Valve-Actuator-Integrated Reference Electrode for an Ultra-Long-Life Rumen pH Sensor
Abstract
1. Introduction
2. Design
2.1. Potential Stability When Switching the Liquid Junction
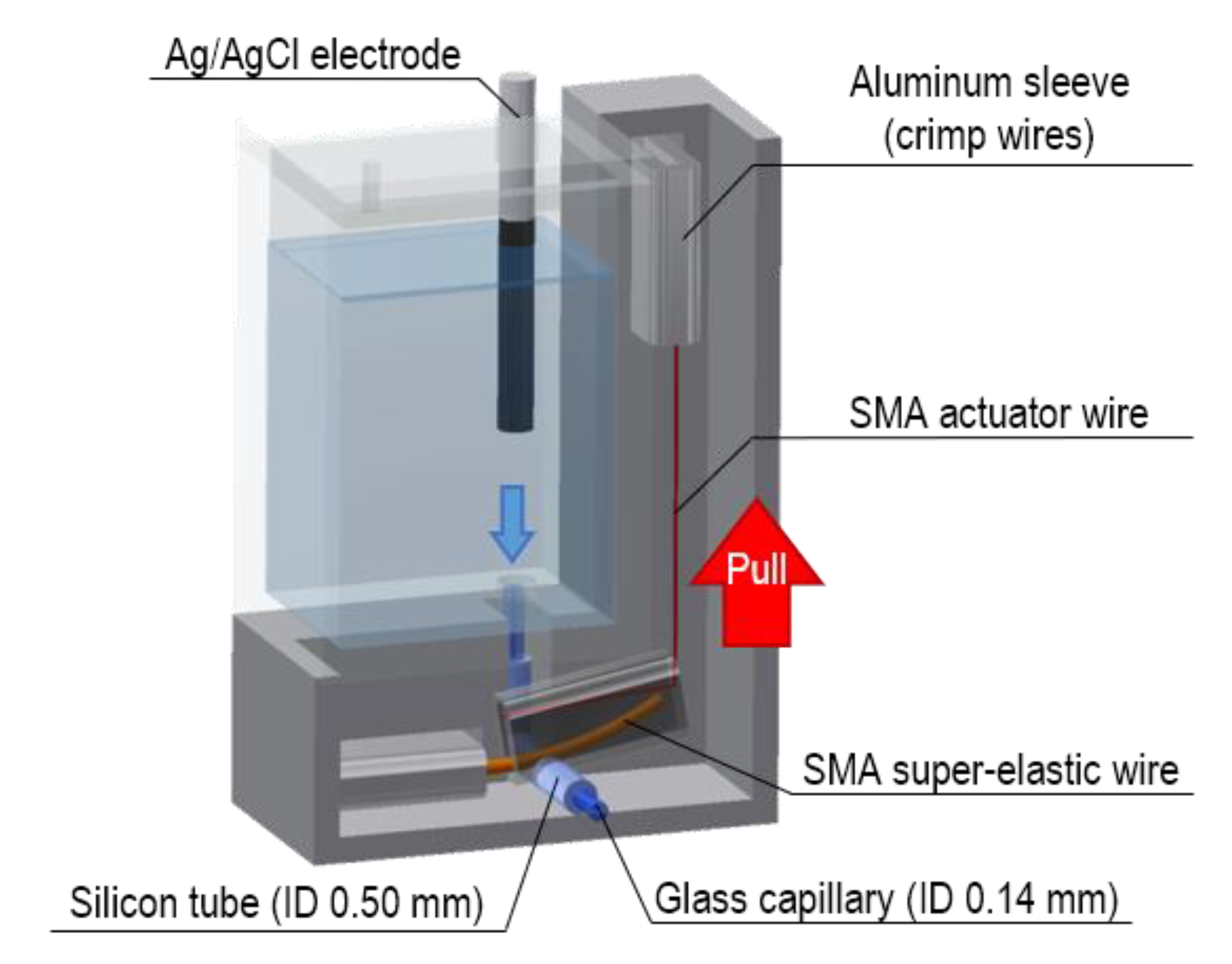
2.2. Design of the Valve-Actuator-Integrated Reference Electrode
2.3. Estimation of the Electrolyte Outflow
3. Materials and Methods
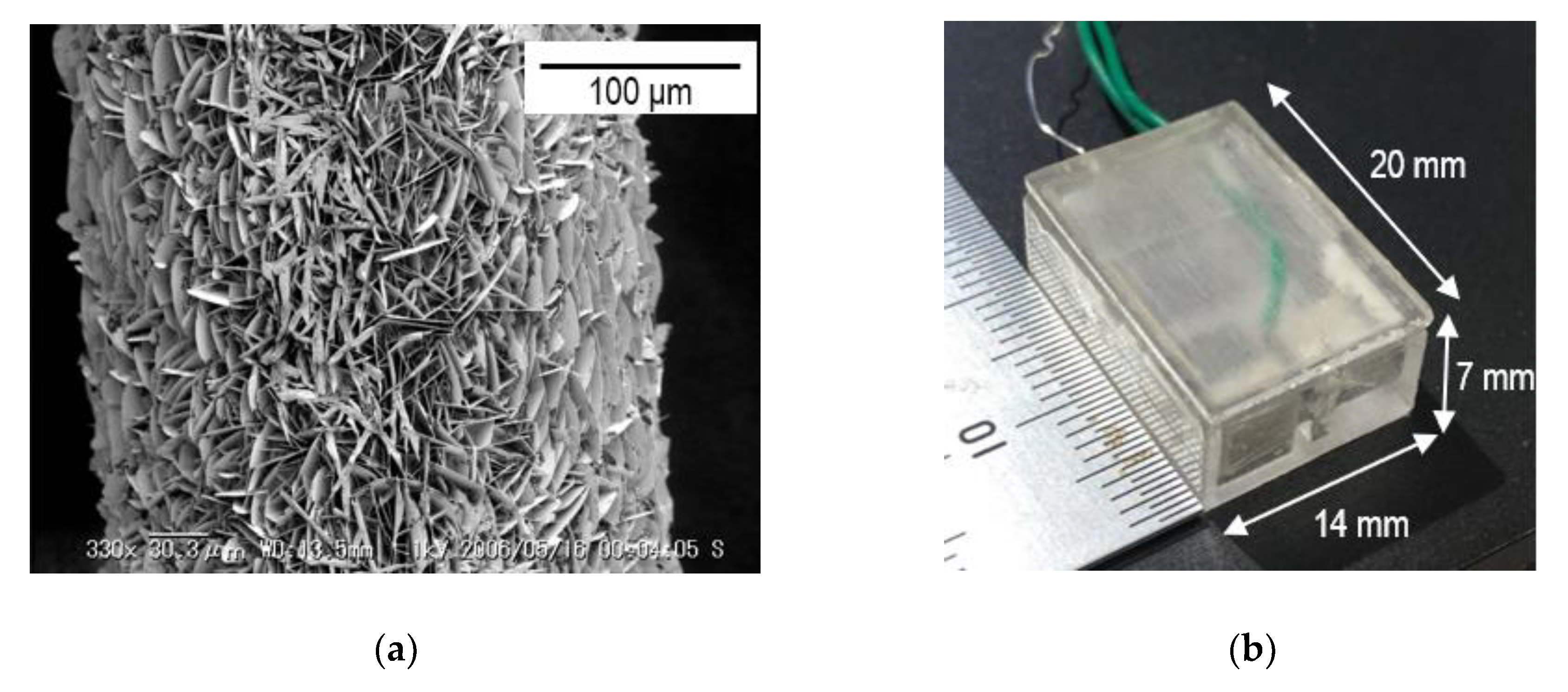
3.1. Fabrication of the Valve-Actuator-Integrated Reference Electrode
3.2. Measurement
4. Results and Discussion
4.1. Impedance of the Liquid Junction
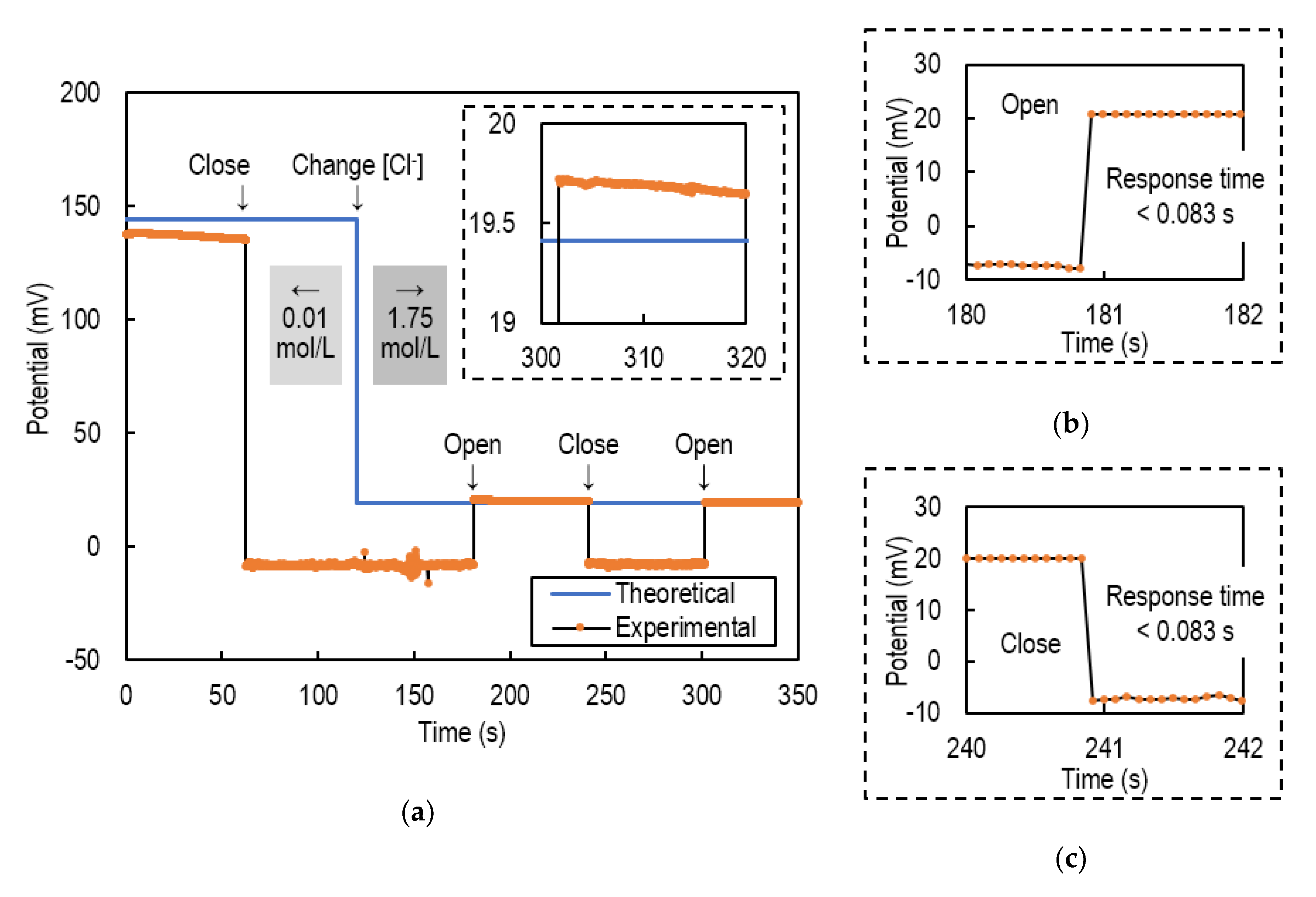
4.2. Intermittent Potential Measurement
4.3. Iintermittent pH Measurement
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, L.; Lu, J.; Okada, H.; Nogami, H.; Itoh, T.; Arai, S. Low-Power Highly Sensitive pH Sensor With mu dots Protective Structures for Monitoring Rumen in Cows in Real-Time. IEEE Sens. J. 2017, 17, 7281–7289. [Google Scholar] [CrossRef]
- Mottram, T.; Lowe, J.; McGowan, M.; Phillips, N. Technical note: A wireless telemetric method of monitoring clinical acidosis in dairy cows. Comput. Electron. Agric. 2008, 64, 45–48. [Google Scholar] [CrossRef]
- Sato, S.; Mizuguchi, H.; Ito, K.; Ikuta, K.; Kimura, A.; Okada, K. Technical note: Development and testing of a radio transmission pH measurement system for continuous monitoring of ruminal pH in cows. Prev. Vet. Med. 2012, 103, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, J.; Nogami, H.; Okada, H.; Itoh, T.; Arai, S. Solid-state pH sensor prototype for real-time monitoring of the rumen pH value of Japanese cows. Microsyst. Technol. Micro-Nanosyst. Inf. Storage Process. Syst. 2018, 24, 457–463. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, J.; Nogami, H.; Okada, H.; Itoh, T. Developing a Solid-State pH Sensor for Wagyu-Rumen Monitoring. Sens. Mater. 2016, 28, 1273–1281. [Google Scholar]
- Guth, U.; Gerlach, F.; Decker, M.; Oelssner, W.; Vonau, W. Solid-state reference electrodes for potentiometric sensors. J. Solid State Electrochem. 2009, 13, 27–39. [Google Scholar] [CrossRef]
- Hu, J.B.; Stein, A.; Buhlmann, P. Rational design of all-solid-state ion-selective electrodes and reference electrodes. Trac-Trends Anal. Chem. 2016, 76, 102–114. [Google Scholar] [CrossRef]
- Kim, T.Y.; Hong, S.A.; Yang, S. A Solid-State Thin-Film Ag/AgCl Reference Electrode Coated with Graphene Oxide and Its Use in a pH Sensor. Sensors 2015, 15, 6469–6482. [Google Scholar] [CrossRef]
- Suzuki, H.; Shiroishi, H.; Sasaki, S.; Karube, I. Microfabricated liquid junction Ag/AgCl reference electrode and its application to a one-chip potentiometric sensor. Anal. Chem. 1999, 71, 5069–5075. [Google Scholar] [CrossRef]
- Vonau, W.; Oelssner, W.; Guth, U.; Henze, J. An all-solid-state reference electrode. Sens. Actuators B-Chem. 2010, 144, 368–373. [Google Scholar] [CrossRef]
- Shitanda, I.; Kiryu, H.; Itagaki, M. Improvement in the long-term stability of screen-printed planar type solid-state Ag/AgCl reference electrode by introducing poly(dimethylsiloxane) liquid junction. Electrochim. Acta 2011, 58, 528–531. [Google Scholar] [CrossRef]
- Shitanda, I.; Komoda, M.; Hoshi, Y.; Itagaki, M. An instantly usable paper-based screen-printed solid-state KCl/Ag/AgCl reference electrode with long-term stability. Analyst 2015, 140, 6481–6484. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Hirakawa, T.; Sasaki, S.; Karube, I. Micromachined liquid-junction Ag/AgCl reference electrode. Sens. Actuators B-Chem. 1998, 46, 146–154. [Google Scholar] [CrossRef]
- Safari, S.; Selvaganapathy, P.R.; Deen, M.J. Microfluidic Reference Electrode with Free-Diffusion Liquid Junction. J. Electrochem. Soc. 2013, 160, B177–B183. [Google Scholar] [CrossRef]
- Ito, S.; Hachiya, H.; Baba, K.; Asano, Y.; Wada, H. Improvement of the silver/silver chloride reference electrode and its application to pH measurement. Talanta 1995, 42, 1685–1690. [Google Scholar] [CrossRef]
- Mousavi, M.P.S.; Saba, S.A.; Anderson, E.L.; Hillmyer, M.A.; Buhlmann, P. Avoiding Errors in Electrochemical Measurements: Effect of Frit Material on the Performance of Reference Electrodes with Porous Frit Junctions. Anal. Chem. 2016, 88, 8706–8713. [Google Scholar] [CrossRef]
- Dohner, R.E.; Wegmann, D.; Morf, W.E.; Simon, W. Reference electrode with free-flowing free-diffusion liquid junction. Anal. Chem. 1986, 58, 2585–2589. [Google Scholar] [CrossRef]
- Peters, G. A reference electrode with free-diffusion liquid junction for electrochemical measurements under changing pressure conditions. Anal. Chem. 1997, 69, 2362–2366. [Google Scholar] [CrossRef]
- Higuchi, S.; Takamatsu, S.; Itoh, T. Ultra-long-life miniature ph sensor with a sma valve-actuator integrated liquid junction for rumen monitoring. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers), Berlin, Germany, 23–27 June 2019; pp. 1411–1414. [Google Scholar]
- Kohl, A.; Gobes, J.; Krevet, B. Normally-closed shape memory microvalve. Int. J. Appl. Electromagn. Mech. 2000, 12, 71–77. [Google Scholar] [CrossRef]
- Oh, K.W.; Ahn, C.H. A review of microvalves. J. Micromech. Microeng. 2006, 16, R13–R39. [Google Scholar] [CrossRef]
- Brewer, P.J.; Leese, R.J.; Brown, R.J.C. An improved approach for fabricating Ag/AgCl reference electrodes. Electrochim. Acta 2012, 71, 252–257. [Google Scholar] [CrossRef]
- Safari, S.; Selvaganapathy, P.R.; Derardja, A.; Deen, M.J. Electrochemical growth of high-aspect ratio nanostructured silver chloride on silver and its application to miniaturized reference electrodes. Nanotechnology 2011, 22, 315601. [Google Scholar] [CrossRef]





| Open | Close | |
|---|---|---|
| (A) Impedance of the liquid junction | 17 kΩ | 1 × 105 kΩ |
| (B) Impedance of the glass capillary 1 | 8.6 kΩ | 8.6 kΩ |
| Impedance of the pinched silicon tube ((A)–(B)) | 7.3 kΩ | 1 × 105 kΩ |
| Inner diameter of the pinched silicon tube | 6.8 × 10−2 mm | 6 × 10−4 mm |
| SMA Actuator Wire | SMA Super-Elastic Wire | |
|---|---|---|
| Material | Ni-Ti | Ni-Ti |
| Diameter | 0.15 mm | 0.40 mm |
| Length | 10 mm | 10 mm |
| Transformation temperature | 70 °C | 5 °C |
| Power | 54 mW | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higuchi, S.; Okada, H.; Takamatsu, S.; Itoh, T. Valve-Actuator-Integrated Reference Electrode for an Ultra-Long-Life Rumen pH Sensor. Sensors 2020, 20, 1249. https://doi.org/10.3390/s20051249
Higuchi S, Okada H, Takamatsu S, Itoh T. Valve-Actuator-Integrated Reference Electrode for an Ultra-Long-Life Rumen pH Sensor. Sensors. 2020; 20(5):1249. https://doi.org/10.3390/s20051249
Chicago/Turabian StyleHiguchi, Shogo, Hironao Okada, Seiichi Takamatsu, and Toshihiro Itoh. 2020. "Valve-Actuator-Integrated Reference Electrode for an Ultra-Long-Life Rumen pH Sensor" Sensors 20, no. 5: 1249. https://doi.org/10.3390/s20051249
APA StyleHiguchi, S., Okada, H., Takamatsu, S., & Itoh, T. (2020). Valve-Actuator-Integrated Reference Electrode for an Ultra-Long-Life Rumen pH Sensor. Sensors, 20(5), 1249. https://doi.org/10.3390/s20051249





