 4.0(1.3) | 0.04 | * |
| Amputated Side | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hip Joint | Knee Joint | Ankle Joint | Mean (°) | |||||||
| Protocol | Flex/Ext | Ab/Ad | In/Ex | Flex/Ext | Ab/Ad | In/Ex | Dorsi/Plntar | Ab/Ad | Inv/Evr | |
| PiG | 9.6 | 3.8 | 38.3 | 10.0 | 11.1 | 12.7 | 14.0 | 18.3 | 40.7 | 17.6 |
| SCM | 10.6 | 4.6 | 22.4 | 8.2 | 9.3 | 15.5 | 3.5 | 5.2 | 4.4 | 9.3 |
| Sound Side | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hip Joint | Knee Joint | Ankle Joint | Mean (°) | |||||||
| Protocol | Flex/Ext | Ab/Ad | In/Ex | Flex/Ext | Ab/Ad | In/Ex | Dorsi/Plntar | Ab/Ad | Inv/Evr | |
| PiG | 11.4 | 5.7 | 19.0 | 10.5 | 8.1 | 22.6 | 17.6 | 22.0 | 21.4 | 15.4 |
| SCM | 11.8 | 3.8 | 19.3 | 7.4 | 10.3 | 14.7 | 6.7 | 6.6 | 6.5 | 9.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samala, M.; Rowe, P.; Rattanakoch, J.; Guerra, G. A Comparison of the Conventional PiG Marker Method Versus a Cluster-Based Model when recording Gait Kinematics in Trans-Tibial Prosthesis Users and the Implications for Future IMU Gait Analysis. Sensors 2020, 20, 1255. https://doi.org/10.3390/s20051255
Samala M, Rowe P, Rattanakoch J, Guerra G. A Comparison of the Conventional PiG Marker Method Versus a Cluster-Based Model when recording Gait Kinematics in Trans-Tibial Prosthesis Users and the Implications for Future IMU Gait Analysis. Sensors. 2020; 20(5):1255. https://doi.org/10.3390/s20051255
Chicago/Turabian StyleSamala, Manunchaya, Philip Rowe, Jutima Rattanakoch, and Gary Guerra. 2020. "A Comparison of the Conventional PiG Marker Method Versus a Cluster-Based Model when recording Gait Kinematics in Trans-Tibial Prosthesis Users and the Implications for Future IMU Gait Analysis" Sensors 20, no. 5: 1255. https://doi.org/10.3390/s20051255
APA StyleSamala, M., Rowe, P., Rattanakoch, J., & Guerra, G. (2020). A Comparison of the Conventional PiG Marker Method Versus a Cluster-Based Model when recording Gait Kinematics in Trans-Tibial Prosthesis Users and the Implications for Future IMU Gait Analysis. Sensors, 20(5), 1255. https://doi.org/10.3390/s20051255






