An Opto-Electronic Sensor-Ring to Detect Arthropods of Significantly Different Body Sizes
Abstract
1. Introduction
2. Materials and Methods
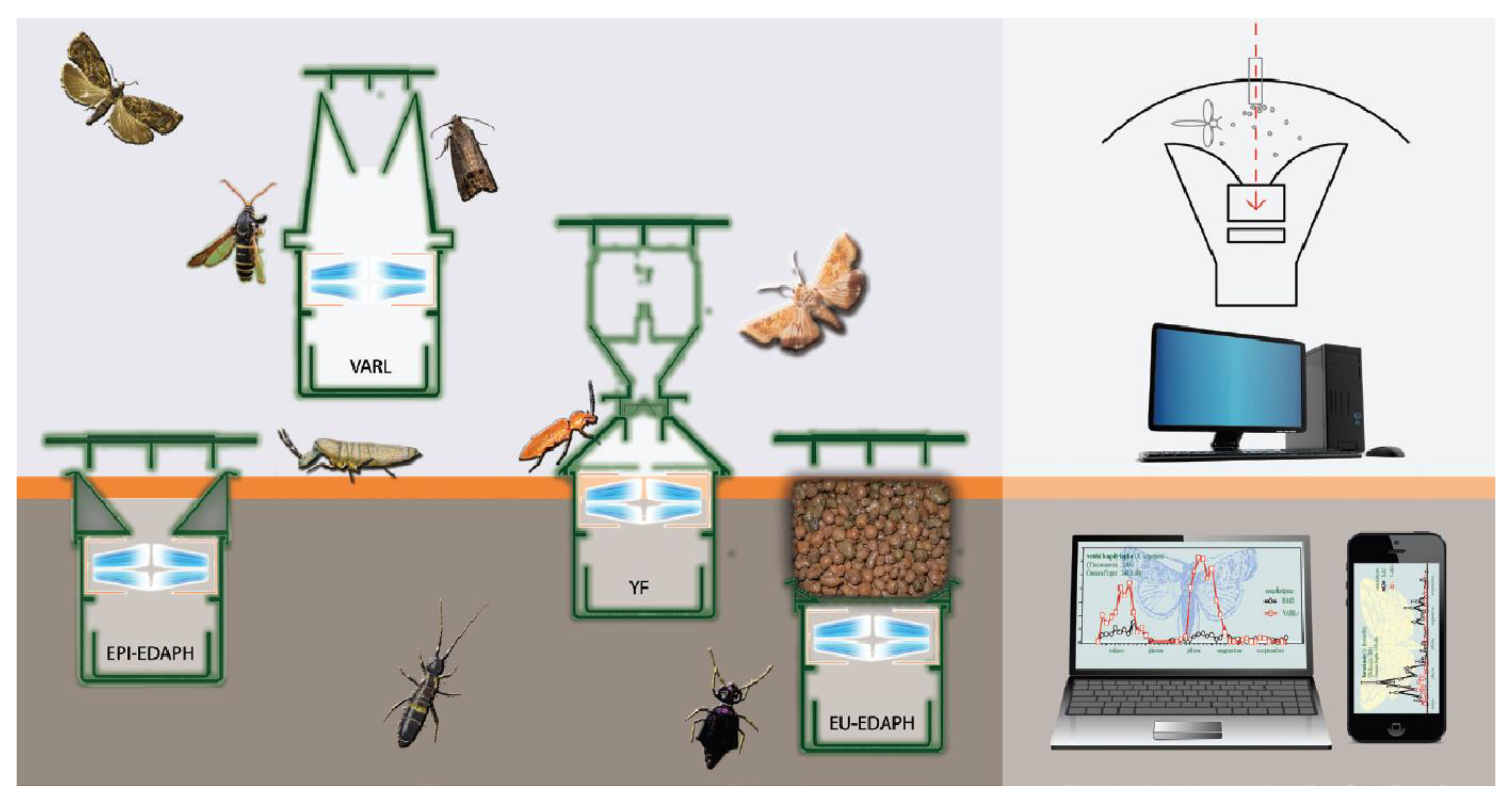
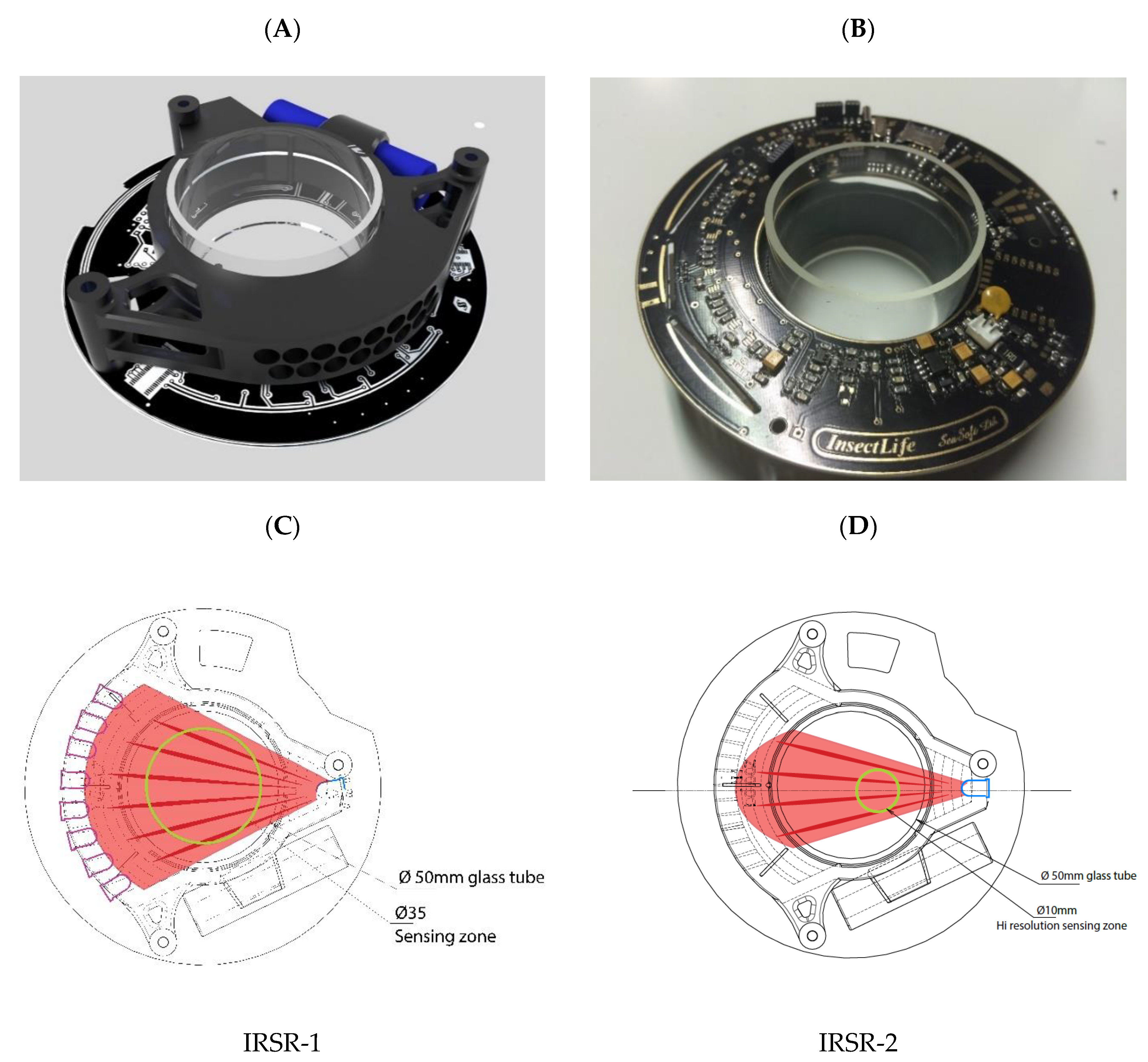
2.1. Mechanical Construction and Operation of the Opto-Electronic IR Sensor-Ring
2.2. Sensor Electronics
2.3. Detection Algorithm
2.4. Settings of the Detection Procedure
2.5. Laboratory Testing of Detection Efficiency
2.5.1. Detectability Investigations with Particles
2.5.2. Detectability Investigations with Arthropods
Detection Accuracy of IRSR-1 for Large Arthropods
Detection Accuracy of IRSR-2 for Small Arthropods
Sensitivity Test of IRSR-2
2.6. Statistics
3. Results
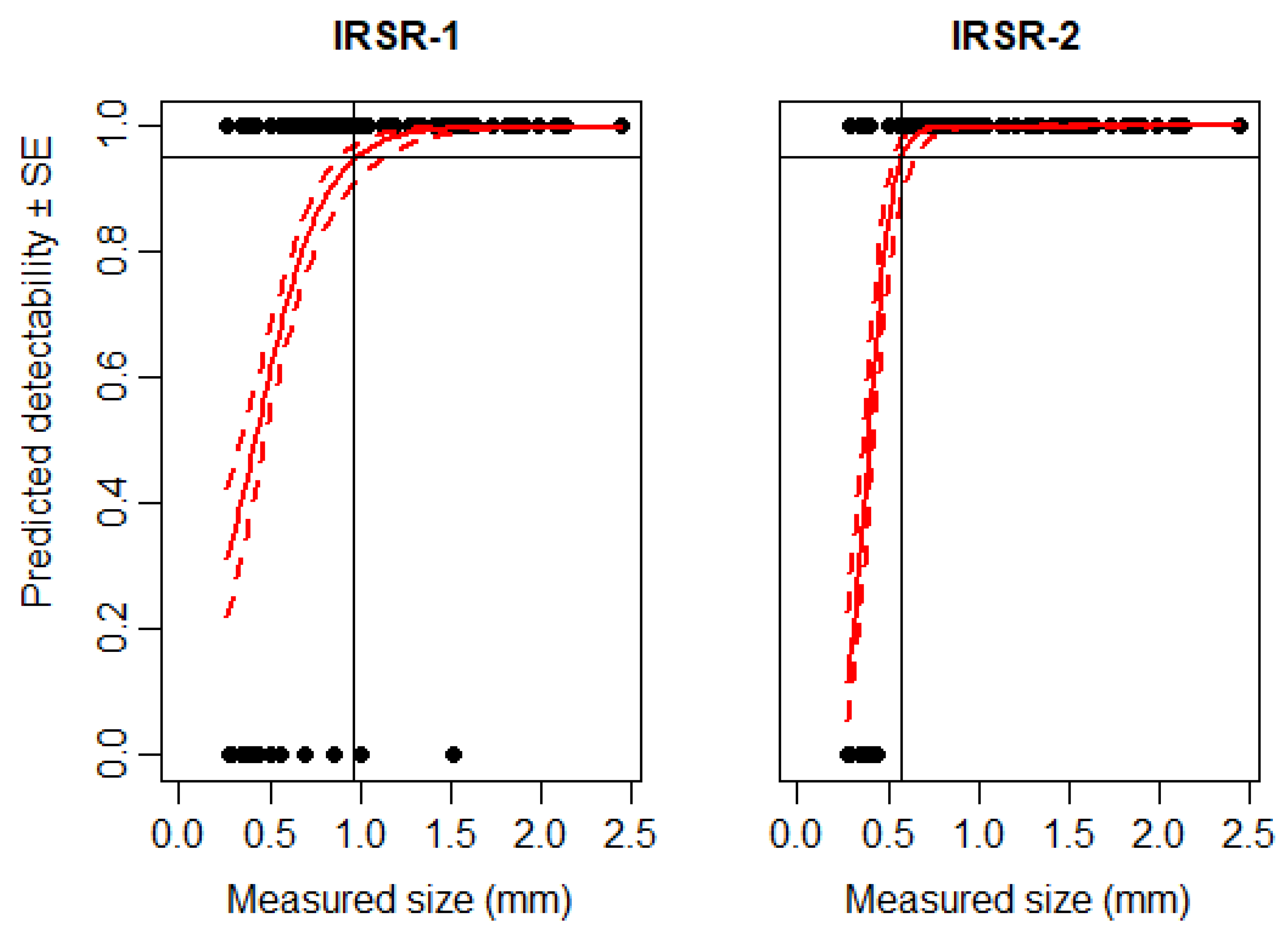
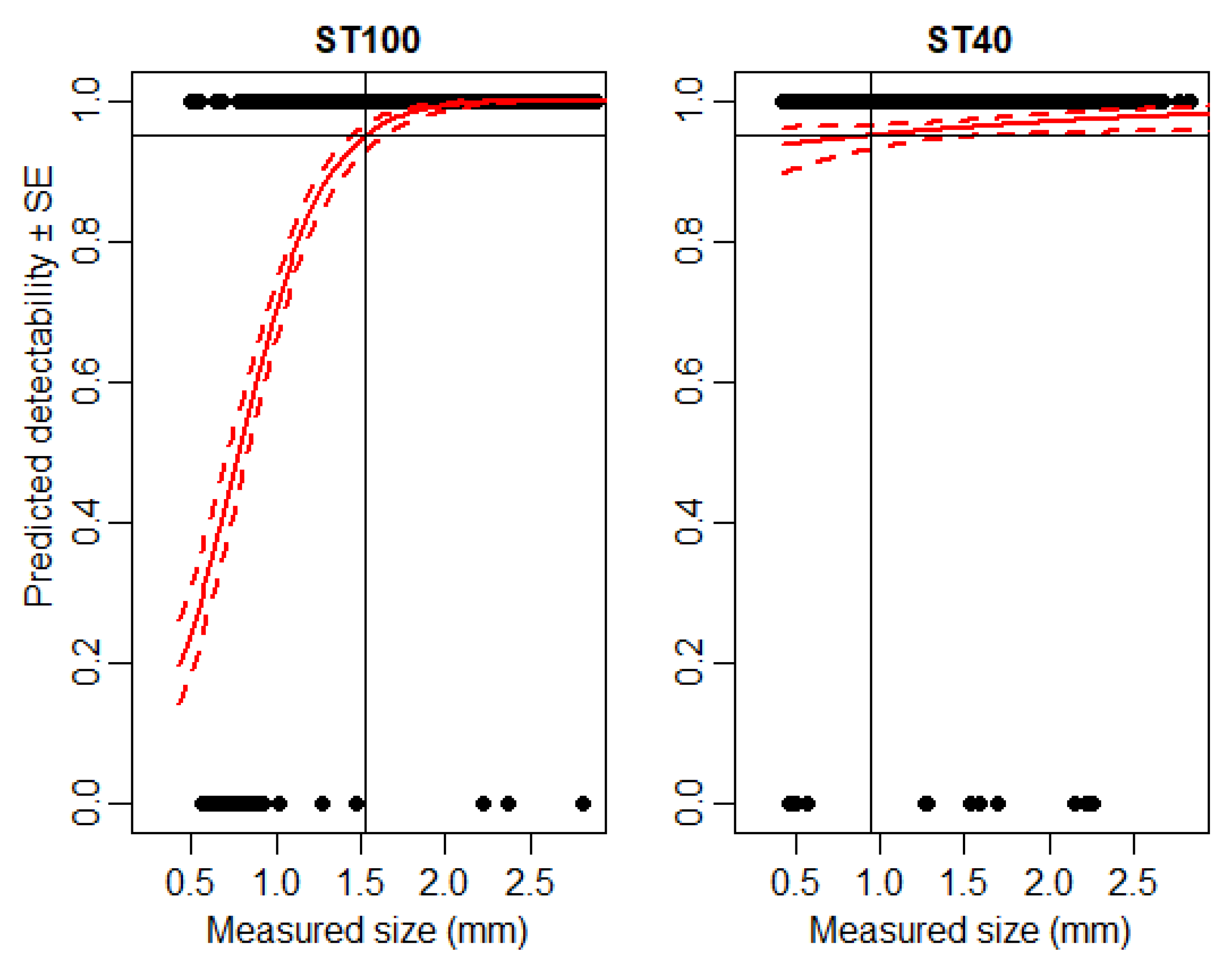
3.1. Detectability of Particles Using IRSR-1 and IRSR-2
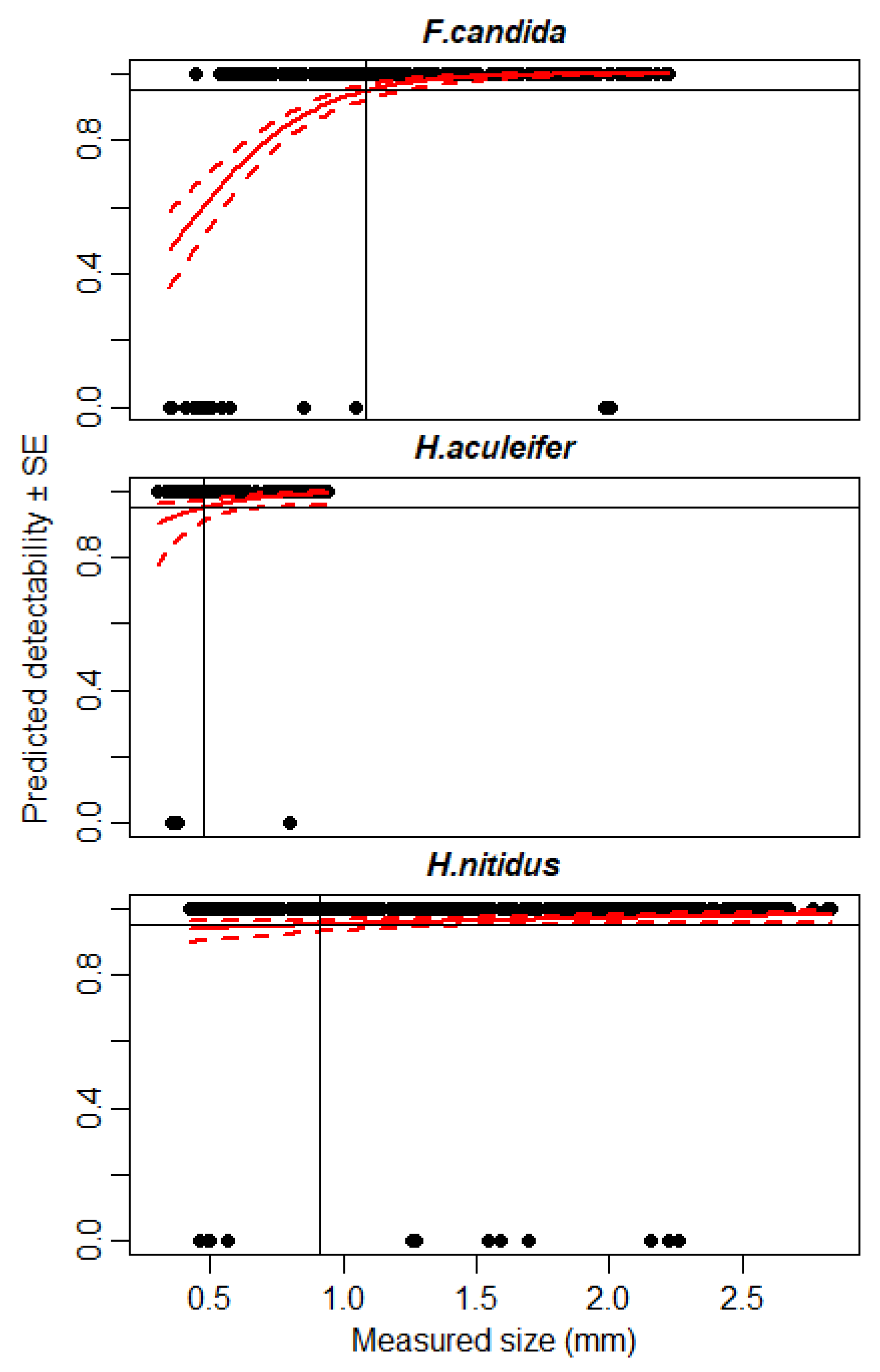
3.2. Detectability of Small Microarthropods Using IRSR-2
3.3. Sensitivity Analysis of IRSR-2
3.4. Detectability of Large Arthropods Using IRSR-1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tibesigwa, B.; Siikamäki, J.; Lokina, R.; Alvsilver, J. Naturally available wild pollination services have economic value for nature dependent smallholder crop farms in Tanzania. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wall, D.; Nielsen, U. Biodiversity and ecosystem services: Is it the same below ground. Nat. Educ. Knowl. 2012, 3, 8. [Google Scholar]
- Powney, G.D.; Carvell, C.; Edwards, M.; Morris, R.K.; Roy, H.E.; Woodcock, B.A.; Isaac, N.J. Widespread losses of pollinating insects in Britain. Nat. Commun. 2019, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Bari, M.; Haque, S.; Kabir, M.; Afrin, S.; Nowrin, F.; Islam, M.; Landis, D. Establishing next-generation pest control services in rice fields: Eco-agriculture. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Dara, S.K. The new integrated pest management paradigm for the modern age. J. Integr. Pest Manag. 2019, 10, 12. [Google Scholar] [CrossRef]
- Pretty, J.; Benton, T.G.; Bharucha, Z.P.; Dicks, L.V.; Flora, C.B.; Godfray, H.C.J.; Goulson, D.; Hartley, S.; Lampkin, N.; Morris, C. Global assessment of agricultural system redesign for sustainable intensification. Nat. Sustain. 2018, 1, 441–446. [Google Scholar] [CrossRef]
- Reynolds, D.; Riley, J. Remote-sensing, telemetric and computer-based technologies for investigating insect movement: A survey of existing and potential techniques. Comput. Electron. Agric. 2002, 35, 271–307. [Google Scholar] [CrossRef]
- Liu, H.; Lee, S.-H.; Chahl, J.S. A review of recent sensing technologies to detect invertebrates on crops. Precis. Agric. 2017, 18, 635–666. [Google Scholar] [CrossRef]
- Chen, Y.; Why, A.; Batista, G.; Mafra-Neto, A.; Keogh, E. Flying insect detection and classification with inexpensive sensors. J. Vis. Exp. 2014, e52111. [Google Scholar] [CrossRef]
- Eliopoulos, P.A.; Potamitis, I.; Kontodimas, D.C. Estimation of population density of stored grain pests via bioacoustic detection. Crop Prot. 2016, 85, 71–78. [Google Scholar] [CrossRef]
- Mankin, R.; Hagstrum, D.; Smith, M.; Roda, A.; Kairo, M. Perspective and promise: A century of insect acoustic detection and monitoring. Am. Entomol. 2011, 57, 30–44. [Google Scholar] [CrossRef]
- Mankin, R.; Machan, R.; Jones, R. Field testing of a prototype acoustic device for detection of Mediterranean fruit flies flying into a trap. In Proceedings of the 7th International Symposium on Fruit Flies of Economic Importance, Salvador, Brazil, 10–15 September 2006; pp. 10–15. [Google Scholar]
- Raman, D.R.; Gerhardt, R.R.; Wilkerson, J.B. Detecting insect flight sounds in the field: Implications for acoustical counting of mosquitoes. Trans. ASABE 2007, 50, 1481–1485. [Google Scholar] [CrossRef]
- Njoroge, A.; Affognon, H.; Richter, U.; Hensel, O.; Rohde, B.; Chen, D.; Mankin, R. Acoustic, Pitfall Trap, and Visual Surveys of Stored Product Insect Pests in Kenyan Warehouses. Insects 2019, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Batista, G.E.; Hao, Y.; Keogh, E.; Mafra-Neto, A. Towards automatic classification on flying insects using inexpensive sensors. In Proceedings of the 2011 10th International Conference on Machine Learning and Applications and Workshops, Honolulu, HI, USA, 18–21 December 2011; pp. 364–369. [Google Scholar]
- De Souza, V.M.; Silva, D.F.; Batista, G.E. Classification of data streams applied to insect recognition: Initial results. In Proceedings of the 2013 Brazilian Conference on Intelligent Systems, Fortaleza, Brazil, 19–24 October 2013; pp. 76–81. [Google Scholar]
- Arbogast, R.T.; Kendra, P.E.; Weaver, D.K.; Shuman, D. Insect infestation of stored oats in Florida and field evaluation of a device for counting insects electronically. J. Econ. Entomol. 2000, 93, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.-A.; Tseng, C.-L.; Lu, F.-M.; Yang, E.-C.; Wu, Z.-S.; Chen, C.-P.; Lin, S.-H.; Lin, K.-C.; Liao, C.-S. A GSM-based remote wireless automatic monitoring system for field information: A case study for ecological monitoring of the oriental fruit fly, Bactrocera dorsalis (Hendel). Comput. Electron. Agric. 2008, 62, 243–259. [Google Scholar] [CrossRef]
- Potamitis, I.; Rigakis, I.; Fysarakis, K. The electronic McPhail trap. Sensors 2014, 14, 22285–22299. [Google Scholar] [CrossRef]
- Potamitis, I.; Rigakis, I.; Vidakis, N.; Petousis, M.; Weber, M. Affordable bimodal optical sensors to spread the use of automated insect monitoring. J. Sens. 2018. [Google Scholar] [CrossRef]
- Rigakis, I.; Potamitis, I.; Tatlas, N.-A.; Livadaras, I.; Ntalampiras, S. A Multispectral Backscattered Light Recorder of Insects’ Wingbeats. Electronics 2019, 8, 277. [Google Scholar] [CrossRef]
- Shieh, J.-C.; Wang, J.-Y.; Lin, T.-S.; Lin, C.-H.; Yang, E.-C.; Tsai, Y.-J.; Tsai, H.-T.; Chiou, M.-T.; Lu, F.-M.; Jiang, J.-A. A GSM-based field monitoring system for Spodoptera litura (Fabricius). Eng. Agric. Environ. Food 2011, 4, 77–82. [Google Scholar] [CrossRef]
- Bánszegi, O.; Kosztolányi, A.; Bakonyi, G.; Szabó, B.; Dombos, M. New method for automatic body length measurement of the collembolan, Folsomia candida Willem 1902 (insecta: Collembola). PLoS ONE 2014, 9, e98230. [Google Scholar] [CrossRef]
- Fukatsu, T.; Watanabe, T.; Hu, H.; Yoichi, H.; Hirafuji, M. Field monitoring support system for the occurrence of Leptocorisa chinensis Dallas (Hemiptera: Alydidae) using synthetic attractants, Field Servers, and image analysis. Comput. Electron. Agric. 2012, 80, 8–16. [Google Scholar] [CrossRef]
- Guarnieri, A.; Maini, S.; Molari, G.; Rondelli, V. Automatic trap for moth detection in integrated pest management. Bull. Insectology 2011, 64, 247–251. [Google Scholar]
- Selby, R.; Gage, S.; Whalon, M. Precise and low-cost monitoring of plum curculio (Coleoptera: Curculionidae) pest activity in pyramid traps with cameras. Environ. Entomol. 2014, 43, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wang, J.; Liu, H.; Mi, H. Insect Identification and Counting in Stored Grain: Image Processing Approach and Application Embedded in Smartphones. Mob. Inf. Syst. 2018. [Google Scholar] [CrossRef]
- Fanioudakis, E.; Geismar, M.; Potamitis, I. Mosquito wingbeat analysis and classification using deep learning. In Proceedings of the 2018 26th European Signal Processing Conference (EUSIPCO), Rome, Italy, 3–7 September 2018; pp. 2410–2414. [Google Scholar]
- Genoud, A.P.; Gao, Y.; Williams, G.M.; Thomas, B.P. Identification of gravid mosquitoes from changes in spectral and polarimetric backscatter cross-sections. J. Biophotonics 2019. [Google Scholar] [CrossRef]
- Holguin, G.A.; Lehman, B.L.; Hull, L.A.; Jones, V.P.; Park, J. Electronic traps for automated monitoring of insect populations. IFAC Proc. Vol. 2010, 43, 49–54. [Google Scholar] [CrossRef]
- Goldshtein, E.; Cohen, Y.; Hetzroni, A.; Gazit, Y.; Timar, D.; Rosenfeld, L.; Grinshpon, Y.; Hoffman, A.; Mizrach, A. Development of an automatic monitoring trap for Mediterranean fruit fly (Ceratitis capitata) to optimize control applications frequency. Comput. Electron. Agric. 2017, 139, 115–125. [Google Scholar] [CrossRef]
- Potamitis, I.; Rigakis, I.; Fysarakis, K. Insect biometrics: Optoacoustic signal processing and its applications to remote monitoring of McPhail type traps. PLoS ONE 2015, 10, e0140474. [Google Scholar] [CrossRef]
- Potamitis, I.; Rigakis, I.; Tatlas, N.-A. Automated surveillance of fruit flies. Sensors 2017, 17, 110. [Google Scholar] [CrossRef] [PubMed]
- Moraes, F.S.; Nava, D.E.; da Rosa, V.S. Optoacoustic intelligent sensor for real-time detection of fruit flies in McPhail traps. In Proceedings of the 2018 3rd International Symposium on Instrumentation Systems, Circuits and Transducers (INSCIT), Bento Goncalves, Brazil, 27–31 August 2018; pp. 1–6. [Google Scholar]
- Dombos, M.; Kosztolányi, A.; Szlávecz, K.; Gedeon, C.; Flórián, N.; Groó, Z.; Dudás, P.; Bánszegi, O. EDAPHOLOG monitoring system: Automatic, real-time detection of soil microarthropods. Methods Ecol. Evol. 2017, 8, 313–321. [Google Scholar] [CrossRef]
- Gedeon, C.; Flórián, N.; Liszli, P.; Hambek-Oláh, B.; Bánszegi, O.; Schellenberger, J.; Dombos, M. An Opto-electronic sensor for detecting soil microarthropods and estimating their size in field conditions. Sensors 2017, 17, 1757. [Google Scholar] [CrossRef] [PubMed]
- Shuman, D.; Larson, R.; Epsky, N. A quantitative stored-product insect monitoring system using sensor output analog processing (SOAP). Trans. ASAE 2004, 47, 1857–1864. [Google Scholar] [CrossRef]
- Perles, A.; Mercado, R.; Capella, J.V.; Serrano, J.J. Ultra-Low power optical sensor for xylophagous insect detection in wood. Sensors 2016, 16, 1977. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: www.r-project.org (accessed on 11 December 2019).
| Target Species | Probe/Sensor | Body Size (mm)/ Species Selectivity | Accuracy/ Selectivity of Trapping | Publication/ Tests (F: field, L: laboratory) |
|---|---|---|---|---|
| Flying insects | ||||
| Mediterranean fruit fly [Ceratitis capitate Wiedemann (Diptera: Tephritidae)] | IR sensors placed in a commercial medfly trap | 3.5–5 | --- pheromone | [30] F |
| Oriental fruit fly [Bactrocera dorsalis, Hendel (Diptera: Tephritidae)] | Double-counting IR sensors in a trapping tube placed in a bottle | 7–8 | --- pheromone | [18] F |
| Mediterranean fruit fly [Ceratitis capitate Wiedemann (Diptera: Tephritidae)] | Imagos of insects are attracted and narcotized by toxins, then counted by IR sensors | 3.5–5 | 88%–100% pheromone | [31] F, L |
| Olive fruit fly [Bactrocera oleae Rossi (Diptera: Tephritidae)] | Opto-acoustic sensor and IR sensors in a McPhail trap; the wingbeat recording is based on the interruption of the emitted light | 0.7–1.2 species-specific | 90%–96% detailed accuracy report pheromone | [19,32] L |
| Fruit flies: Olive fruit fly (B. oleae) Mediterranean fruit fly (C. capitate) Oriental fruit fly (B. dorsalis) | IR sensor lines in a McPhail trap, opto-acoustics, with Fast Fourier Transformation analysis | 0.7–8 species-specific | 90%–96% detailed accuracy report pheromone | [33] F, L |
| Fruit flies: Olive fruit fly (B. oleae) Mediterranean fruit fly (C. capitate) Oriental fruit fly (B. dorsalis) | Wingbeat sensing based on Fresnel optics, stereo wingbeat recorder, equipped in a McPhail trap | 0.7–8 species-specific | 98%–99% pheromone | [20] F |
| Fruit flies: South American fruit fly [Anastrepha fraterculus Wiedemann (Diptera: Tephritidae)]; West Indian fruit fly [Anastrepha obliqua Marquart (Diptera: Tephritidae)]; South American cucurbit fruit fly [Anastrepha grandis Marquart (Diptera: Tephritidae)]; C. capitate; Carambola fruit fly [Bactrocera carambolae Drew and Hancock (Diptera: Tephritidae)] | McPhail trap with an opto-acoustic sensor line; | 0.7–5 species-specific | simulation only | [34] L |
| Mosquitos, house fly, and fruit flies: Culex tarsalis Coquilett (Diptera: Culicidae); Culex quinquefasciatus Say (Diptera: Culicidae); Culex stigmatosoma Dyar (Diptera: Culicidae); Aedes aegypti Linnaeus (Diptera: Culicidae); Musca domestica Linnaeus (Diptera: Muscidae); Drosophyla simulans Sturtevant (Diptera: Drosophilidae) | Phototransistor array with laser beams; species classification according to wingbeat diversity analyzed by Bayesian network | 4–15 | 80%–98% | [9] L |
| Drosophila suzukii (Diptera: Drosophilidae); Drosophila melanogaster Meigen (Diptera: Drosophilidae); Zaprionus sp. (Diptera: Drosophilidae) | Multispectral sensor, backscattered light recorder | 4–7 | -- pheromone | [21] L |
| Wingless arthropods | [22] | |||
| Tobacco cutworm larvae: Spodoptera litura Fabricius (Lepidoptera: Noctuidae) | Insect larvae are detected when they move down/fall in the trap tube. Two IR sensors (double-counting) | 2.3–32 | 80%–90% pheromone | L, F |
| Soil microarthropods: Collembola, Acari | Soil microarthropods are caught in a pitfall-like trap, they fall in a small tube and are detected cross-sectionally by one IR sensor through two plano-convex optical lenses | 0.5–10 body size differentiation | 80%–100% no pheromone | [35,36] L, F |
| Four stored-product species: Flat grain beetle [Cryptolestes pusillus Schönherr (Coleoptera: Laemophloeidae)]; Sawtoothed grain beetle [Oryzaephilus surinamensis (Linnaeus, 1758) (Coleoptera: Silvanidae)]; Red flour beetle [Tribolium castaneum Herbst (Insecta: Coleoptera: Tenebrionidae)]; Rice weevil [Sitophilus oryzae Linnaeus (Coleoptera: Curculionidae)] | Trap tubes are placed in stored products equipped with one IR phototransistor | 2–3 | no data no pheromone | [37] L [17] F |
| Termites: Reticulitermes lucifugus Rossi (Blattodea: Rhinotermitida) | Low-power light sensor (white insects on a black background) | 3–4 | [38] L |
| Amplifier | Consumption | Gain-Bandwidth Product | Amplification | Power Supply |
|---|---|---|---|---|
| MCP6141 | 600 nA | 100 kHz | 15x | 3.6 V Li-ion accumulator |
| MCP607 | 60 µA | 600 kHz | 30x |
| Species | Mean Size (mm) | Min. Size (mm) | Max. Size (mm) | Detection |
|---|---|---|---|---|
| Hypoaspis aculeifer (Acari) | 1.32 | 0.26 | 0.92 | 40% |
| Heteromurus nitidus (Collembola) | 1.34 | 0.58 | 2.38 | 83% |
| Meligethes aeneus (Coleoptera) | 2.37 | 1.40 | 2.40 | 95% |
| Cameraria ohridella (Lepidoptera) | 2.77 | 2.18 | 3.19 | 100% |
| Diabrotica virgifera (Coleoptera) | 5.16 | 4.53 | 6.30 | 100% |
| Agriotes ustulatus (Coleoptera) | 8.06 | 7.29 | 10.21 | 100% |
| Ephestia kuehniella (Lepidoptera) | 8.75 | 6.35 | 10.60 | 100% |
| Epicometis hirta (Coleoptera) | 10.53 | 9.13 | 11.70 | 100% |
| Operophtera brumata (Lepidoptera) | 15.97 | 14.14 | 19.28 | 100% |
| Cetonia aurata (Coleoptera) | 16.77 | 14.43 | 20.09 | 100% |
| Autographa gamma (Lepidoptera) | 21.67 | 15.77 | 23.94 | 100% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balla, E.; Flórián, N.; Gergócs, V.; Gránicz, L.; Tóth, F.; Németh, T.; Dombos, M. An Opto-Electronic Sensor-Ring to Detect Arthropods of Significantly Different Body Sizes. Sensors 2020, 20, 982. https://doi.org/10.3390/s20040982
Balla E, Flórián N, Gergócs V, Gránicz L, Tóth F, Németh T, Dombos M. An Opto-Electronic Sensor-Ring to Detect Arthropods of Significantly Different Body Sizes. Sensors. 2020; 20(4):982. https://doi.org/10.3390/s20040982
Chicago/Turabian StyleBalla, Esztella, Norbert Flórián, Veronika Gergócs, Laura Gránicz, Franciska Tóth, Tímea Németh, and Miklós Dombos. 2020. "An Opto-Electronic Sensor-Ring to Detect Arthropods of Significantly Different Body Sizes" Sensors 20, no. 4: 982. https://doi.org/10.3390/s20040982
APA StyleBalla, E., Flórián, N., Gergócs, V., Gránicz, L., Tóth, F., Németh, T., & Dombos, M. (2020). An Opto-Electronic Sensor-Ring to Detect Arthropods of Significantly Different Body Sizes. Sensors, 20(4), 982. https://doi.org/10.3390/s20040982






