Fluorescence-Sensor Mapping for the in Vineyard Non-Destructive Assessment of Crimson Seedless Table Grape Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Sites and Plant Management
2.2. Fluorescence Indices
2.3. Data Acquisition
2.4. Data Filtering
2.5. Statistical Analysis
2.6. Geostatistical Analysis and Mapping
3. Results and Discussion
3.1. Plot Comparison
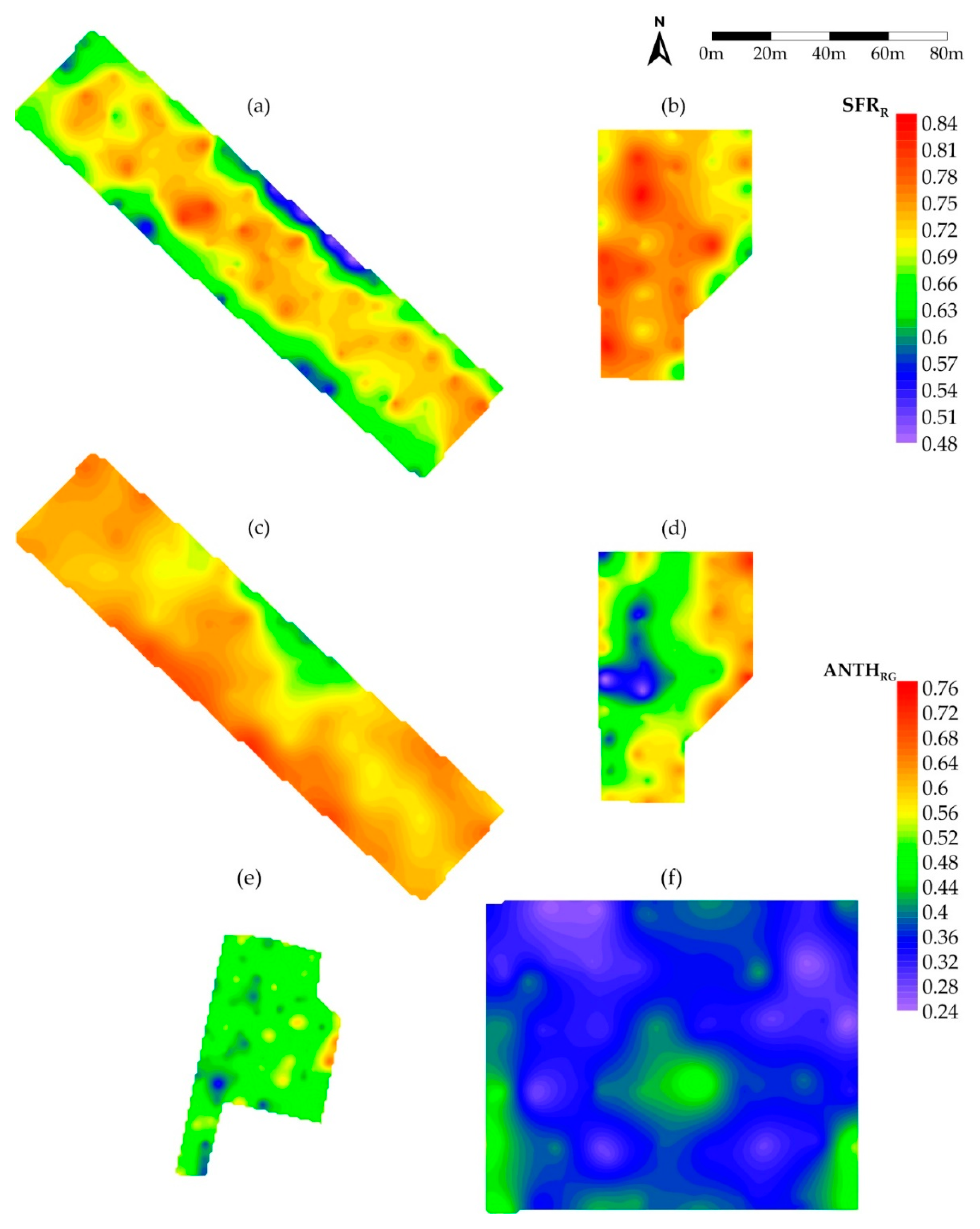
3.2. Spatial Variability
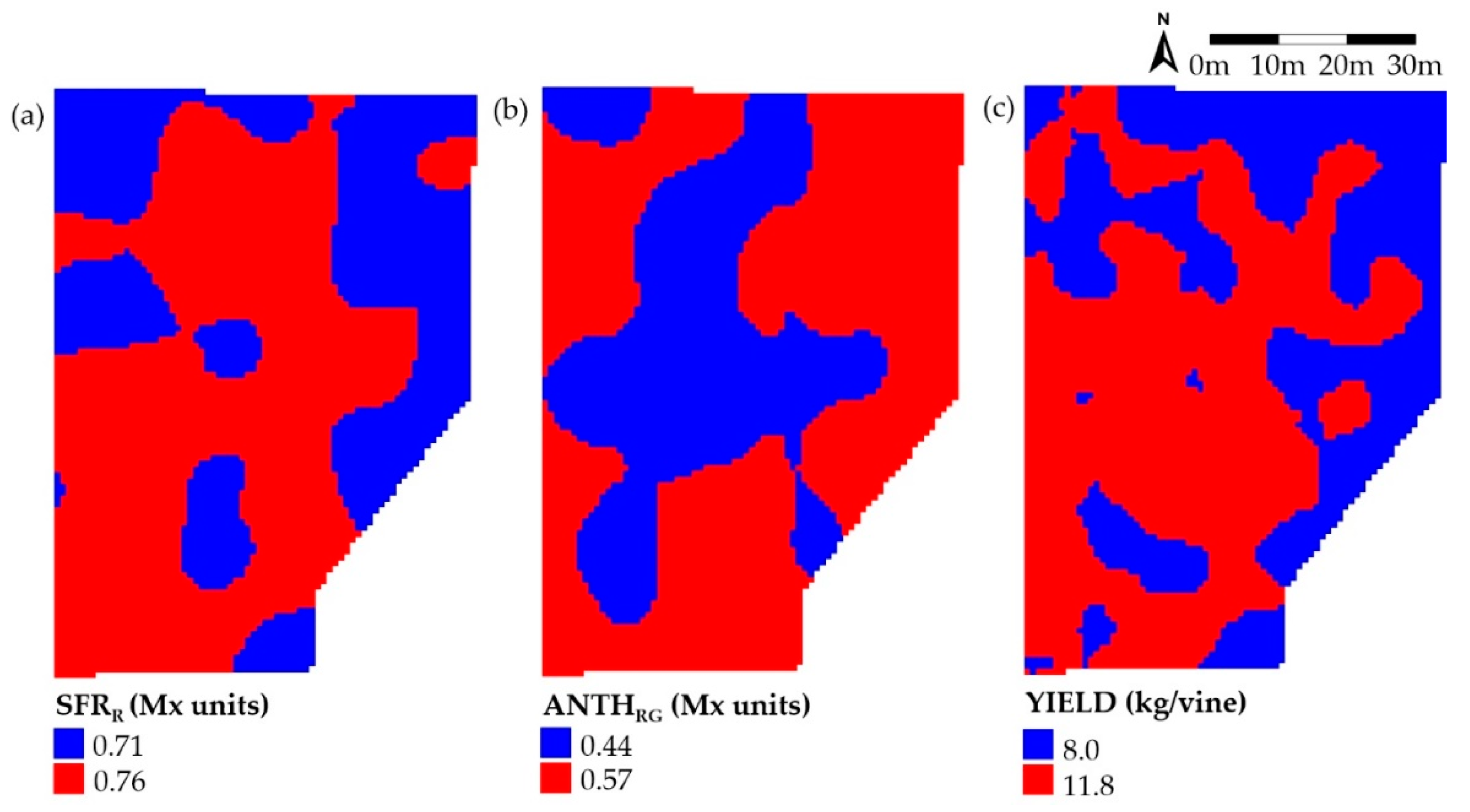
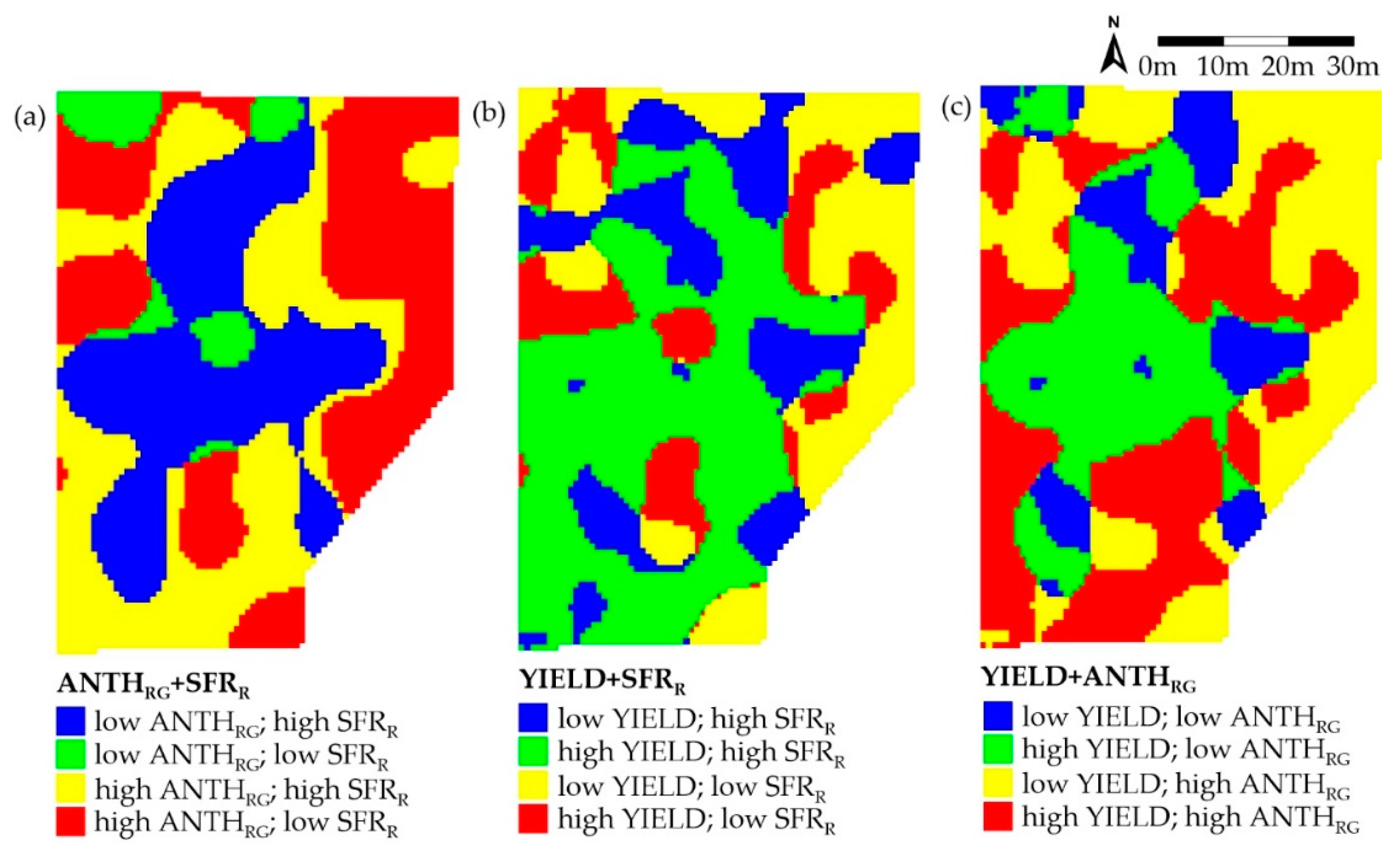
3.3. Zoning
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baseca, C.C.; Sendra, S.; Lloret, J.; Tomas, J. A Smart Decision System for Digital Farming. Agronomy 2019, 9, 216. [Google Scholar] [CrossRef]
- Huang, S. Global trade of fruits and vegetables and the role of consumer demand. In Trade, Food, Diet and Health: Perspectives and Policy Options; Hawkes, C., Blouin, C., Henson, S., Drager, N., Dubé, L., Eds.; Wiley-Blackwell: Oxford, UK, 2010; pp. 60–76. [Google Scholar]
- FAO-OIV Focus 2016. Table and Dried Grapes. Available online: http://www.fao.org/publications/card/en/c/709ef071-6082-4434-91bf-4bc5b01380c6/ (accessed on 12 February 2020).
- Jayasena, V.; Cameron, I. °Brix/Acid Ratio as A Predictor of Consumer Acceptability of Crimson Seedless Table Grapes. J. Food Qual. 2008, 31, 736–750. [Google Scholar] [CrossRef]
- Cavallo, D.P.; Cefola, M.; Pace, B.; Logrieco, A.F.; Attolico, G. Non-destructive and contactless quality evaluation of table grapes by a computer vision system. Comput. Electron. Agric. 2019, 156, 558–564. [Google Scholar] [CrossRef]
- Baiano, A.; Terracone, C.; Peri, G.; Romaniello, R. Application of hyperspectral imaging for prediction of physico-chemical and sensory characteristics of table grapes. Comput. Electron. Agric. 2012, 87, 142–151. [Google Scholar] [CrossRef]
- Rose, J.C.; Kicherer, A.; Wieland, M.; Klingbeil, L.; Töpfer, R.; Kuhlmann, H. Towards Automated Large-Scale 3D Phenotyping of Vineyards under Field Conditions. Sensors 2016, 16, 2136. [Google Scholar] [CrossRef] [PubMed]
- Rist, F.; Herzog, K.; Mack, J.; Richter, R.; Steinhage, V.; Töpfer, R. High-Precision Phenotyping of Grape Bunch Architecture Using Fast 3D Sensor and Automation. Sensors 2018, 18, 763. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, E.; Balafoutis, A.; Darra, N.; Psiroukis, V.; Biniari, A.; Xanthopoulos, G.; Fountas, S. Satellite and Proximal Sensing to Estimate the Yield and Quality of Table Grapes. Agriculture 2018, 8, 94. [Google Scholar] [CrossRef]
- Agati, G.; D’Onofrio, C.; Ducci, E.; Cuzzola, A.; Remorini, D.; Tuccio, L.; Lazzini, F.; Mattii, G. Potential of a Multiparametric Optical Sensor for Determining in Situ the Maturity Components of Red and White Vitis vinifera Wine Grapes. J. Agric. Food Chem. 2013, 61, 12211–12218. [Google Scholar] [CrossRef]
- Bahar, A.; Kaplunov, T.; Zutahy, Y.; Daus, A.; Lurie, S.; Lichter, A. Auto-fluorescence for analysis of ripening in Thompson Seedless and colour in Crimson Seedless table grapes. Aust. J. Grape Wine Res. 2012, 18, 353–359. [Google Scholar] [CrossRef]
- Lichter, A.; Kaplunov, T.; Zutahy, Y.; Daus, A.; Beno, D.; Lurie, S.; Sarig, P.; Stromza, A. Using autofluorescence to follow the effect of abcisic acid and leaf removal on color development of ‘flame seedless’ grapes. Acta Hortic. 2015, 1091, 183–186. [Google Scholar] [CrossRef]
- Bramley, R. Understanding variability in winegrape production systems 2. Within vineyard variation in quality over several vintages. Aust. J. Grape Wine Res. 2005, 11, 33–42. [Google Scholar] [CrossRef]
- Tardaguila, J.; Baluja, J.; Arpon, L.; Balda, P.; Oliveira, M. Variations of soil properties affect the vegetative growth and yield components of “Tempranillo” grapevines. Precis. Agric. 2011, 12, 762–773. [Google Scholar] [CrossRef]
- Pothen, Z.; Nuske, S. Automated Assessment and Mapping of Grape Quality through Image-based Color Analysis. IFAC-PapersOnLine 2016, 49, 72–78. [Google Scholar] [CrossRef]
- Agati, G.; Soudani, K.; Tuccio, L.; Fierini, E.; Ben Ghozlen, N.; Fadaili, E.M.; Romani, A.; Cerovic, Z.G. Management Zone Delineation for Winegrape Selective Harvesting Based on Fluorescence-Sensor Mapping of Grape Skin Anthocyanins. J. Agric. Food Chem. 2018, 66, 5778–5789. [Google Scholar] [CrossRef] [PubMed]
- Bramley, R.; Le Moigne, M.; Evain, S.; Ouzman, J.; Florin, L.; Fadaili, E.; Hinze, C.; Cerovic, Z. On-the-go sensing of grape berry anthocyanins during commercial harvest: Development and prospects. Aust. J. Grape Wine Res. 2011, 17, 316–326. [Google Scholar] [CrossRef]
- Ben Ghozlen, N.; Cerovic, Z.G.; Germain, C.; Toutain, S.; Latouche, G. Non-Destructive Optical Monitoring of Grape Maturation by Proximal Sensing. Sensors 2010, 10, 10040–10068. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, C. Variability and application of the chlorophyll fluorescence emission ratio red/far-red of leaves. Photosynth. Res. 2007, 92, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Meyer, S.; Matteini, P.; Cerovic, Z.G. Assessment of Anthocyanins in Grape (Vitis vinifera L.) Berries Using a Noninvasive Chlorophyll Fluorescence Method. J. Agric. Food Chem. 2007, 55, 1053–1061. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Ounis, A.; Cartelat, A.; Latouche, G.; Goulas, Y.; Meyer, S.; Moya, I. The use of chlorophyll fluorescence excitation spectra for the non-destructive in situ assessment of UV-absorbing compounds in leaves. Plant Cell Environ. 2002, 25, 1663–1676. [Google Scholar] [CrossRef]
- Oliver, M.; Webster, R. A tutorial guide to geostatistics: Computing and modelling variograms and kriging. Catena 2014, 113, 56–69. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Moorman, T.B.; Novak, J.M.; Parkin, T.B.; Karlen, D.L.; Turco, R.; Konopka, A.E. Field-Scale Variability of Soil Properties in Central Iowa Soils. Soil Sci. Soc. Am. J. 1994, 58, 1501–1511. [Google Scholar] [CrossRef]
- Robert, P.; Rust, R.; Larson, W.; Han, S.; Evans, R.G.; Schneider, S.M.; Rawlins, S.L. Spatial Variability of Soil Properties on Two Center-Pivot Irrigated Fields. ACSESS Publ. 1996, 97–106. [Google Scholar] [CrossRef]
- Baluja, J.; Diago, M.; Goovaerts, P.; Tardaguila, J. Spatio-temporal dynamics of grape anthocyanin accumulation in a Tempranillo vineyard monitored by proximal sensing. Aust. J. Grape Wine Res. 2012, 18, 173–182. [Google Scholar] [CrossRef]
- Pinelli, P.; Romani, A.; Fierini, E.; Agati, G. Prediction models for assessing anthocyanins in grape berries by fluorescence sensors: Dependence on cultivar, site and growing season. Food Chem. 2018, 244, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Savi, S.; Poni, S.; Moncalvo, A.; Frioni, T.; Rodschinka, I.; Arata, L.; Gatti, M. Destructive and optical non-destructive grape ripening assessment: Agronomic comparison and cost-benefit analysis. PLOS ONE 2019, 14, e0216421. [Google Scholar] [CrossRef] [PubMed]
- Yamane, T.; Jeong, S.T.; Goto-Yamamoto, N.; Koshita, Y.; Kobayashi, S. Effects of temperature on anthocyanin biosynthesis in grape berry skins. Am. J. Enol. Viticult. 2006, 57, 54–59. [Google Scholar]
- Koshita, Y.; Yamane, T.; Yakushiji, H.; Azuma, A.; Mitani, N. Regulation of skin color in ‘Aki Queen’ grapes: Interactive effects of temperature, girdling, and leaf shading treatments on coloration and total soluble solids. Sci. Hortic. 2011, 129, 98–101. [Google Scholar] [CrossRef]
- Shinomiya, R.; Fujishima, H.; Muramoto, K.; Shiraishi, M. Impact of temperature and sunlight on the skin coloration of the ‘Kyoho’ table grape. Sci. Hortic. 2015, 193, 77–83. [Google Scholar] [CrossRef]
- Brar, H.S.; Singh, Z.; Swinny, E. Dynamics of anthocyanin and flavonol profiles in the ‘Crimson Seedless’ grape berry skin during development and ripening. Sci. Hortic. 2008, 117, 349–356. [Google Scholar] [CrossRef]
- Kuhn, N.; Guan, L.; Dai, Z.W.; Wu, B.-H.; Lauvergeat, V.; Gomès, E.; Li, S.-H.; Godoy, F.; Arce-Johnson, P.; Delrot, S. Berry ripening: Recently heard through the grapevine. J. Exp. Bot. 2013, 65, 4543–4559. [Google Scholar] [CrossRef]
- Baluja, J.; Tardaguila, J.; Ayestarán, B.; Diago, M.P. Spatial variability of grape composition in a Tempranillo (Vitis vinifera L.) vineyard over a 3-year survey. Precis. Agric. 2012, 14, 40–58. [Google Scholar] [CrossRef]
- Serrano, L.; González-Flor, C.; Gorchs, G. Assessment of grape yield and composition using the reflectance based Water Index in Mediterranean rainfed vineyards. Remote. Sens. Environ. 2012, 118, 249–258. [Google Scholar] [CrossRef]
- Filippetti, I.; Allegro, G.; Valentini, G.; Pastore, C.; Colucci, E.; Intrieri, C. Influence of vigour on vine performance and berry composition of cv. Sangiovese (Vitis vinifera L.). OENO One 2013, 47, 21. [Google Scholar] [CrossRef]

| Name | Location (Latitude Longitude) | Mean Altitude (m a.s.l.) | Area (m2) | Planting Distance (m) 1 | Row Orientation | Training System | Plant Age (Years) | Yield (2018) (tn ha−1) |
|---|---|---|---|---|---|---|---|---|
| Katerini | 40°12′52.30″N 22°25′50.06″E | 89 | 7900 | 3.6 × 2.2 | NW–SE | Semi-pergola | 11 | 16 |
| Kavala1 | 40°49′30.88″N 24°16′22.55″E | 15 | 2800 | 2.5 × 1.6 | NNE–SSW | Y-shaped trellis | 8 | 48 |
| Kavala2 | 40°48′38.35″N 24°13′32.48″E | 179 | 2100 | 2.5 × 1.6 | NNW–SSE | Y-shaped trellis | 6 | 45 |
| Kilkis1 | 41° 3′11.64″N 22°57′32.02″E | 257 | 4400 | 3.0 × 2.4 | N–S | Y-shaped trellis | 7 | 20 |
| Kilkis2 | 41°4′41.83″N 22°46′40.58″E | 102 | 14200 | 3.0 × 2.4 | E–W | Pergola | 10 | 35 |
| Index | Plot | Spread (%) | CV (%) | CI (%) | MCD (m) | MSE | RCVP |
|---|---|---|---|---|---|---|---|
| SFRR | |||||||
| Katerini | 73 | 14.2 | 30.00 | 5.51 | 0.005 | 0.583 | |
| Kavala1 | 70 | 10.3 | 100 | 0.00 | -- | -- | |
| Kavala2 | 52 | 9.1 | 69.30 | 1.21 | 0.0046 | 0.099 | |
| Kilkis1 | 62 | 12.7 | 52.53 | 3.04 | 0.007 | 0.452 | |
| Kilkis2 | 49 | 9.7 | 100.00 | 0.00 | 0.005 | 0.199 | |
| ANTHRG | |||||||
| Katerini | 99 | 15.5 | 52.88 | 6.63 | 0.006 | 0.449 | |
| Kavala1 | 136 | 24.5 | 50.00 | 1.58 | 0.011 | 0.342 | |
| Kavala2 | 99 | 20.4 | 74.29 | 1.45 | 0.008 | 0.158 | |
| Kilkis1 | 152 | 29.5 | 30.34 | 5.33 | 0.014 | 0.604 | |
| Kilkis2 | 157 | 31.7 | 53.59 | 6.27 | 0.009 | 0.584 |
| Map | Cluster Area (%) | |||||
|---|---|---|---|---|---|---|
| High–High | Low–Low | Low–High | High–Low | HH + LL 1 | HL + LH 1 | |
| ANTHRG+SFRR | 29.6 | 6.3 | 31.5 | 32.7 | 35.8 | 64.2 |
| Yield+SFRR | 42.7 | 23.2 | 18.4 | 15.7 | 65.9 | 34.1 |
| Yield+ANTHRG | 33.2 | 12.4 | 29.1 | 25.3 | 45.6 | 54.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuccio, L.; Cavigli, L.; Rossi, F.; Dichala, O.; Katsogiannos, F.; Kalfas, I.; Agati, G. Fluorescence-Sensor Mapping for the in Vineyard Non-Destructive Assessment of Crimson Seedless Table Grape Quality. Sensors 2020, 20, 983. https://doi.org/10.3390/s20040983
Tuccio L, Cavigli L, Rossi F, Dichala O, Katsogiannos F, Kalfas I, Agati G. Fluorescence-Sensor Mapping for the in Vineyard Non-Destructive Assessment of Crimson Seedless Table Grape Quality. Sensors. 2020; 20(4):983. https://doi.org/10.3390/s20040983
Chicago/Turabian StyleTuccio, Lorenza, Lucia Cavigli, Francesca Rossi, Olga Dichala, Fotis Katsogiannos, Ilias Kalfas, and Giovanni Agati. 2020. "Fluorescence-Sensor Mapping for the in Vineyard Non-Destructive Assessment of Crimson Seedless Table Grape Quality" Sensors 20, no. 4: 983. https://doi.org/10.3390/s20040983
APA StyleTuccio, L., Cavigli, L., Rossi, F., Dichala, O., Katsogiannos, F., Kalfas, I., & Agati, G. (2020). Fluorescence-Sensor Mapping for the in Vineyard Non-Destructive Assessment of Crimson Seedless Table Grape Quality. Sensors, 20(4), 983. https://doi.org/10.3390/s20040983







